A novel evaluation model of image registration for cone-beam computed tomography guided lung cancer radiotherapy
Yimei Liu, Meining Chen, and Jianlan Fang contributed equally to this study and are cofirst authors.
Abstract
Background
The aim of the study was to establish a weighted comprehensive evaluation model (WCEM) of image registration for cone-beam computed tomography (CBCT) guided lung cancer radiotherapy that considers the geometric accuracy of gross target volume (GTV) and organs at risk (OARs), and assess the registration accuracy of different image registration methods to provide clinical references.
Methods
The planning CT and CBCT images of 20 lung cancer patients were registered using diverse algorithms (bony and grayscale) and regions of interest (target, ipsilateral, and body). We compared the coverage ratio (CR) of the planning target volume (PTVCT) to GTVCBCT, as well as the dice similarity coefficient (DSC) of the GTV and OARs, considering the treatment position across various registration methods. Furthermore, we developed a mathematical model to assess registration results comprehensively. This model was evaluated and validated using CRFs across four automatic registration methods.
Results
The grayscale registration method, coupled with the registration of the ipsilateral structure, exhibited the highest level of automatic registration accuracy, the DSC were 0.87 ± 0.09 (GTV), 0.71 ± 0.09 (esophagus), 0.74 ± 0.09 (spinal cord), and 0.91 ± 0.05 (heart), respectively. Our proposed WCEM proved to be both practical and effective. The results clearly indicated that the grayscale registration method, when applied to the ipsilateral structure, achieved the highest CRF score. The average CRF scores, excellent rates, good rate and qualification rates were 58 ± 26, 40%, 75%, and 85%, respectively.
Conclusions
This study successfully developed a clinically relevant weighted evaluation model for CBCT-guided lung cancer radiotherapy. Validation confirmed the grayscale method's optimal performance in ipsilateral structure registration.
INTRODUCTION
Image-guided radiotherapy (IGRT) ensures the precise implementation of advanced radiation techniques, such as intensity-modulated radiation therapy (IMRT) and stereotactic body radiotherapy (SBRT). Bissonnette et al.1 confirmed that the verification and adjustment of radiotherapy setup, guided by cone-beam computed tomography (CBCT), can effectively enhance the geometric accuracy of lung cancer radiotherapy and minimize the external margin of the planning target volume (PTV). The accuracy of CBCT-guided radiotherapy positioning is influenced by various factors, primarily observer-related differences in judgment,2, 3 the quality of images used for registration,4-6 different registration algorithms,7-9 and the choice of registration regions.10-12 Some studies have emphasized that selecting different regions of interest (ROIs) for online image registration in planning images can lead to varying displacements. Incorrect displacement adjustments might not only fail to correct errors in dose distribution during treatment but also result in inadequate dosing to the target or excessive radiation exposure to organs at risk (OARs).13, 14 Previous studies exploring the impact of different registration regions on registration accuracy during CBCT-guided radiotherapy for lung cancer are limited, highlighting the need for further research.
Another challenge in the clinical application of IGRT lies in the evaluation of registration results. Various methods have been employed for IGRT registration evaluations, including the assessment of position deviations (setup error) through comparing CBCT scan images before and after the correction of setup errors in planning CT images,15 as well as the evaluation and guidance of setup correction based on position deviations in bone structures9 and internal metal markers.16 However, these methods primarily focus on assessing errors at specific geometric points and fail to provide comprehensive insights such as volume overlap rates of anatomical structures after registration, coverage ratios (CRs) of the PTV to targets, or other volume error parameters. Additionally, they lack the ability to comprehensively consider and balance registration accuracy between the target and OARs, thus limiting their clinical utility in providing adequate setup correction information. Furthermore, real-world patients may experience relative displacement of organs, tumor regression, and changes in body shape during fractionated radiotherapy. Consequently, the position deviations of different parts and organs may vary during each treatment setup. Therefore, it is crucial to select appropriate IGRT image registration and evaluation methods based on clinical requirements, taking into account the geometric accuracy demands of both the target and various OARs to guide setup corrections.
In summary, we collected planning CT images and online CBCT images from lung cancer patients, accurately delineating both target areas and OARs on these images. Initially, we conducted a thorough analysis of the dice similarity coefficient (DSC) and the coverage ratio (CR) of the PTV to target volume in CBCT images, employing various registration algorithms and combinations of registration regions. This allowed us to compare the registration accuracy of different methods. Subsequently, we introduced a method that takes into account both the geometric accuracy of the target volume and OARs, enabling a comprehensive evaluation of registration results through a weighted assessment approach. By developing a mathematical model, we quantified this assessment using the weighted comprehensive evaluation model (WCEM), providing clinicians with valuable references for selecting and evaluating registration methods in CBCT-guided radiotherapy.
METHODS
General clinical data
Between September 2013 and February 2015, 20 lung cancer patients were treated with IGRT at our cancer center. Of these patients, 14 were male and six were female, with a median age of 66 years (range: 40–83 years). The distribution of patients by cancer stage were as follows: six patients with stage I, eight with stage II, four with stage III, and two with stage IV. Lesions were observed in the right lung in eight patients and the left lung in 12 patients; 10 lesions were detected in the upper lobe, four in the middle lobe, and six in the lower lobe. For treatment plans of all patients, the gross target volume (GTV) varied from 4.29 to 182.36 cc (average: 48.34 cc).
Acquisition of the treatment planning CT and organs delineation
A CT scanner (Brilliance Big Bore, Philips) was utilized to perform four-dimensional CT (4DCT) scanning for simulation positioning. The patients were positioned in a supine posture and immobilized using vacuum and mold. The CT scanning parameters were meticulously set at 140 kV and 250 mAs, with slice thickness and interval precisely adjusted to 5 and 3 mm, respectively. Once acquired, the planning CT images were promptly transferred to the Monaco treatment planning system (TPS, version V3.2, Elekta AB). Drawing from the ICRU Report 83,17 the GTVCT and OARCT (inclusive of lungs, heart, esophagus, and spinal cord) were initially automatically delineated. These contours were then manually refined and verified by a radiation oncologist using the treatment planning CT (4D-CT) images. Subsequently, the GTVCT was expanded by 5 mm to derive the PTVCT. Following the completion of contour delineation, treatment planning was commenced. Once the plan was approved by a physician, the delineated GTV and OARs from the planning CT images were transmitted to the accelerator's image-guided system workstation. These contours served as references during the online CBCT image registration process.
Acquisition of the CBCT and organs delineation
The setup utilized laser markers commonly employed during the initial treatment of patients. The accelerator's X-ray volume imaging system, XVI (version R4.2, Elekta AB), was utilized for CBCT scanning. The scanning parameters were set to 120 kV and 650 mAs. The scan was executed in a counterclockwise rotation, ranging from 179.9° to 180.1°, utilizing an M20 collimator with a field of view (FOV) of 42.6 cm. The images were reconstructed at a medium resolution, featuring a slice thickness of 5 mm. Upon completion of reconstruction, these images were transferred to the XVI image registration workstation, serving as floating images for the registration process. Concurrently, the initial setup CBCT images were acquired and sent to the radiation treatment planning system after reconstruction. On the CBCT, we initially performed rigid registration between the CBCT and CT to delineate the contours of the GTV and OARs. These contours were then refined and verified by the same physician on the CBCT. The visible GTVCBCT and OARCBCT on the CBCT images were utilized for the analysis of volume overlap.
Image registration with different methods
- Target registration group: The registration region encompassed an expansion of 1 cm in all directions—left–right, up-down, and front-back—from the perimeter of the PTV.
- Ipsilateral registration group: In the left–right direction, the registration region encompassed the ribs situated on the side where both the spine and the tumor were located. For the up-down direction, it included the apex and base of the lung on the affected side. And in the front-back direction, it encompassed the sternum and the outermost portion of the vertebrae.
- Body registration group: This registration region encompassed the entire scanned area of the CBCT image.
According to different registration regions, 6D (translation and rotation) rigid registration method was used to image register by selecting bony registration and gray registration algorithms respectively. Therefore, six groups of automatic registration results were obtained. Furthermore, utilizing the registration method commonly employed in clinical settings, we obtained the registration results for the clinical group. Additionally, we documented and compared the registration times as well as the couch shift data for each respective group.
Parameters for the evaluation of registration accuracy
The impact of different registration methods on the positioning accuracy of image-guided radiation therapy for lung cancer was evaluated by comparing the CR of the PTVCT to the corresponding GTVCBCT. Additionally, the DSC between the target volume (GTVCT) and OARs (OARCT) on the planning CT with the GTVCBCT and OARCBCT were examined in each registration group. The image registration times of various methods were also compared.
The evaluation indices are defined as follows:
Establishment of the weighted comprehensive evaluation model of image registration
To establish the WCEM that assigns clinical treatment priority based on registration accuracy in target volumes and OARs, as well as scenarios demanding balanced consideration, we evaluated the registration results approved by clinicians during the image-guided setup for the 20 previously mentioned patients utilizing CBCT (clinic group). The distribution of the DSC for the target volume and OARs (including heart, esophagus, and spinal cord) was analyzed to determine the average DSC for each organ structure, establishing a standard DSC (DSCS).
- The weight of the evaluation was determined by the ratio of the standard DSC (DSCS) for each organ structure in the statistical results of clinically accepted registrations.
- The ratio of the standard DSCS to the DSC of the registration was evaluated to assess the registration effectiveness. This ratio indicates the relative quality of the registration outcome being evaluated in comparison to the average result typically accepted in clinical practice.
FGTV is defined as a target coverage factor. According to the clinical requirements for lung cancer radiotherapy, the GTV should be completely covered by 100% of the prescription dose. Therefore, if the PTV in the treatment planning CT does not fully encompass the GTV (based on the CBCT image) during treatment, that is, if the coverage ratio CRGTV ≠ 100%, then FGTV = 0. Conversely, if it provides complete coverage, or CRGTV = 100%, then FGTV = 1. Herein, CRGTV represents the CR of the PTVCT of the GTV during treatment.
FOARi is defined as the safety factor for OARs. In SBRT treatments, as the entire PTV is subjected to a high dose of radiation, the presence of an OAR within this range could lead to serious complications. Therefore, if the planned region of PTVCT does not overlap with the OAR in the setup registered CBCT, then FOARi = 1. If there is an overlap, then FOARi = 0. No such restriction applies to conventional radiotherapy; therefore, FOARi = 1 is true for all conventional radiotherapy.
wGTV and wOARi are defined as weight factors. Depending on the level of clinical importance placed on the GTV and various OARs, a weight factor value was assigned to each GTV and OAR. The values of these weight factors can also be customized based on the specific conditions of the patient. In this study, the initial weight factors for GTV and OARs were assigned according to the registration results from the IGRT clinicians in our hospital. Through statistical analysis, the ratio of average DSC for each organ structure in the reference registration results was used to determine the registration weight of the GTV and each organ. A higher average DSC in standard registration implies a greater requirement for clinical accuracy, which results in a larger weight assignment.
KGTV and KOARi represent the registration effect factors for each organ. There are the ratio of the average DSC from the clinical manual registration of each organ to the DSC of the registration under evaluation (DSCS/DSC). When K < 1, the registration result for that structure surpasses the average of the standard clinical manual registration, the smaller the value, the higher the registration accuracy. When K >1, the registration result is inferior, with a larger value indicating a lower registration accuracy. Considering the relatively large volume of the heart, the effect of registration error on its DSC is minimal (with the average DSCheart by standard manual registration being 0.90 ± 0.04). Therefore, the registration effect factor for the heart was assigned a fixed value of 1/2.
Determination of weight factors for WCEM
The weight factors for WCEM were defined through a statistical analysis of the registration results data, which were accepted by a group of clinical physicians (clinic group). The specific method is that we calculate the ratio of the average DSC value of each organ to obtain the weight factor of each organ, and the weight factor ranges from 0 to 1.
WCEM model validation
In accordance with the previously stated evaluation methods and formula definitions, it is anticipated that under ideal registration conditions—wherein the GTV and the evaluated OAR exhibit perfect overlap—the CRF will attain a maximum value of 100. Conversely, a CRF of 0 signifies a failed registration that falls short of clinical standards. To guarantee complete target coverage and organ safety (wherein FGTV and FOARi values are set at 1), it is imperative to assess the overlap of organs during registration. This evaluation should determine whether the volume of overlap adheres to clinical requirements, particularly when the DSC of all organs matches the average of those observed in clinically acceptable standard registrations. Furthermore, the validation criteria are derived from DSC values reported in prior literature: a DSC of 0.7 or greater for an organ indicates effective registration of that specific organ.18 If the DSC for all organs exceeds 0.7 in the registration, it is deemed excellent; in such cases, a CRF value exceeding 70 signifies an outstanding registration performance. Upon establishing the weight factors for the WCEM as indicated above, the CRF scores of each automatic registration method were calculated for validation.
Statistical analysis
SPSS 17.0 software was used to perform the Wilcoxon signed-rank test on the registration results of various automatic registration methods. p < 0.05 was considered statistically significant.
RESULTS
Comparison CR of PTV among different registration methods
Across the three tested registration regions, the comparison of the CR of the PTVCT with its corresponding GTV (GTVCBCT) in the CBCT revealed that grayscale registration either outperformed bony registration or demonstrated comparable results. Specifically, when utilizing bony registration, the CR of the PTV in the target registration group was significantly lower than that in the ipsilateral registration group and the body registration group (p < 0.05). Conversely, with grayscale registration, the ipsilateral registration group exhibited the highest CR of the PTV, albeit the differences among registration groups across various regions were not statistically significant (p > 0.05) as shown in Figure 1.
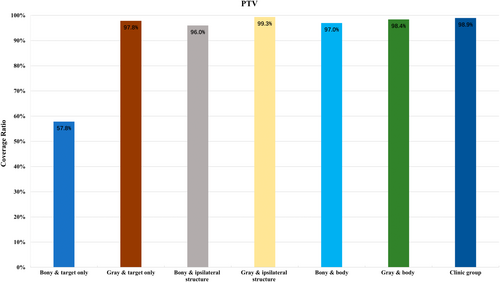
Comparison of DSC among different registration methods
Similar to the CR of PTV, in groups with different registration regions, the DSC results of the organs using grayscale registration were either significantly superior to or equivalent to those from bony registration. Within the various groups, the DSC for GTV within the ipsilateral registration group was the highest, significantly surpassing that of the body registration group (p < 0.05). However, no statistically significant difference was observed when compared to the target registration group (p > 0.05). Upon comparing the DSC of each OAR across different registration region groups, the target registration group exhibited the lowest DSC, while no significant differences were detected between the other two groups. Overall, the grayscale registration method proved optimal for automatically registering the ipsilateral structure. The similarity indices for the GTV and various OARs were as follows: 0.87 ± 0.09 for GTV, 0.71 ± 0.09 for esophagus, 0.74 ± 0.09 for spinal cord, and 0.91 ± 0.05 for heart as shown in Figure 2.
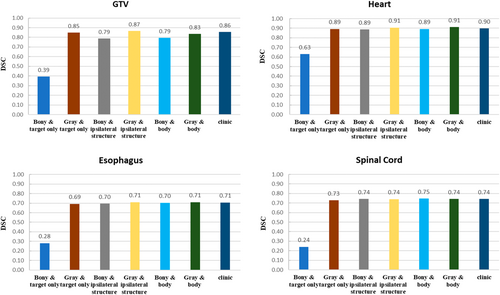
Weight factors of WCEM
The standard DSC (DSCS) ratios for the GTV, heart, esophagus, and spinal cord in the standard registration group (clinic group) were 0.86, 0.90, 0.71, and 0.74, respectively. Evaluation weights were determined based on these DSCS ratios, which were 26.79, 28.04, 22.12, and 23.05, summing up to 100 (Table 1).
| GTV | Heart | Esophagus | Spinal cord | |
|---|---|---|---|---|
| DSC | 0.86 ± 0.11 | 0.90 ± 0.06 | 0.71 ± 0.09 | 0.74 ± 0.10 |
| Weights | 26.79 | 28.04 | 22.12 | 23.05 |
Registration evaluation of WCEM in clinical group
In the course of treatment, the GTV was fully encompassed by the GTV during setup. When the registration DSC for all evaluated organ structures (including the GTV and OARs) exceeded the DSCS ratio of the group, the CRF would exceed 70 points (patients nos. 1–5), and the registration was considered to be excellent. When the DSC for the GTV exceeded 0.7 and the DSC for at least two or more organ structures surpassed the standard group's DSCS, the CRF score was >60 points, thus suggesting that the registration was considered good, and there were 12 patients over 60 points. However, if the registration failed to ensure that the planned PTV completely encompassed the GTV in the treatment setup, the CRF score would be 0. During the manual registration of patients nos. 19 and 20, challenges arose. Patient no. 19 developed atelectasis, making the GTV difficult to identify. On the other hand, patient no. 20 had two GTVs with significant relative displacement, and the originally planned PTV could not simultaneously cover both GTVs, necessitating a plan adjustment (Figure 3). In both cases, the planned PTV failed to cover the GTV on the setup CBCT, resulting in a CRF score of 0 and classifying them as registration failures. Moreover, barring two exceptional cases, the CRF scores of all other clinical groups were found to be above 50 (Figure 4) for details. Consequently, a CRF score of 50 can serve as a qualified benchmark for assessing the performance of alternative registration methods.
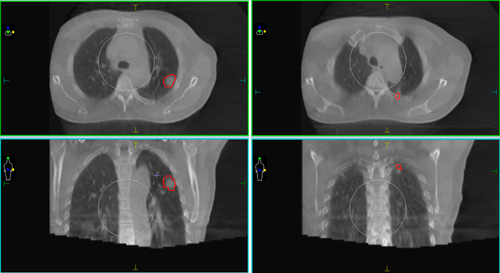
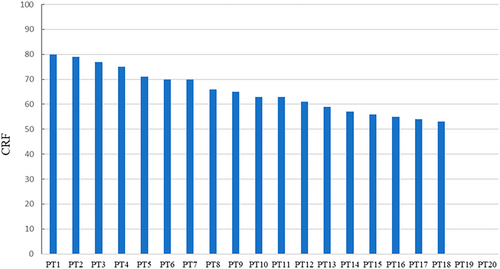
WCEM model validation among different registration methods
In a study involving 20 lung cancer patients, the model's accuracy was tested using registration results from four distinct automatic registration methods, as detailed in Table 2. Notably, the grayscale registration algorithm yielded the highest average CRF score for patient structures. When comparing the average CRF scores and registration quality rates across the four methods, the following trends were observed in descending order: ipsilateral/grayscale (with an average CRF of 58 ± 26, 40% excellent registration, 75% good registration, and 85% qualification registration), followed by body/grayscale (53 ± 28, 30%, 65%, and 70%, respectively). The body/bony method came in third (44 ± 34, 25%, 55%, and 60%, respectively), while the ipsilateral/bony method registered the lowest scores (39 ± 37, 35%, 50%, and 55%, respectively). Remarkably, the ipsilateral/grayscale group recorded the lowest number of registration failures (CRF = 0) among all patients, with only 3 cases. Conversely, the two bony registration groups exhibited higher proportions of registration failures, specifically 35% and 45%, respectively. These findings suggest that the grayscale registration algorithms, especially when combined with the ipsilateral approach, offer superior performance in lung cancer patient registration.
| Ipsilateral/bony | Ipsilateral/grayscale | Body/bony | Body/grayscale | |
|---|---|---|---|---|
| CRF | 39 ± 37 | 58 ± 26 | 44 ± 34 | 53 ± 28 |
| CRF = 0 | 9 (45%) | 3 (15%) | 7 (35%) | 4 (20%) |
| CRF ≥ 50 | 11 (55%) | 17 (85%) | 12 (60%) | 14 (70%) |
| CRF ≥ 60 | 10 (50%) | 15 (75%) | 11 (55%) | 13 (65%) |
| CRF ≥ 70 | 7 (35%) | 8 (40%) | 5 (25%) | 6 (30%) |
- Abbreviation: CRF, comprehensive registration factor.
DISCUSSION
CBCT-guided radiotherapy has the potential to reduce setup errors, improve radiotherapy accuracy, minimize the external margin of the PTV, and decrease the dose delivered to the OARs.19-21 However, the accuracy of image registration is influenced by various factors, including the registration algorithm,22 the region of interest,10, 23 and the evaluation criteria.24 Richter et al.22 reported that gray-level registration has higher accuracy registration than bony registration. Cao et al.10 advocated the utilization of the registration body or ipsilateral structure in CBCT image guidance for lung cancer. Consistent with these findings, our results confirmed that, considering both registration time and accuracy, the grayscale algorithm for ipsilateral registration surpassed other groups (Figure 1). However, it is worth noting that most of these studies point out that the registration results of different registration methods are different, but they do not provide an objective and standard evaluation index of registration accuracy, so it is difficult to objectively evaluate the image registration accuracy. Aiming at the objective evaluation index of image registration, this study innovatively proposed to use the dice similarity coefficient (DSC) after image registration to objectively evaluate the registration accuracy of different registration methods, providing a very intuitive and reliable evaluation index and method for the registration accuracy of CBCT images.
CBCT-guided setup correction primarily aims to guarantee precision in the GTV and achieve a 100% coverage ratio for the PTV following correction. Beyond meeting the CR requirements for the PTV, IGRT setup corrections should strive to optimize the geometric similarity of each organ structure. However, due to inevitable geometric deformations and changes in the relative positions of the GTV and OARs in patients, setup corrections are confined to the movement and rotation of the treatment couch, limiting them to rigid registration, often resulting in suboptimal correction results.25-27 In clinical IGRT, it is imperative to comprehensively evaluate the coverage ratio of the target after registration correction, as well as the DSC of each anatomical structure, making adjustments based on clinical treatment priorities. At present, all accelerator loaded IGRT image registration systems can only perform general registration based on the pixel features of the registration image; no registration method has been established that aligns with the clinical evaluation standards. This discrepancy leads to confusion in clinical applications, diminishing its relevance for precision-guided radiotherapy. Through analysis of manual registrations conducted by radiation oncologists and examination of approved registration results in clinical IGRT, this study summarized the requirements for geometric registration accuracy and priority conditions for the GTV and OARs during IGRT. This study is the first to apply the concept and method of weighted comprehensive evaluation of image registration that considers the geometric precision for the GTV and OARs. The WCEM developed based on the actual clinical registration outcomes and accuracy standards for treatment doses, determines and computes the registration weight factors for distinct anatomical structures. This provides a quantifiable mathematical model for weighted comprehensive evaluation, offering an objective and quantifiable method for the assessment of image registration results.
This study comprehensively assessed and verified the practicality and precision of the WCEM, utilizing diverse clinical results from automatic registration processes. The grayscale registration algorithm demonstrated superior performance compared to bony registration in image-guided lung cancer radiotherapy. Notably, the grayscale algorithm exhibited a significantly lower registration failure rate (defined as a CRF of 0) than bony registration. Furthermore, the qualification rate (where CRF is at least 50) and overall CRF score of grayscale registration surpassed those of bony registration, accompanied by a reduced standard deviation, indicating greater stability. These findings suggest that grayscale registration offers enhanced reliability and is a more appropriate choice for image registration in IGRT for thoracic tumors.28-30 Additionally, the WCEM can effectively evaluate the precision of image registration and determine its feasibility (i.e., whether registration was successful or not). Among the four groups of automatic registration that were analyzed, the “ipsilateral/grayscale” group demonstrated the most promising outcomes. Specifically, it achieved an average CRF score of 58, exceeding the qualification standard of 50. By employing the CRF quantitative scoring method, the consistency and reliability of the “ipsilateral/grayscale” registration method were thoroughly validated, establishing it as a suitable choice for clinical applications.
Based on our evaluation of registration accuracy for image-guided setup correction in lung cancer radiotherapy, we observed that even with the most precise “ipsilateral/grayscale” method, 15% (3/20) of automatic registrations failed to meet the planned PTV coverage for the treatment GTV. This underscores the need for vigilance in clinical IGRT procedures, as excluding the PTV contour from registration evaluation may lead to potential oversights in GTV after setup correction. Such oversights could significantly reduce treatment efficacy or even result in treatment failure. Patient no. 20, for instance, could not achieve 100% target coverage regardless of the registration method used. Notably, all registration methods yielded a CRF value of 0, which falls below clinical standards. Further analysis of this lung cancer patient's treatment plan revealed the concurrent treatment of two distinctly positioned GTVs. One target was located at the lung apex, minimally affected by motion, while the other was situated on the chest wall, highly susceptible to setup traction. However, due to the significant positional differences between these two GTVs, the impact of their positional variations remained inconsistent. As a result, a standard PTV expansion boundary may not suffice to guarantee complete coverage of each GTV in the event of positioning errors. Therefore, for lung cancer patients undergoing IGRT, we recommend either enlarging the PTV expansion boundary or developing separate plans and setup treatments for each GTV. This approach can help mitigate the challenges posed by multiple target areas and enhance the accuracy and effectiveness of radiotherapy treatments. Additionally, it is worth noting that in such complex cases, the CRF method can effectively detect registration errors, providing valuable insights for guiding clinical IGRT applications.
This study still had certain limitations. First, the definition of organ weight factors within our established WCEM model lacks theoretical rigor. However, the flexibility of these weight factors offers a distinct advantage as it allows adaptability to a wider array of radiotherapy techniques and clinical settings. Physicians can customize weight factor combinations for individual patients, tailoring them to specific radiotherapy techniques, thereby enhancing the clinical evaluation of registration accuracy to meet each patient's unique needs. Second, it is noteworthy that the CRF score range mentioned in this article does not encompass all possible score values. Instead, the enumerated scores primarily aim to illustrate the variations of CRF in diverse scenarios, rather than providing a comprehensive score distribution chart. Consequently, to fully assess the performance of the CRF metric, further data enrichment and deeper exploration are imperative. Finally, while we have not validated the model using additional external data, we intend to undertake more extensive and comprehensive validations across various patient types and radiotherapy techniques in the future.
In conclusion, in this study, a WCEM was developed to align with clinical standards and subsequently validated through rigorous clinical data analysis to ascertain its suitability and practicality for lung cancer IGRT image registration. The WCEM serves as a reliable tool for assessing the precision of image registration, leveraging the CRF value as a metric. Furthermore, the study reveals that when employed for registering the ipsilateral structure, the grayscale registration algorithm achieves optimal results for the GTV and various OARs. Consequently, the grayscale registration algorithm is highly recommended for registering the ipsilateral structure in CBCT-guided lung cancer radiotherapy.
AUTHOR CONTRIBUTIONS
Yimei Liu: Project administration, conceptualization, methodology, formal analysis, investigation, data curation, writing–original draft/review and editing. Meining Chen and Jianlan Fang: Methodology, formal analysis, investigation, data curation, writing–original draft/review and editing. Liangjie Xiao, Songran Liu, Qiwen Li, Bo Qiu and Runda Huang: Investigation, data curation and visualization. Jun Zhang: Conceptualization, methodology, formal analysis, draft editing. Yinglin Peng: Supervision, project administration, conceptualization, methodology, formal analysis, investigation, data curation, writing–original draft/review and editing, visualization.
ACKNOWLEDGMENTS
This work was jointly supported by National Key Research and Development Program of China (no. 2022YFC2402300), Science and Technology Program of Guangzhou, China (202206010154 and 202206010180).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The datasets are backed up on the Research Data Deposit (RDD no. RDDA2023663396, https://www.researchdata.org.cn) and are available upon reasonable request.




