Effect of percutaneous radiofrequency ablation after thoracoscopic pleurodesis for treating non-small cell lung cancer patients with malignant pleural effusion and/or pleural dissemination
Abstract
Background
The purpose of this study was to retrospectively evaluate percutaneous radiofrequency ablation (RFA) combined with palliative thoracoscopic pleurodesis (TP) for malignant pleural effusion and/or pleural disseminated non-small cell lung cancer (NSCLC), diagnosed by thoracoscopy.
Methods
The study was composed of 40 patients with primary NSCLC with malignant pleural effusion and/or pleural dissemination recognized for the first time during thoracoscopic exploration. Twenty patients received TPR (TP plus RFA group), while the remaining 20 patients who underwent TP (TP group) represented the control. We evaluated technical success, safety, initial response rate, progression-free survival (PFS), and overall survival (OS).
Results
No procedure-related deaths or major complications occurred in any of the 22 ablation procedures. Complete response was observed in 15% of patients, partial response in 50%, stable disease in 25%, and progressive disease in 15% of patients. The mean follow-up was 15.5 months. The PFS at years one, two, and three were 77.5%, 38.8%, and 25.8%, respectively. The OS at years one, two, and three were 77.5%, 41.4%, and 27.6%, respectively. The PFS and OS were longer in the TP-R group, indicating a better prognosis than that of the patients who underwent TP only (OS 24 vs. 18 months, P = 0.030; PFS 22 vs. 15 months, P = 0.036).
Conclusions
Palliative TP combined with percutaneous RFA is a safe, feasible, and effective procedure in patients with malignant pleural effusion and/or pleural disseminated NSCLC.
Introduction
Lung cancer has increased in incidence and is now the most common malignancy and the leading cause of cancer death in China.1 Although the advancement of diagnostic tools has meant that cancer can be detected in early stages, malignant pleural effusion and/or pleural dissemination are not always detectable preoperatively and are often recognized for the first time during surgery. Malignant pleural effusion and/or pleural dissemination are common complications of advanced lung cancer that may severely affect performance status in patients and carries a poor prognosis when treated by pleura-pneumonectomy.2 Therefore, patients with non-small cell lung cancer (NSCLC) and carcinomatous pleuritis are generally not considered suitable candidates for surgery.3 On the other hand, it has been reported that a combination of thoracoscopic pleurodesis (TP) and postoperative chemotherapy or epidermal growth factor receptor (EGFR)-targeted therapy has improved five-year survival rates in such a patient population.4, 5 In addition, good results have been reported with the use of radiofrequency ablation (RFA) during TP in patients with NSCLC and dissemination or carcinomatous pleurisy.6 In this study, we retrospectively evaluated the feasibility, safety, and effectiveness of palliative TP combined with percutaneous RFA (TP-R) and TP alone in NSCLC patients with malignant pleural effusion and/or pleural dissemination diagnosed by thoracoscopy.
Methods
Patients
In this retrospective study, conducted at the Department of General Thoracic Surgery, Xuanwu Hospital of Capital Medical University, between 2008 and 2014, we performed thoracoscopy for the diagnosis and treatment of undiagnosed exudative pleural effusions with NSCLC. Undiagnosed exudative pleural effusion was defined as failure to achieve an etiologic diagnosis by initial pleural fluid microscopic and biochemical analysis, including protein, lactate dehydrogenase (LDH), and cytology. All patients underwent detailed clinical evaluation of their medical history and clinical examinations. A lung mass is clinically staged by thoracic computed tomography (CT), abdominal ultrasound, brain magnetic resonance imaging (MRI), and isotopic bone scanning. According to 7th edition of the Tumor Node Metastasis (TNM) Classification of Lung Cancer, clinically staged T1–T3N0M0 patients are eligible for thoracoscopic exploration.
Patients were included in the study if they met the following criteria: (i) no cancer history; (ii) no previous history of chest surgery; (iii) normal lung and heart function; (iv) able to tolerate general anesthesia and one lung ventilation with an American Society of Anesthesiologists score of I–III; and (v) malignant pleural effusion pleural and/or dissemination was verified during thoracoscopic exploration.
Patients were excluded for any of the following reasons: (i) failure of lung expansion after insertion of intercostal tube (trapped lung); (ii) central tumors; (iii) advanced malignancy with limited predicted life expectancy (<90 days); or (iv) bleeding diathesis, hemodynamic instability, or dysrhythmia.
Written informed consent was obtained from all patients before undergoing TP-R. The ethics committee of Xuanwu Hospital of Capital Medical University approved this research and granted a waiver for individual patient consent for patients who were retrospectively evaluated.
Surgery
Thoracoscopic pleurodesis (TP)
All patients underwent general anesthesia, and selective bronchial intubation maintaining the collapse in the affected lung by placing the patient in the lateral decubitus position, contralateral to the affected lung, using two 12 mm ports (7th or 8th intercostal space in the mid-axillary line for the thoracoscope; 4th or 5th intercostal space in the anterior axillary line for the instrument as biopsy forceps). Keeping the lung deflated and allowing adequate visualization of the pleural surfaces, all of the pleural fluid was evacuated and adhesiolysis was performed when necessary to achieve better lung re-expansion. In the majority of cases, except when diagnosis had been established preoperatively, pleural biopsy was performed to confirm the morphological diagnosis. Biopsies were taken from the parietal pleura where macroscopic abnormalities were obvious. Once pleural dissemination was verified by frozen section during thoracoscopic exploration, TP was then performed by means of two techniques: mechanical pleural abrasion and intrapleural administration of anticancer drugs. Abrasion was conducted using a sterile sponge with iodopovidone, adapted to the entrance ports to irritate the parietal pleura by friction until hemorrhage was observed. Electrocautery or other devices controlled hemostasis. Normal saline solution (20 mL) containing cisplatin (30–40 mg) was administered into the pleural cavity through the second ports. After the procedure was completed, the thoracoscope and other port(s) were removed and a 28 Fr chest tube was inserted through the same incision. A chest drain was connected to negative pressure suction to achieve complete drainage of the effusion and/or complete lung expansion. Chest X-ray was used to confirm complete re-expansion.
Radiofrequency ablation (RFA)
Radiofrequency ablation was conducted under sedation using pethidine hydrochloride or morphine injected intramuscularly the day before chest tube removal. The patient was positioned on the CT table according to the location of the lesion in order to obtain the shortest access route. Intravenous access was available in all patients. Patients’ cardiac status and vital signs were continuously monitored: non-invasive blood pressure was checked every 3–5 minutes; peripheral blood oxygen saturation was monitored and recorded via a pulse oximeter; and by electrocardiogram during and for two hours following the procedure. Oxygen was administered by facemask at a flow rate of 4–5 L/minute. Grounding pads were placed in the lumbar and gluteal regions, or on the thighs, according to the position of the nodule to be treated.
All patients underwent chest CT immediately prior to RFA. Selected transverse images were obtained within the area of interest of 5–10 mm section thickness, depending on the size of the lesion, and to select optimal percutaneous needle access. After the skin was cleansed with iodine and alcohol and draped in a sterile fashion, local anesthesia was administered with 5–15 mL 1% lidocaine, intradermal and subcutaneous down to the pleura along the predetermined puncture site.
Radiofrequency ablation was performed using the RITA system (RITA Medical Systems Inc., AngioDynamics, Manchester, GA, USA). The deployment of the electrode needle was staged according to the size of the tumor. Under CT guidance, the probes were introduced into the tumor and the path was planned to avoid vessels, bronchi, blebs, and fissures. The electrode needles were pushed forward and unfolded gradually until they reached or crossed the borders of the tumor. Once proper electrode positioning was confirmed by CT scan, electrodes were attached to an RF generator with an ablation algorithm with staged deployment at a target temperature of 90°C. Completion of the RFA procedure involves retraction of the electrodes and the routine performance of track ablation, which cauterizes the access track at the completion of each lesion ablation, to reduce the risk of tumor seeding and lower the risk of hemorrhage. In lesions larger than 3 cm, overlapping ablations are required. Multiple repositioning and repeated RFA at different sites within the tumor were required to ensure ablation of the entire lesion. RFA was terminated when the following had occurred: (i) at least 0.5–1 cm tumor margins were achieved; (ii) ground-glass opacification was seen in the normal lung parenchyma surrounding the tumor at intra-procedural CT; and (iii) sufficient time had elapsed, according to probe manufacturer recommendations.
After the procedure, patients were instructed to stay in bed for two to four hours in the supine position. Once the lung had expanded and drained and output decreased to less than 150 mL per 24 hours, the chest drain was removed. A day after chest tube removal, a chest radiograph was taken. Patients were discharged in the case of minimal or no residual pleural effusion and general status allowing outpatient care.
Each patient received chemotherapy or EGFR-targeted therapy (EGFR mutant) following RFA.
Follow-up
Patients were followed-up one and three months after treatment, and then at three-month intervals with: a physical examination; radiological imaging for tumor assessment, including CT of the chest (the same technique as that used at baseline); and selectively, with positron emission tomography or single-photon emission CT scans. The primary end points of this study were time to progression and patient death, calculated from the date of the RFA procedure. We evaluated technical success, safety, clinical response rate, progression-free survival (PFS), and overall survival (OS). Because the aim of RFA was to produce a volume of coagulation necrosis exceeding that of the native tumor, the one-month follow up CT scan (in which the high-density area representing the ablation zone was usually larger than the native tumor) was taken as a term of reference.7
Technical success was defined as correct placement of the ablation device into all target tumors with completion of the planned ablation protocol; that is, maintenance of the target temperature of 90°C for the required time according to tumor size.
The safety assessment included identification of puncture-related and ablation-related complications occurring within 30 days from treatment. Complications were assessed on a per-procedure basis. Minor complications were defined as those resulting in no sequelae or needing nominal treatment or a short hospital stay for observation. Major complications were defined as those resulting in readmission to the hospital for treatment, an unplanned increase in the level of care, extended hospitalization, permanent adverse sequelae, or death.
Local disease progression of the treated nodule was assessed in accordance with modified Response Evaluation Criteria in Solid Tumors (RECIST) in comparison with baseline diameter.7 Assessment of the target tumor response was based on CT analysis of lesion size, geometry, and enhancement. Areas of hypoattenuation that did not enhance were considered to represent the ablation zone. Focal enhancement of soft tissue of more than 15 HU when compared with the initial post-ablation non-enhanced series was considered to indicate local tumor progression. Typically, when tumor progression was identified at initial post-ablation CT or subsequent follow-up CT, a second session of RFA was performed. Initial response was determined by modified RECIST criteria.8
Data collection and statistical analysis
Information on patient demographics, tumor characteristics, treatment, comorbidities and clinical outcomes of both groups were collected from the clinical database of Thoracic Surgery, Xuanwu Hospital, Capital Medical University.
A comparative study was conducted between both groups, using SPSS version 19.0 (IBM Corp., Armonk, NY, USA) for statistical analysis comparing variables such as procedure time and postoperative in-hospital length of stay with an unpaired t-test. Other variables, such as recurrence rate and postoperative complications, including pneumothorax, hemorrhage, pleural effusion, and mortality, were studied applying Fisher's exact tests. PFS and OS were evaluated by means of the Kaplan–Meier method and any difference between the groups was evaluated with a log-rank test. Statistical significance was defined as P < 0.05. All analyses were performed from the time of the TP.
Results
Clinical features
This study included 40 patients with primary NSCLC with malignant pleural effusion and/or pleural dissemination, recognized for the first time during thoracoscopic exploration. None of the patients could be diagnosed with malignant pleural effusion by CT before thoracoscopic exploration. The patients were randomly divided into two groups: 20 patients received TP-R (TP-R group) and 20 patients underwent TP (TP group). In terms of patient demographics, the two groups were comparable in age, gender, tumor size, histological type, and postoperative treatment (Table 1).
| Item | TP-R (n = 20) | TP (n = 20) | P |
|---|---|---|---|
| Gender (male/female) | 8/12 | 7/13 | 0.744 |
| Mean age (years) | 64.7 ± 9.9 (44–81) | 66.6 ± 8.1 (44–77) | 0.512 |
| Tumor size (cm) | 3.5 ± 1.6 (2–9) | 3.5 ± 1.2 (1.5–6) | 0.887 |
| Tumor location (right/left) | 14/6 | 12/8 | 0.507 |
| Histological type (Ad/Sq/B) | 17/2/1 | 16/4/0 | 0.428 |
| Postoperative treatment (C/T/S) | 10/6/4 | 9/4/7 | 0.530 |
- Ad, adenocarcinoma; B, big cell carcinoma; C, chemotherapy; Sq, squamous cell carcinoma; S, supportive therapy; T, targeted therapy; TP, thoracoscopic pleurodesis; TP-R, percutaneous radiofrequency ablation.
After surgery, 12 patients (6 patients in the TP, 6 patients in the TP-R group) were administered oral targeted therapy (gefitinib or erlotinib) as a result of gene analysis for EGFR mutation; 21 patients (11 in TP, 10 in TP-R) received chemotherapy (e.g. docetaxel plus cisplatin) for four to six cycles; and seven patients (3 in TP, 4 in TP–R) received supportive therapy, including pain control or nutrition support.
Feasibility
Radiofrequency ablation was successfully completed in all patients with a median of six days (range 4–12) postoperatively. The median tumor size was 3 cm (range 2–9; mean 3.5 ± 1.6). The ablation time varied from 12.2 to 50 minutes (mean 22.1 ± 9.9), depending on the difficulty of accessing the lesion and patient cooperation. The mean time to reach the target temperature was 2.9 minutes (range 1.5–7.7).
Safety
No procedure-related deaths, 30-day mortality, or major complications occurred. Minor complications included pneumothorax in one patient (that did not require treatment), chest pain in one, and cough in one patient. Side effects (moderate grade fever <38.5°C, and/or chest pain) were the most common complications; however, most of these were cured within a couple of days. The TP-R and TP groups were completely free from pleural effusion during the follow-up period.
The hospital observation period after RFA was 24 hours for most patients in the TP-R group. The median hospital stay after surgery was seven days (range 5–18) for the TP-R and seven days for the TP group (range 6–14).
Effectiveness
Survival data were available for all patients. The mean follow-up was 15.5 months (median 13, range 3–56). In the TP-R group, 20 patients survived over three months after the surgery. CT scans showed that three patients (15%) achieved complete remission, 10 (50%) achieved partial remission, five (25%) remained stable, and two (10%) had progressive disease. The total disease control rate (DCR) was 90%.
After follow-up, tumor progression occurred in 12 patients in the TP-R group, including two local recurrences, four contralateral lung metastases, two bone metastases, one brain metastasis, one breast metastasis, and two cases of multi-organ metastasis. In the TP group, none of the patients were free from tumor progression: eight cases of local recurrence, five of bone metastasis, three of brain metastasis, two of lung dissemination, and two cases of multi-organ metastasis occurred during follow-up.
The PFS for the TP-R and TP groups were 77.5% and 57.6% at one year, 38.8% and 19.8% at two years, and 25.8% and 0% at three years, respectively. The median PFS in the TP-R was longer than in the TP group (P = 0.036), with median PFS of 22 (95% confidence interval [CI] 18–26) and 15 months (95% CI 9–21), respectively (Fig 1).
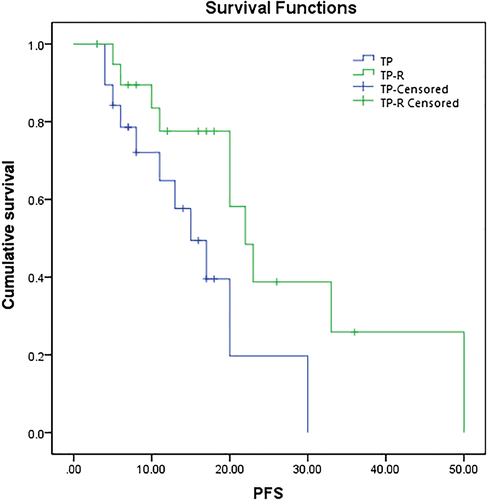
The OS for the TP-R and TP groups were 77.5% and 64.3% at one year, 41.4% and 15.0% at two years, and 27.6% and 0% at three years, respectively. The median OS in the TP-R group was longer than in the TP group (P = 0.030), with median OS of 24 (95% CI 21–27) and 18 months (95% CI 12–24), respectively (Fig 2).
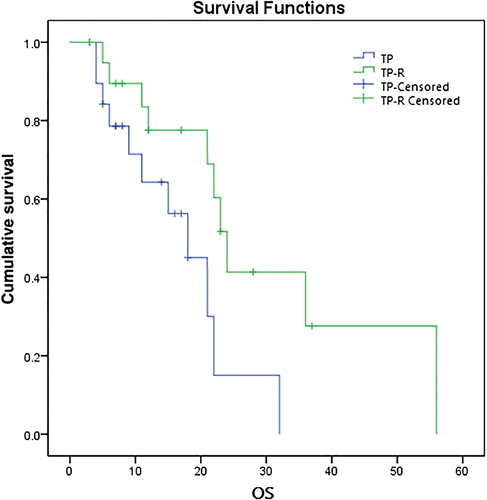
Discussion
Malignant pleural effusion and/or pleural dissemination are common complications of advanced lung cancer that may severely affect performance status in patients and are associated with poor prognosis. However, they are not always detectable preoperatively and are often only recognized for the first time during surgery. TP is standard care and is an effective method to treat malignant pleural effusions and/or pleural dissemination.9 TP is a procedure that obliterates the visceral and parietal pleural surfaces, with the intention of preventing the accumulation of either air or fluid in the pleural space. Symphysis of the pleural surface can be achieved using either chemical agents or physical abrasion of the surface during thoracoscopy. Chest drainage with negative pressure suction helps to expel the residual gas and pleural effusion, adhere visceral and parietal pleura, and, thus, reduce the incidence of pleural hypertrophy and the formation of encapsulated pleural effusion. Although TP has been shown to be safe and effective, the prognoses of patients who undergo TP remain unchanged. Hence, postoperative chemotherapy or EGFR-targeted therapy are required to improve the survival of patients with advanced-stage NSCLC.10 Nevertheless, responses to these modern-agent systemic therapies are incomplete. In nonsurgical candidates, RFA is an alternative to surgery for local treatment to eradicate the tumor. Since first reported by Dupuy et al., RFA has been shown to obtain remarkable outcomes, especially in patients with early stage cancer who cannot tolerate standard surgery.11-16 However, whether RFA could obtain the same remarkable outcomes in advanced lung cancer as it does for early stage lesions remains unclear. Good results have been reported with the use of RFA during TP in patients with malignant pleural effusion and/or pleural disseminated NSCLC.6 Computed tomography is currently the only accurate imaging technique for guidance during lung RFA. The correct planning of the needle track is a key factor for technical success. In this study, we compared the difference in clinical outcomes between percutaneous RFA (TP-R) and TP groups in patients with malignant pleural effusion and/or pleural dissemination diagnosed by thoracoscopy.
In our study, the OS at years one, two, and three were 77.5%, 41.4%, and 27.6%, respectively. The PFS and OS were longer in the TP-R group, indicating a better prognosis than that of patients who underwent TP only.
According to a review by Zhu et al., complete tumor control rates by RFA range from 38–97%.17 A number of studies have shown a higher risk of local recurrence in larger tumors.13, 14, 18 In our study, however, the local or disease progression did not seem to depend on tumor size. The total effective rate was 65% in the TP-R group. The exact reason for this observation cannot be determined; however, CT allows precise tumor localization and the type of electrode used may have been a factor. Over the past several years, advances in delivery mechanisms that can either increase the amount of energy deposited or the conduction of heat through the tissue have increased the sphere of tissue that can be ablated. Multiprobe array electrodes, in which multiple tines apply current simultaneously, achieve coagulation zones of 5–7 cm. Wet electrodes using saline (either isotonic or hypertonic) infused through the electrode into surrounding tissue increase conductivity with greater amounts of infusion of ions in the tissue, increasing current flow and, thus, allowing a longer duration of current flow and an increased volume of coagulation.19, 20
We reported a technical success of 100%, with no 30 day mortality. Moreover, the application of RFA in combination with TP did not increase the risk of perioperative morbidity and mortality, according to our data. Therefore, combined TP-R treatment for pleural disseminated NSCLC does not increase the risk of higher morbidity.
The most common complication of CT-guided percutaneous RFA is pneumothorax (range 4.5–61.1%), which is usually self-limiting. Approximately 3.3–38.9% of patients require chest tube insertion for drainage.17, 21 In our study, only one case (5%) occurred in the TP-R group. Although the study was based on small population, the relatively low incidence of complications suggested that palliative TP combined with TP-R could be considered as safe and did not increase the postoperative hospital stay in patients with malignant pleural effusion and/or pleural disseminated NSCLC.
This study had some limitations, including the study design, number of cases examined, and follow-up duration.
In conclusion, we believe that our experience indicates that palliative TP combined with percutaneous RFA is a safe, feasible, and effective procedure in patients with malignant pleural effusion and/or pleural disseminated NSCLC. In future, properly designed prospective, randomized multicenter trials are necessary to define the role of RFA in multimodality treatment for advanced NSCLC.
Disclosure
No authors report any conflict of interest.




