Study of efficacy and safety of pulsatile administration of high-dose gefitinib or erlotinib for advanced non-small cell lung cancer patients with secondary drug resistance: A single center, single arm, phase II clinical trial
Abstract
Background
The objective of the study was to observe the efficacy and safety of pulsatile administration of high-dose gefitinib or erlotinib in patients with advanced non-small cell lung cancer (NSCLC) with secondary drug resistance to standard doses of tyrosine kinase inhibitor (TKI) treatment.
Materials and methods
We recruited 42 NSCLC patients from our hospital, between August 2014 and December 2015, who had experienced drug resistance after one year of conventional treatment with gefitinib or erlotinib. The gefitinib group (29 patients) received one dose of 1000 mg gefitinib every four days. The erlotinib group (13 patients) received one dose of 450 mg erlotinib every three days. Treatments continued until disease progression according to Response Evaluation Criteria In Solid Tumors 1.1 or development of intolerable toxicity.
Results
Median progression-free survival (PFS) was 30 months (gefitinib vs. erlotinib: 31 vs. 24 months; P > 0.05). After high-dose pulsatile administration, eight patients achieved a partial response (PR), 11 had stable disease (SD), and 23 had progressive disease (PD; relative risk 19.0%; disease control rate 45.2%; median PFS six months). Patients were categorized based on epidermal growth factor receptor gene mutation: exon 19 (no patients achieved complete response [CR], 4 PR, 6 SD, and 17 PD) and exon 21 mutation groups (no patients achieved CR, 4 PR, 5 SD, and 6 PD).
Conclusion
High-dose TKI pulsatile treatment is safe, efficient, and can improve prognoses for certain patients with advanced NSCLC.
Introduction
Lung cancer currently has one of the highest mortality rates in the world.1 Non-small cell lung cancer (NSCLC), the most common type of lung cancer, has been of considerable interest to medical researchers and developers for some time. Epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs), such as gefitinib and erlotinib, have become indispensable for treating advanced NSCLC. These drugs suppress the proliferation of tumor cells by blocking EGFR-mediated anti-apoptotic signaling pathways; about 90% of the EGFR mutation in NSCLC is characterized by an exon 19 deletion or a substitution of leucine on the 858 site of exon 21 to arginine (L858R).2, 3 EGFR-TKIs are clinically effective in about 35% of patients with unknown EGFR mutations, as well as in 80% of patients with defined mutations.4 To this effect, EGFR-TKIs are recommended as a standard second-line or third-line chemotherapeutic agent for patients with advanced NSCLC who have not responded to traditional treatment, and as first-line or maintenance treatment for patients with EGFR gene mutations.4-7
Drug resistance does inevitably occur in patients receiving EGFR-TKI treatment, which eventually allows the disease to progress. In patients who have acquired drug resistance, EGFR-TKIs may exhibit a better therapeutic effect after suspension for a period of time.8 It has been also reported that pulsatile administration of high-dose TKI could benefit patients with NSCLC with brain metastasis.9 The objective of the present study was to improve the rate of control and progression-free survival (PFS) of patients in advanced stages of NSCLC by multiplying the dose pulse of EGFR-TKI.
Methods
Objects of study
Patients with stage IV NSCLC (tumor node metastasis [TNM] classification 7) were enrolled in this study at the First Affiliated Hospital of Anhui Medical University from August 2014 to December 2015. All patients had experienced disease progression after initial treatment with EGFR-TKIs (gefitinib or erlotinib) for a year, which was then followed by corresponding high-dose TKI pulsatile treatment. In all patients: (i) identification of disease progression was confirmed by chest computed tomography (CT), head magnetic resonance imaging (MRI), bone scan, or abdominal ultrasound or CT tests; (ii) local treatment (i.e. surgery, radiotherapy, interventional therapy) was not provided in the course of TKI treatment; (iii) Eastern Cooperative Oncology performance scores (PS) were less than 3 and patients had more than one detected lesion; and (iv) no abnormalities were found via electrocardiogram (ECG) of liver, kidney, and bone marrow hematopoietic function. Tumor response rate and PFS was measured for all patients. The final follow-up survey was given to all participants on February 2016. The Ethics Committee of the First Affiliated Hospital of Anhui Medical University approved this study, and all patients signed informed consent forms before treatment.
Characteristics of patients
Patient characteristics included gender, age, PS score, smoking status, metastasis, and gene mutation. The adenocarcinoma patient sample contained an equal number of men and women ranging in age from 42 to 81, with PS scores of 0–3 points. The patients were divided into gefitinib (29 patients) or erlotinib groups (13 patients), as listed in Table 1. No significant difference was observed between the two groups (P > 0.05).
| Characteristics | Gefitinib group | Erlotinib group |
|---|---|---|
| Gender | ||
| Women | 15 (52) | 6 (46) |
| Men | 14 (48) | 7 (54) |
| Median age (years) | ||
| <65 | 15 (52) | 8 (62) |
| ≥65 | 14 (48) | 5 (38) |
| PS score | ||
| 0–1 | 24 (83) | 10 (77) |
| ≥2 | 5 (17) | 3 (23) |
| Smoker | ||
| Yes | 13 (45) | 8 (62) |
| No | 16 (55) | 5 (38) |
| Number of metastasized organs | ||
| 1–2 | 25 (86) | 11 (85) |
| ≥3 | 4 (14) | 2 (15) |
| EGFR gene mutation | ||
| Exon 19 | 20 (69) | 7 (54) |
| Exon 21 | 9 (31) | 6 (46) |
| Initial treatment | ||
| First-line | 12 (41) | 4 (31) |
| Second-line | 11 (38) | 5 (38) |
| Third-line | 6 (21) | 4 (31) |
- EGFR, epidermal growth factor receptor; PS, performance status.
Treatment strategies
The patients were treated by oral administration with 1000 mg of gefitinib once every four days, or 450 mg of erlotinib once every three days. Treatment continued until the disease progressed according to Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 standards or intolerable toxicity, which were recorded as adverse reactions.
Efficacy and toxicity
Treatment efficacy in all patients was assessed monthly until disease progression. According to RECIST 1.1, the objective response rate includes complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). The disease control rate (DCR) was calculated as CR + PR + SD. PFS was defined as the duration between gefitinib or erlotinib administration and the occurrence of PD or the last follow-up. Toxicity was assessed using Toxicity Criteria Version 4.0 (CTC 4.0) from the United States National Cancer Institute (which includes skin rash, fatigue, vomiting, diarrhea, liver damage, interstitial pneumonia, and other factors).
Statistical analysis
Statistical analysis was performed using SPSS 19.0 (IBM Corp., Armonk, NY, USA). Chi-square tests were performed to compare the rates and rank-by-rank sum test results. Survival analysis was observed using the Kaplan–Meier method and log-rank sequential inspection. Differences in the data were considered statistically significant when P < 0.05, as shown in Figure S1.
Results
Effect of treatment with normal dose of tyrosine kinase inhibitors (TKI)
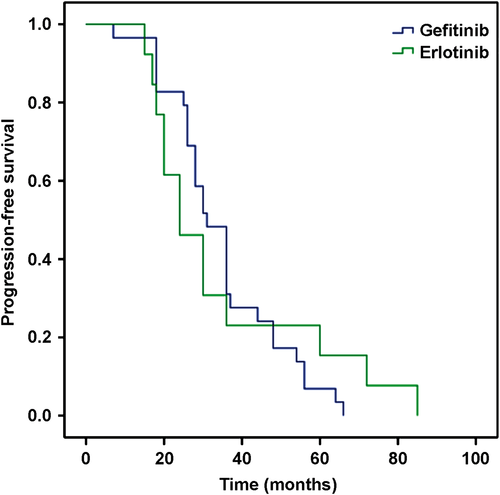
All 42 patients showed PD after having received gefitinib or erlotinib for a year prior to this study. The PFS of the patients ranged from 12 to 85 months, with a mean of 30 months. The median PFS was 31 months in the gefitinib and 24 months in the erlotinib group. No significant difference between the two groups (P = 0.81) was noted, as shown in Figure 1.
Effect of pulsatile administration with high-dose TKI
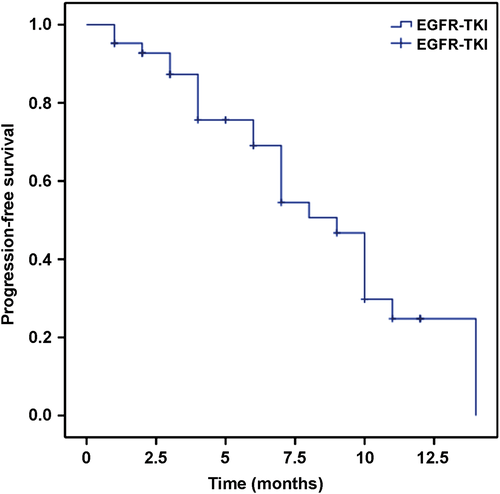
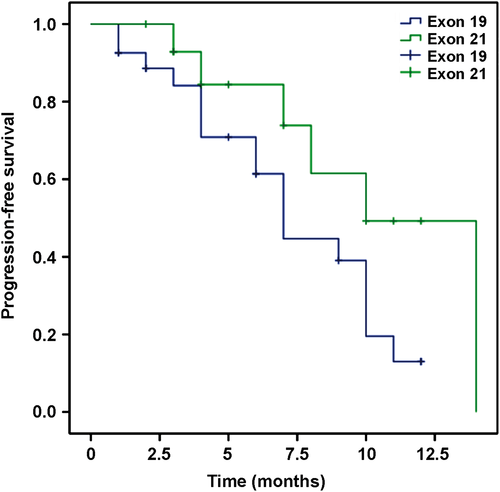
None of the patients achieved CR. Eight patients achieved PR, 11 had SD, and 23 experienced PD. In the entire patient sample, the response rate (RR) was 19% and the DCR was 45.2%. In the gefitinib group, six patients achieved PR, nine patients had SD, and 14 patients experienced PD, with a RR of 20.7% and a DCR of 51.7%. In the erlotinib group, two patients achieved PR, two patients had SD, and nine patients experienced PD, with a RR of 15.4% and a DCR of 30.8%. No significant difference between the two groups with regard to RR (P = 0.37) or DCR (P = 0.46) was observed, as shown in Table S1. Figure 2 shows that the median PFS in all patients was six months. Figure 3 shows that the median PFS was eight months in the gefitinib and six months in the erlotinib group, which were not significantly different (P = 0.25). Based on EGFR exon mutation, six and five patients had SD, and 17 and 6 patients with PD harbored exon 19 and exon 21 mutations, respectively. However, PR was observed in four patients in each of the two groups. As shown in Figure 4, the median PFS was six and seven months in exon 19 and exon 21 mutation groups, respectively (P = 0.07).

To demonstrate, an image of a chest CT scan of a patient who achieved PR after pulsatile administration of gefitinib is provided in Figure 5.

Toxicities
Slight toxicities were observed in both initial TKI and high-dose TKI pulsatile administration treatments (grade 1–2; P > 0.05), including rash, fatigue, anorexia, and skin dryness, which is consistent with treatment with either gefitinib or erlotinib. No expiratory dyspnea or neurotoxicity was observed. A few individuals showed increases in transaminase levels, an indicator of liver function, which was alleviated through supplementary treatment to protect the liver and reduce enzyme activity. The difference between the two groups was not statistically significant (P > 0.05), as shown in Table S3.
Discussion
Since the discovery of the EGFR gene mutation in lung cancer, the focus of most therapeutic approaches with regard to advanced NSCLC has been to target the EGFR gene. NEJGSG, WJTOG3405, First-SIGNA, IPASS, EURTACA, OPTIMAL, LUX Lung3, and LUX Lung6 studies have all indicated that TKI treatment has significant advantages compared with other chemotherapeutic drugs for the first-line treatment of advanced lung cancer in patients with positive EGFR gene mutation.4-6, 10-14 However, the median PFS in patients with EGFR mutations who receive EGFR-TKI (gefitinib or erlotinib) treatment is about 9–13 months in the clinic, after which acquired drug resistance inevitably emerges.
In 2010, Jackman et al. provided the definition of EGFR-TKI acquired drug resistance as disease progression in patients with EGFR mutation post-treatment resulting in CR or PR, or SD for >6 months.15 The mechanisms by which tumor cells are resistant to EGFR-TKI could be T790M mutation of the EGFR gene, C-Met gene amplification, phosphatidyl inositol-3-kinase activation, epithelial-mesenchymal transition, human epidermal growth factor-2 gene amplification, progression into small-cell lung cancer, or other unidentified mechanisms.16 According to National Comprehensive Cancer Network guidelines, the clinical pattern of drug resistance progression is either slow or rapid, and local development is dependent on the duration of disease control, tumor load, and patient symptoms, which determine the course of treatment. EGFR-TKIs should be continually used for patients with slow disease progression.17
Two main methods of treatment are typically administered in an effort to overcome acquired resistance to EGFR-TKIs. The first is continued use of the original TKI treatment. The ASPIRATION study showed that continuous treatment of erlotinib in patients with tumor progression, resulting in erlotinib therapy failure, does extend PFS for 3.1 months.18 By repeatedly applying the same TKI to patients with NSCLC with disease progression who had received prior TKI treatment (20 patients with gefitinib; 13 patients with erlotinib), Song et al. demonstrated that continued TKI treatment improves the rate of tumor control in patients who had positively responded to the prior treatment.19 The second main approach is the application of second and third generation TKIs. Second generation EGFR-TKIs, such as afatinib and dacomitinib, can irreversibly bind to EGFR receptors and target multiple ErbB family members at the same time; but unfortunately, the drugs are expensive and have caused serious adverse effects in some patients.20 Third generation TKIs, such as AZD9291, CO1686, and HM61713, were developed to target T790M mutation and have been shown to shrink tumors in patients with EGFR mutations (mainly with T790M) or EGFR-TKI resistance in phase III clinical trials.21-23
Given the severe toxicity of second generation TKIs and the fact that third generation TKIs are not yet commercially available, treatment for patients with acquired EGFR-TKI drug resistance has been problematic for clinical researchers for some time. However, studies have shown that pulsatile administration is effective for patients with EGFR-TKI acquired drug resistance. Pulsatile, also known as “strategic” administration supplies a high dose of medication in a short period of time. The advantage of this technique is that the drug concentration in blood and tissues accumulates very rapidly, thus, the drugs work rapidly. Normal EGFR-TKI administration is performed daily at a certain dosage, whereas pulsatile administration is provided using multifold dosages of TKIs for lengthier intervals. Milton et al. studied 21 patients with advanced lung cancer who developed PD after chemotherapy and treated them with pulsatile administration of erlotinib (2000 mg) weekly, after which their duration of survival averaged 9.5 months.24 No severe toxicity was observed, although the objective response rate did not reach the expected goal. By investigating the effect of pulsatile administration of erlotinib on patients with NSCLC with meningeal metastasis and acquired erlotinib resistance, Kuiper et al. found that meningeal metastases shrink significantly, accompanied by marked reduction in pulmonary lesions, as erlotinib concentration increases in the ncurolymph.25 Grommes et al. examined pulsatile administration of erlotinib (1500 mg/week) in nine patients with brain metastases from lung cancer in which EGFR-TKI treatment had failed, and found that six patients achieved PR, one patient had SD, and two patients experienced PD, all of which were associated with EGFR gene mutations.9 In short, research has shown that pulsatile administration of TKIs in patients with acquired drug resistance is safe and, for some patients, very effective.
In this study, pulsatile TKI administration was utilized to improve the rate of tumor control and prolong the duration of PFS in the sample population – 42 patients who had not responded to normal TKI treatment. After pulsatile administration of TKI, eight patients achieved PR in the entire group, with a RR of 19.0% and a DCR of 45.2%. Further, the median PFS of all patients reached six months. The PFS in patients who achieved PR in the two groups was more than four months until the final follow-up. In addition, the PFS of normal TKI treatment was positively correlated with that of pulsatile administration, which confirms that this study can be used as a reference for selecting pulsatile administration of TKI in clinics. There was no difference in regard to adverse reactions between pulsatile and regular administration of TKI, suggesting that pulsatile administration of high-dose TKIs is a relatively safe therapeutic strategy.
Some limitations of this study should be discussed. First, few patients received erlotinib, which may have affected the accuracy of the results. Second, the diversity of times of initial TKI treatment may have influenced the survival analysis after pulsatile administration. Third, the mechanism of TKI resistance after initial TKI treatment is yet to be investigated. We intend to continue following up with the patients who participated in this study (especially those who achieved PR and may benefit from continued treatment) to collect data related to EGFR gene mutation in an effort to reveal detailed mechanisms. It is also expected that the ongoing investigation will allow us to enroll more patients, enlarging the sample and, thus, obtaining more convincing conclusions.
The data gathered from this study confirms that certain patients benefit from pulsatile administration of TKIs, under the condition that they are resistant to regular TKI treatment. Pulsatile administration is safe and effective in patients who show continued disease progression after TKI treatment, and can control the development of disease without inducing severe adverse effects. These findings suggest that pulsatile administration of high-dose TKI may be a better option for patients with TKI resistance than the third generation TKI drugs soon to come onto the market. As such, is worthy of further clinical research.
Acknowledgments
This work was granted by Special Funds for Anhui Provincial Science and Technology Agency Foundation of China (No. 11070403061 and No. 1301042214) and Anhui Provincial Natural Science Research Program of Higher Education Institutions Foundation of China (grant no. KJ2012A157).
Disclosure
No authors report any conflict of interest.




