Clinical outcome of node-negative oligometastatic non–small cell lung cancer
Abstract
Introduction
The concept of “oligometastasis” has emerged as a basis on which to identify patients with stage IV non–small cell lung cancer (NSCLC) who might be most amenable to curative treatment. Limited data have been available regarding the survival of patients with node-negative oligometastatic NSCLC.
Patients and methods
Consecutive patients with advanced NSCLC who attended Kindai University Hospital between January 2007 and January 2016 were recruited to this retrospective study. Patients with regional lymph node–negative disease and a limited number of metastatic lesions (≤5) per organ site and a limited number of affected organ sites (1 or 2) were eligible.
Results
Eighteen patients were identified for analysis during the study period. The most frequent metastatic site was the central nervous system (CNS, 72%). Most patients (83%) received systemic chemotherapy, with only three (17%) undergoing surgery, for the primary lung tumor. The CNS failure sites for patients with CNS metastases were located outside of the surgery or radiosurgery field. The median overall survival for all patients was 15.9 months, with that for EGFR mutation–positive patients tending to be longer than that for EGFR mutation–negative patients.
Conclusion
Cure is difficult to achieve with current treatment strategies for NSCLC patients with synchronous oligometastases, although a few long-term survivors and a smaller number of patients alive at last follow-up were present among the study cohort. There is an urgent clinical need for prospective evaluation of surgical resection as a treatment for oligometastatic NSCLC, especially negative for driver mutations.
Introduction
Lung cancer is the most common cause of cancer-related death worldwide, with non–small cell lung cancer (NSCLC)1 being the major histological subtype of this malignancy. Approximately 50% of patients with NSCLC present with metastatic disease at diagnosis, and the most common distant metastatic sites are the brain, bone, liver, and adrenal gland.1 Platinum-based chemotherapy regimens are the standard first-line treatment for individuals with advanced NSCLC who do not harbor identifiable driver oncogenes, such as mutant forms of the epidermal growth factor receptor (EGFR) gene or rearrangements of the anaplastic lymphoma kinase (ALK) gene.2 However, the efficacy of these regimens has reached a plateau, with most such patients not being amenable to curative treatment.3, 4
The concept of “oligometastasis” has been introduced to describe a restricted locoregional tumor load and has been applied to the identification of patients with NSCLC of stage IV who are potentially amenable to curative treatment.5 The implication is that local cancer treatments, such as surgical resection or radiation therapy, are potentially curative in a proportion of patients with distant metastases. Retrospective studies have evaluated the clinical outcome of patients with oligometastatic NSCLC regardless of the presence or absence of regional lymph nodes metastases,6-9 however, with patients positive for regional lymph node metastases tending to have a shorter overall survival (OS) than those without regional lymph node involvement. Although the clinical manifestations of lung cancer usually follow a multistep progression including contiguous spread of the primary tumor, intrathoracic lymph node spread, and distant metastasis, patients with a small primary lung tumor and synchronous distant metastases but without any regional lymph node metastasis are encountered in clinical practice.10 Given that limited data are available regarding the survival of patients with node-negative oligometastatic NSCLC, we have now evaluated the clinical outcome of such individuals.
Methods
Patients
The present retrospective study recruited consecutive patients with advanced NSCLC who attended Kindai University Hospital between January 2007 and January 2016, given that EGFR mutation tests have been commercially available with insurance coverage since 2007 in Japan. Patients met all of the following criteria: a histological diagnosis of NSCLC, a clinical stage of IV negative for node metastasis, and clinical T1–T3 disease according to the 7th edition of the AJCC/UICC TNM staging system. Given that most clinical T4 disease is not a contraindication for surgery, which is the reason for its continued classification as stage IIIB, patients with T4 disease were excluded from the study. With regard to clinical nodal assessment, clinical N0 means nonenlarged lymph nodes on a computed tomography (CT) scan, and no tracer uptake if positron emission tomography (PET)–CT was performed. Patients with a limited number of metastatic lesions (≤5) per organ site and with a limited number of affected organ sites (1 or 2) were eligible. Synchronous brain metastasis was evaluated by contrast-enhanced magnetic resonance imaging (MRI) if possible. No restrictions on NSCLC histology, subsequent or previous treatment, performance status, or other factors were imposed. Treatment response was determined on the basis of Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. The study was approved by the Institutional Review Board of Kindai University Faculty of Medicine.
Detection of EGFR mutations and ALK rearrangement analysis
EGFR mutations that confer sensitivity to EGFR tyrosine kinase inhibitors (TKIs) were identified either by the Scorpion Amplified Refractory Mutation System (ARMS) method (SRL, Tokyo, Japan), by the polymerase chain reaction (PCR)–Invader method (BML, Tokyo, Japan), or by the peptide nucleic acid (PNA)–locked nucleic acid (LNA) PCR clamp method (Mitsubishi Chemical Medience, Tokyo, Japan). ALK rearrangement was detected by fluorescence in situ hybridization analysis with break-apart probes for ALK, or by ALK immunohistochemistry judged by a commercial clinical laboratory (SRL, Tokyo, Japan).
Statistical analysis
OS was calculated from the date of diagnosis to the date of death from any cause or to the date of last contact. The probability of survival as a function of time was estimated with the Kaplan-Meier method, and the difference in survival between subgroups of patients was evaluated with the log-rank test. A P value of <0.05 was considered statistically significant. All statistical analysis was performed with GraphPad Prism software (version 5.0; GraphPad Software, San Diego, CA).
Results
Patient characteristics
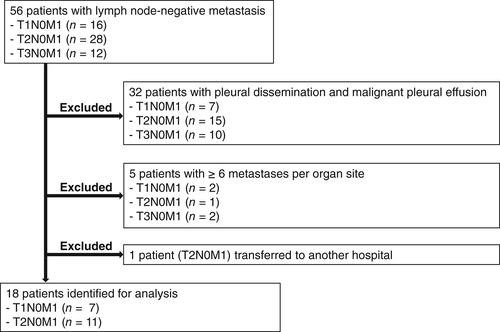
Between January 2007 and January 2016, 56 individuals with lymph node–negative (N0) metastatic NSCLC were identified through the electronic pharmacy record system at Kindai University Hospital (Fig 1), which includes the medication history of all treated patients. Thirty-eight of these 56 patients were subsequently excluded from the study because they had pleural dissemination and malignant pleural effusion (n = 32), had ≥6 metastases per organ site (n = 5), or were transferred to another hospital (n = 1). Eighteen patients were thus identified for analysis during the study period (Table 1). Eleven (61%) patients were male and 14 (78%) were smokers, with the median age of all patients being 67 years (range, 50–80 years). Most (83%) patients had a good Eastern Cooperative Oncology Group performance status (0 or 1) at the time of diagnosis. Thirteen (72%) patients had adenocarcinoma, and 17 (94%) underwent a PET-CT scan. EGFR mutations were identified in six (33%) patients, whereas ALK rearrangements were not detected. Seven (39%) and 11 (61%) patients had T1 and T2 tumors, respectively. With regard to the distribution of synchronous metastatic sites, a single metastatic site was detected in 12 (67%) patients, and the most frequent metastatic site was the central nervous system (CNS) at 72%.
| Characteristic | Subset | Number of patients (%) |
|---|---|---|
| Median (range) age in years | 67 (50–80) | |
| Sex | Male | 11 (61) |
| Female | 7 (39) | |
| Smoking status | Never | 4 (22) |
| Smoker | 14 (78) | |
| ECOG PS | 0–1 | 15 (83) |
| 2 | 3 (17) | |
| Tumor histology | Adenocarcinoma | 13 (72) |
| Squamous cell carcinoma | 5 (28) | |
| EGFR mutation | Positive | 6 (33) |
| Negative | 10 (56) | |
| Unknown | 2 (11) | |
| ALK rearrangement | Positive | 0 (0) |
| Negative | 10 (56) | |
| Unknown | 8 (44) | |
| Number of patients who underwent PET-CT | 17 (94) | |
| T status | T1a/T1b | 2 (11)/5 (28) |
| T2a/T2b | 8 (44)/3 (17) | |
| T3 | 0 (0) | |
| N status | N0 | 18 (100) |
| M status | M1a/M1b | 0 (0)/18 (100) |
| Number of metastatic organ sites | 1 | 12 (67) |
| 2 | 6 (33) | |
| Metastatic organ | CNS | 13 (72) |
| Liver | 3 (17) | |
| Adrenal | 2 (11) | |
| Bone | 4 (22) | |
| Other | 2 (11) |
- ECOG PS, Eastern Cooperative Oncology Group performance status.
Treatment intervention
Table 2 shows the treatments applied for the primary lung tumor and metastatic lesions in the study subjects. Most (83%) patients received systemic chemotherapy, with three (17%) undergoing aggressive surgery without postoperative adjuvant chemotherapy, for the primary lung tumor. Among the 13 patients with synchronous metastatic CNS lesions, 11 individuals received local CNS therapy, including stereotactic radiotherapy (SRT), whole-brain radiotherapy (WBRT), surgery, or surgery followed by WBRT. In contrast, 10 of the 11 extra-CNS metastatic lesions were treated with palliative chemotherapy. Of note, all six patients who tested positive for EGFR mutations had developed synchronous CNS metastases at diagnosis, and five of these individuals underwent local treatment (SRT, surgery, or WBRT) for the CNS lesions.
| Tumor site | Treatment | Number of patients (%) |
|---|---|---|
| Primary lung tumor | Radiotherapy | 0 (0) |
| Surgery | 3 (17) | |
| Systemic chemotherapy | 15 (83) | |
| Metastatic lesions CNS (n = 13) | SRT | 4 (22) |
| WBRT | 3 (17) | |
| Surgery | 1 (6) | |
| Surgery → WBRT | 3 (17) | |
| Systemic chemotherapy | 2 (11) | |
| Extra-CNS site (n = 11) | Radiotherapy | 1 (6) |
| Surgery | 0 (0) | |
| Systemic chemotherapy | 10 (56) |
Outcome
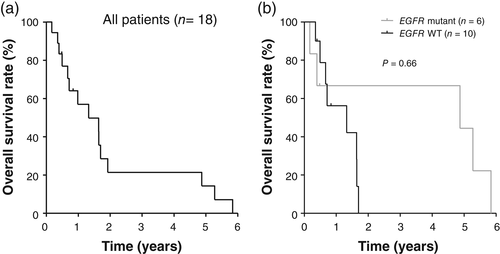
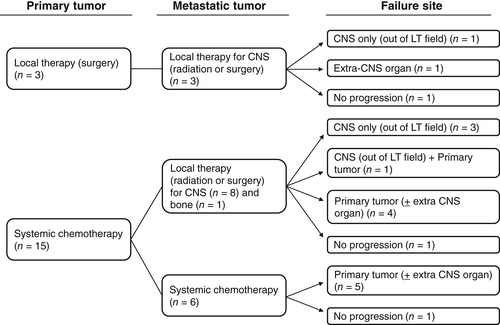
The median follow-up for the study patients was 10.9 months, with three of the 18 patients still being alive at the time of analysis. The median OS for all patients was 15.9 months (Fig 2). Among patients whose tumors were evaluated for EGFR mutations, OS in the EGFR mutation–positive patients (58.5 months) tended to be longer than that in the EGFR mutation–negative patients (15.9 months), although this difference was not statistically significant (P = 0.66) (Fig 2). Failure pattern is shown in Figure 3. Among the three patients who received local therapy for both the primary tumor and metastatic lesions, the disease progression sites included the CNS only (n = 1) and an extra-CNS organ (n = 1). Among the nine patients who received chemotherapy for the primary tumor and local therapy for the metastatic sites, the disease progression sites included the CNS only (n = 3), the CNS plus the primary tumor (n = 1), and the primary tumor with or without an extra-CNS organ (n = 4). For the six patients who received only systemic chemotherapy, the failure site was the primary lung tumor with or without an extra-CNS organ. The CNS failure sites in all patients manifesting such failure were located outside of the surgery or radiosurgery field.
Discussion
The prognosis of patients with stage IV NSCLC remains poor, with new strategies based on a better understanding of clinical characteristics being needed to maximize the efficacy of current treatments. The concept of oligometastasis has been proposed to encompass patients with a small number of distant metastases who might be able to achieve long-term survival or be potentially cured by local treatment.5 The potential benefit of liver resection for removal of metastases in patients with colorectal cancer has become increasingly evident, with 10–30% of such patients having potentially resectable disease that can be treated with curative intervention at diagnosis.11-14 Those patients who undergo resection of all evident metastatic disease appear to experience long-term survival, although there have been no randomized studies assessing outcome following resection compared with chemotherapy alone or other therapeutic modalities in patients with resectable liver metastases. With regard to lung cancer, several retrospective studies have evaluated the clinical outcome of patients treated for oligometastatic NSCLC regardless of the presence or absence of regional lymph nodes metastases, and they have shown modest clinical efficacy with a median OS of ~7 to 24 months.6-9, 15-18 These previous studies suggested that lymph node involvement is an indicator of poor prognosis for oligometastatic NSCLC patients with known lymph node status. We therefore focused on the clinical course of NSCLC patients selected for node-negative oligometastases, given that such individuals might be expected to have a greater chance for a cure than those with node-positive oligometastases. Most patients in the present study underwent systemic chemotherapy with or without local therapy for metastatic sites, with the analysis thus being potentially informative with regard to the natural history of node-negative oligometastatic NSCLC. Our results indicate that a cure is difficult to achieve with current treatment modalities for NSCLC patients with synchronous oligometastases, although there were a few long-term survivors and a smaller number of patients alive at last follow-up.
Although we recruited consecutive stage IV NSCLC patients with node-negative clinical T1–T3 disease, all patients with clinical T3 disease were excluded from further analysis for various reasons including pleural dissemination and the presence of ≥6 metastases per organ site (Fig 1). Clinical T3 disease, which is more widespread than T1 or T2 disease, is potentially associated with a less aggressive approach to treatment of the primary tumor as well as distant metastases. Although the pathological and molecular features of node-negative oligometastatic disease are unclear, the frequency of EGFR mutations among oligometastatic patients with adenocarcinoma in the present study (6/13, 46%) is consistent with the prevalence in clinically unselected patients,19 suggesting that this molecular classification does not correlate with cancer spread from the primary tumor to distant organ sites without the involvement of nearby lymph nodes. No clinical parameters could be established to assist the selection of patients who might benefit from surgical treatment of the primary tumor. The tendency for OS to be longer in EGFR mutation–positive patients than in EGFR mutation–negative patients suggests that it will be important to prospectively evaluate local therapy for the primary tumor as well as metastatic lesions in driver mutation–negative, regional lymph node–negative oligometastatic NSCLC.
The CNS is the most frequent metastatic site for oligometastatic NSCLC. Most such patients with CNS metastases (11/13, 85%) in the present study received local therapy for these metastases, with about half of these individuals experiencing progressive disease in the CNS after local therapy for metastatic sites. Of note, the CNS failure sites for all of these patients were located outside of the surgery or radiosurgery field, indicating that CNS metastases identified at diagnosis were well controlled by local therapy. The primary tumor is likely to spread to the CNS according to the “seed and soil” hypothesis, which states that cancer cell “seeds” have intrinsic compatibilities with specific organ microenvironment “soils”.20 Such patients might thus benefit from local therapy for the primary lung tumor in order to prevent seed formation.
The retrospective nature of most studies performed to date makes it difficult to draw definitive conclusions concerning the effectiveness of surgical intervention for oligometastatic disease in NSCLC, regardless of the presence or absence of regional lymph nodes metastases. This limitation is reflected in the wide variability of reported results among these studies. Given the rarity of oligometastatic disease in patients with NSCLC, it is unlikely that large randomized prospective studies will be performed to compare different treatment strategies. Systemic chemotherapy was administered in most patients in the present study, which reflects the natural history of node-negative NSCLC. Chemotherapy showed only modest efficacy in terms of OS for EGFR mutation–negative NSCLC patients with synchronous oligometastases, which may encourage prospective consideration of surgical resection for the primary tumor as well as metastatic lesions in such patients.
Disclosure
This study was supported in part by Japan Society for the Promotion of Science (JSPS) KAKENHI grant number 15K21525.




