New agent to treat advanced or metastasized thyroid cancer, its efficacy, safety, and mechanism
Abstract
Background
Vandetanib is the first US Food and Drug Administration approved agent to treat advanced or metastasized thyroid cancer. To gain a better understanding of the drug, we conducted a systematic review of its efficacy and safety in patients with thyroid cancers.
Methods
Trial data was retrieved from Pubmed, EMBASE, Medline, China National Knowledge Infrastructure, and the Cochrane database without restrictions on language. A systematic review of the literature was performed to assess median progression-free survival (PFS) and adverse events associated with vandetanib therapy for advanced or metastasized thyroid cancers.
Results
Vandetanib statistically prolonged PFS in comparison with the placebo (30.5 vs. 19.3 months, hazard ratio 0.46), It even prolonged PFS in surgically unresectable or metastatic differentiated thyroid cancer cases compared with the placebo (11.1 vs. 5.9 months, hazard ratio 0.63). Rash, diarrhea, neutropenia, and hypertension were the most frequent side effects.
Conclusion
Vandetanib can significantly improve PFS. Though it has some side effects, it is still a promising agent in the treatment of advanced or metastasized thyroid cancer, especially in those with metastasized or advanced medullary thyroid carcinoma.
Introduction
Thyroid cancer (TC) is a common endocrine malignancy that has rapidly increased in global incidence in recent decades. Differentiated thyroid cancer (DTC), which includes papillary, follicular, and poorly differentiated carcinomas, accounts for about 90% of all cases of TC. Papillary and follicular carcinomas are generally associated with a favorable prognosis. However, the recurrence of TC occurs in 10–15% of patients after surgery.1 Distant metastases are detected in less than 10% of patients at diagnosis, and are the main cause of mortality.2 Vandetanib (Caprelsa, Astra-Zeneca, Wilmington, DE, USA) is a once daily oral anticancer drug that selectively targets rearranged during transfection (RET), vascular endothelial growth factor receptor (VEGFR), and EGFR tyrosine kinases.3, 4 The activity against RET makes it a good choice in the treatment of certain types of TCs. By targeting both VEGFR and EGFR simultaneously, vandetanib could potentially offer greater antitumor efficacies than the inhibitors aimed at either of the pathways alone. Moreover, vandetanib can inhibit primary and acquired resistance to EGFR-tyrosine kinase inhibitors (TKIs). Other multikinase inhibitors targeting VEGFR, RET, and other tyrosine kinases are associated with response rates of 14–59% in DTC.5-8 To gain a better understanding of the efficacy, safety, and mechanism of vandetanib in the treatment of advanced or metastasized TC, we carried out a systematic review of RCTs comparing vandetanib-containing and non-vandetanib-containing regimens in TCs to offer a comprehensive overview of the available literature.
Materials and method
Search strategy
Pubmed, EMBASE, Medline, China National Knowledge Infrastructure (CNKI), Web of Science, the Cochrane database, and the reference lists of relevant studies were searched for the terms “thyroid cancer” in combination with “vandetanib,” “zd6474,” “Zactima,” and “AstraZeneca.” There was no restriction on language or whether the articles had been published.
Study selection
Eligible articles met the following criteria: (i) patients with advanced or metastasized TC; (ii) studies combining chemotherapy with vandetanib versus without vandetanib, and studies on the efficacy and safety of vandetanib; and (iii) case-control studies. Exclusion criteria included: (i) non case-control studies; and (ii) animal studies. Two researchers assessed the eligibility of the clinical trials for inclusion in our systematic review. All work was checked by two reviewers (Fig 1).
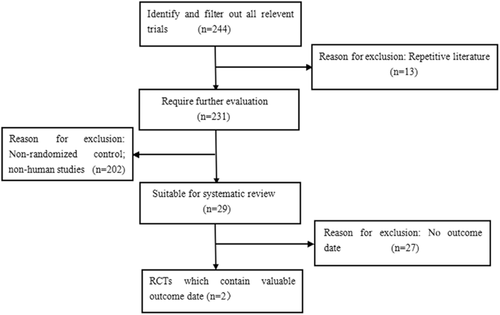
Search and screening studies regarding the efficacy of vandetanib in the treatment of thyroid cancer.
Quality assessment and data extraction
The quality of the studies was assessed and two reviewers independently extracted the data (Table 1). Potential disagreements were resolved by consensus. The following information was extracted: study authors, publication year, number of patients, gender, treatment, median progression-free survival (PFS), and related endpoints (Table 2). The primary outcome was PFS; the secondary was side effects.
| Entries | |
|---|---|
| Was the aim of the study clear? | Yes |
| Was the distribution in the intervention and control groups random? | Yes |
| Were all study participants accounted for by the study's conclusion? | Yes |
| Were the object and researchers of the study blinded? | Yes |
| Was the baseline data between groups at the start of the test similar? | Yes |
| In addition to the intervention of the studies, were other processing factors the same? | Yes |
| The effect of the intervention | PFS increased and more adverse reactions |
| Accuracy of the evaluation of the intervention effect | Qualitative analysis |
| Can the test results be applied to local people? | Yes |
| Did the study takes consider all important clinical trial outcomes | No |
| Were the benefits of therapy comparable to the cost? | Yes |
| Author | Year of publication | Female | Male | Median age | Diarrhea | Nausea | Rash | Fatigue | Blurred vision | PFS (Month) |
|---|---|---|---|---|---|---|---|---|---|---|
| Leboulleux et al.22 | 2012 | 97 | 134 | 50.7 | 130 | 77 | 103 | 54 | 19 | 30.5 |
| 44 | 56 | 53.4 | 6 | 16 | 11 | 12 | 0 | 19.5 | ||
| Wells et al.23 | 2012 | 33 | 39 | 63 | 54 | 18 | 18 | 17 | 5 | 11.1 |
| 34 | 39 | 64 | 12 | 18 | 3 | 13 | 1 | 5 |
- PFS, progression-free survival.
Results
Efficacy of vandetanib
Vandetanib statistically prolonged PFS in comparison with the placebo (30.5 vs. 19.3 months, hazard ratio 0.46), and the response rate was higher (45% vs. 13%, P < 0.001). The results of a placebo-controlled phase 2 trial of vandetanib in the treatment of surgically unresectable or metastatic DTC showed that vandetanib prolonged PFS in comparison with the placebo (11.1 vs. 5.9 months, hazard ratio 0.63). The tolerability of vandetanib in subjects with solid tumors was assessed in a phase 1 trial conducted abroad. Because of the small number of events at the time of the analysis and the fact that the design was open label after progression in the placebo group, the results did not show a marked difference in overall survival. In addition, because of the long PFS of 19.3 months in the placebo group, it was supposed that the tumor growth rate in the study population was relatively slow, which might be another reason no significant difference in overall survival was found.
Safety of vandetanib
A comparison of the side effects in the experimental and control groups is detailed in Table 2. Diarrhea was the most common adverse event, with a higher incidence than other side effects. Diarrhea, rashes, fatigue, and nausea were noted in approximately 60–70% of the patients.
Discussion
Thyroid cancer is the most common endocrine malignancy, representing over 90% of all endocrine cancers.9 Most TCs present as differentiated subtypes, including papillary and follicular carcinomas that respond well to surgery followed by radioactive iodine ablation. Medullary thyroid carcinoma (MTC) arises from the parafollicular C-cells of the thyroid gland that secrete calcitonin and accounts for 5% of all thyroid malignancies.10, 11 MTC can be either sporadic or hereditary, with about 75% of all new cases being sporadic. The remaining 25% hereditary cases involve one of three autosomal-dominant hereditary syndromes: multiple endocrine neoplasia (MEN) 2A, MEN2B, and familial MTC. Treatment for locally advanced and metastatic MTC continues to pose a significant challenge. Patients with locally advanced or metastatic MTC are incurable, and chemotherapy and radiation therapy have been largely ineffective. Therefore, the ability to substantially prolong PFS would benefit such patients. These limitations in the treatment of MTC patients provide a wonderful opportunity for novel drug development. A critical need exists for the development of better therapeutics that improve efficacy and survival, yet cause limited toxicity in these patients. Increasing data suggest that RET tyrosine kinase phosphorylation is a key signaling event in MTC proliferation. Early studies with TKIs have indicated that RET activity inhibition could restrain the proliferation of MTC cells in vitro.12 The mechanism of RET in MTC therapy is that TKIs are a hammerhead ribozyme, a catalytic ribonucleic acid enzyme that specifically cleaves complementary ribonucleic acid sequences.13 Ribozyme targets a particular mutation (C634R) of RET, demonstrating that the function of the kinase proteins could be inactivated, leading to the inhibition of MTC cell proliferation. The potential therapeutic implications of disrupting RET autophosphorylation were researched in vitro and in vivo by transfecting TT MTC cells (product CRL-1803, American Type Culture Collection, Manassas, VA, USA), which were derived from a patient with advanced MTC with adenoviral vectors expressing a dominant-negative truncated form of RET.14 Previous open-label phase 2 trials of other TKIs (Motesanib diphosphate) involving patients with DTC have reported median PFS durations ranging 3.7–18.2 months.15, 16 Following preclinical studies demonstrated that vandetanib inhibited signaling by blocking RET kinase and prolonging PFS of advanced or metastasized TC patients significantly.17, 18 The significant improvements in PFS suggest that vandetanib might be an effective treatment option for long-term stabilisation of advanced DTC, especially for patients with papillary TC. Partial remissions were confirmed in 20% of advanced MTC patients and the disease remains unprogressive for more than 24 weeks in 73% of patients with advanced MTC.19
Vandetanib is an oral agent. It has inhibitory activity against all EGFRs, except VEGFRs, which are vital to tumor growth and metastasis, as well as having RET inhibitory activity. Vandetanib was first developed as “ZD6474” by Astra-Zeneca who demonstrated its potent anti-angiogenesis effects in vitro and in vivo to target and inhibit VEGFR tyrosine kinase.9 Subsequently, its anti-tumor effects via the inhibition of EGFR have become apparent. Early clinical applications of vandetanib were to tumors that expressed high proportions of VEGFR and EGFR, including non-small cell lung and colorectal cancers.20 Vandetanib's anti-proliferative effects via inhibition of VEGFR and EGFR kinase-mediated pathways were demonstrated in an in vitro model of VEGF-A-stimulated human umbilical vein endothelial cells and lung cancer.3 It inhibits cancer cell-proliferation in prostate, breast, lung, and colorectal cancers, and in melanoma. Its anti-angiogenic, anti-tumorigenic, and anti-metastatic properties were further defined in orthotopic murine models of gastric, pancreatic, and renal cancers. Based on these promising results, vandetanib was studied in MTC and was shown to inhibit the activity of the RET tyrosine kinase receptor. An early advance leading to RET-molecular targeting was the recognition that the molecular structures of RET and VEGFR kinases were similar and that inhibitors of VEGFR could also inhibit RET tyrosine kinase activity.21 The most common adverse events of vandetanib are diarrhea, rash, acne, fatigue, nausea, and hypertension. Almost all patients experienced at least one adverse event. Rash, diarrhea, neutropenia, and hypertension occurred more frequently, whereas rates of vomiting, nausea, fatigue, dyspnea, and anemia were relatively low. One of the common and severe adverse reactions associated with vandetanib treatment was skin toxicity, particularly rash. There is a consensus that the inhibition of EGFR signaling on epidermal and adnexal epithelium results in skin toxicities, such as rash and diarrhea. However, the mechanisms are still unclear. Hypertension, a common side effect of VEGF/VEGFR signaling inhibitors, also occurred more frequently in the vandetanib-based therapy group compared with the placebo group. The occurrence of protocol-defined QTc prolongation was higher in the vandetanib-based therapy group. These side effects were generally manageable with dose interruption or reduction.
Conclusion
MTC, with a poor response to chemotherapy and radiotherapy, has challenged clinicians to determine effective treatment, especially in advanced stages. Vandetanib, originally developed as a VEGFR and EGFR inhibitor, was finally shown to inhibit RET kinase activity, and to have efficacy in various tumors, including in MTC. Vandetanib became the first approved agent by the US Food and Drug Administration for targeted therapy of advanced MTC. It can significantly improve PFS. However, vandetanib therapy is associated with some side effects. Given the toxicity profile and cost considerations of vandetanib therapy, patients with advanced or metastasized TC should be evaluated individually for risk-benefit stratification and consideration for the most appropriate therapy option based on clinical judgment.
Acknowledgments
This work was supported by grants for the Development of Science and Technology of Shandong Provincial (No. 2010GSF10228, 2012GGH11862, 2014GSF118118), the National Natural Science Foundation of China (no. 81070637), and the Natural Science Foundation of Shandong Province (No.Y2006C76, Y2008C73, ZR2010HM044).
Disclosure
No authors report any conflict of interest.




