Causal association between several gender-driven factors and gout: A bidirectional Mendelian randomization study
Yanshuang Ren and Yuqing Jiang contributed equally to this work and share the first authorship.
Plain Language Summary
- Serum uric acid (SUA) and urate concentrations play an essential role in the development of gout. Our study corroborates the causal influence of genetically predicted testosterone levels on SUA and urate concentrations, which are crucial in gout development.
- Prior research indicates estradiol may lower serum SUA levels and enhance urinary excretion; our findings do not establish a genetically significant causal link between estradiol and SUA.
- Hormone levels are intimately linked to breast cancer development, with a notably higher incidence in women than men. Our research indicates that breast cancer elevates the risk of increased urate levels, potentially adding to the disease burden for patients.
Gout was more prevalent in men than women, with incidence rates four times higher in male patients under 65 years.1 Testosterone was linked to higher gout rates in men than in women, but the specific relationship between testosterone and gout remains ambiguous. Nevertheless, postmenopausal women displayed a notably higher prevalence compared to their premenopausal peers. This gender-based prevalence gap narrowed for those over 65 years old persons, primarily driven by an uptick in incidence among older women.2 Prior observational research and fundamental assays highlighted estrogen's potential protective effect against gout.3 The malignant neoplasm of the breast stood as the leading oncological cause of female mortality. A meta-analysis of 33 distinct studies deduced a significant correlation between gout and elevated cancer incidence (relative risk [RR] = 1.19; 95% confidence interval [CI], 1.12–1.25). Hyperuricemia or gout correlated with a surge in cancer incidence and mortality, suggesting a potential linear relationship.4 To understand the gender-driven disparities in gout incidence, examining the hormonal profiles of both men and women was crucial. In this study, we utilized a two-sample Mendelian randomization (MR) approach to investigate the causal effects of testosterone, estradiol, and malignant neoplasm of the breast on serum uric acid (SUA), urate, and gout. Our study employed variants linked to the exposure as instrumental variables (IVs) and conducted the bidirectional MR, thus mitigating reverse causation and enhancing conclusion validity.
The study first estimated the causal effects of gender-driven indicators (total testosterone level, estradiol, malignant neoplasm of breast-human epidermal growth factor receptor [HER]-negative and HER-positive) on SUA, urate, and gout, respectively. Genetic variants qualify as IVs only if three strict conditions are met. First, a robust correlation between the genetic variant and the exposure must exist. Second, the variants should not be associated with confounding variables such as body mass index, tobacco and alcohol consumption, or age. Finally, the direct effects of genetic variants on outcomes were not considered. Their effects were mediated exclusively through the exposure pathway. This investigation was built on recently pooled statistical data from a genome-wide association study (GWAS) focusing on indicators of gender-driven factors and gout-related factors (SUA, urate, and gout). The quality control and ethical procedures applied to the GWAS data utilized in this research have been thoroughly documented in prior publications.5-7
This study utilized GWAS data of serum total testosterone levels in 199 569 European ancestry participants, containing 12 321 875 single nucleotide polymorphisms (SNPs). We also used GWAS summary statistics for estradiol levels, which included 147 690 individuals (including 13 367 cases with 134 323 controls), malignant neoplasm of the breast with HER-negative from 102 359 participants (3092 cases and 99 267 controls), and malignant neoplasm of the breast with HER-positive from 103 530 participants (4263 cases and 99 267 controls). GWAS datasets from the Integrative Epidemiology Unit (IEU) GWAS database, UKbiobank, BioBank Japan Project, and FinnGen were utilized.8-11 Sample characteristics of the study population are detailed in Table S1. We extracted outcome-associated variants, all of which reached genome-wide significance (p < 5 × 10−8), and tested for linkage disequilibrium (LD) for these SNPs. Genetic variants with LD (LD; r2 < .001) were not included in the study.12 To measure the strength of the IVs, we computed r2 and F-statistics and excluded failed IVs.
MR instruments of gender-driven factors served as the exposure factor to examine reverse causality for SUA, urate, and gout. Given that the F-statistics of all SNPs surpassed 10, weak instrumental variable bias was deemed negligible. Outcomes were determined using MR-Egger and inverse variance weighted (IVW) methods.13 The MR-Egger intercept test and Cochran's Q test were employed to test the pleiotropy and heterogeneity of each analysis. Outliers identified by the MR-PRESSO test were excluded. We used forest plots to reveal the causal effect of each SNP on gout-related factors.14 Similarly, estradiol level and malignant neoplasm of the breast (HER-positive and HER-negative) were analyzed as exposure factors for causal relationships with SUA, urate, or gout.
Our study employed the TwoSampleMR package with R software to conduct MR analysis.15 This study took the IVW method as the primary MR analysis approach, estimating the causal effect of genetic-prediction exposures on outcomes. The MR-Egger regression (MR-Egger) and weighted median (WM) methods were used to test the sensitivity. The WM method took the median estimate to assess the causal effect, and the MR-Egger provided a consistent causality estimate even if none of the genetic variants was valid for IVs. The IVW method integrates Wald ratios from individual SNPs to generate a composite estimate. This study utilized robust IVs to enhance the reliability of the IVW method's results. Consequently, it is concluded that the outcomes derived from the IVW method are more credible.13 This study applied Cochran's Q statistic and the I2 statistic to quantify the level of heterogeneity, with larger I2 values indicating greater heterogeneity. To bolster the robustness of our findings, we executed a leave-one-out analysis to exclude each SNP iteratively. Reverse MR was utilized, considering SUA, urate, and gout as exposures, with testosterone, estradiol, and breast cancer as outcomes (Table S2).
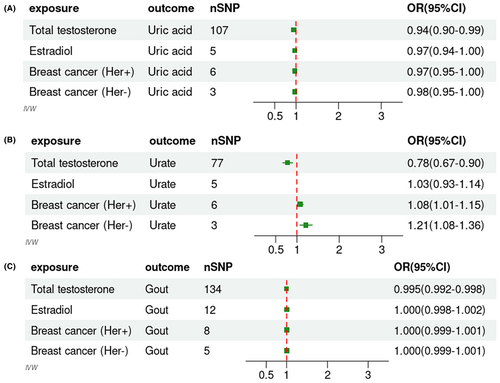
Outliers were excluded after removing IVs in LD. Our investigation identified 107 SNPs correlated with SUA as IVs from the GWAS analysis (R2 < .001, p < 5 × 10−8). The F-statistics for all SNPs under study exceeded 10, underscoring the reliability of the outcomes derived from our research. Notably, any minor bias was deemed negligible.16 The statistics indicated that genetically predicted total testosterone levels and SUA levels were causally related (IVW, odds ratios [OR]: 0.94, 95% CI: 0.90–0.99, p: .0236). Moreover, the MR-Egger intercept test (p: .8413) and the horizontal pleiotropy test (p: .3749) showed no pleiotropy. For urate, we found that after excluding outliers, genetically predicted total testosterone levels were causally related to urate levels (IVW, OR: 0.78, 95% CI: 0.67–0.90, p: .0017), with no evident pleiotropy (p: .1552). Our research robustly substantiated a causal impact of genetically predicted total testosterone levels on gout (IVW, OR: 0.995, 95% CI: 0.992–0.998, p: .0007), with on evidence of pleiotropy (p: 0.9853). Comprehensive statistics are described in Figure 1 and Table 1. Figures S1–S3 displayed forest plots and scatter plots for SUA, urate, or gout based on genetic predictions at total testosterone levels. Additionally, we revealed all the details of the sensitivity and pleiotropy analyses in Table 1. We found no evidence for a causal effect of genetically predicted estradiol on SUA, urate, and gout (p for IVW: .1263, .5401, and .9793, Table 1). The remaining pooled analysis methods did not provide strong evidence for the correlation between estradiol levels and gout. Figures S4–S6 incorporated scatterplots and forest plots.
| Exposure | Outcome | MR analysis | Heterogeneity test IVW Qpval | Pleiotropy test pval | |
|---|---|---|---|---|---|
| IVW pval | MR-egger pval | ||||
| Tes | SUA | .0166 | .6289 | 9.611742e−13 | .3750 |
| Urate | .0017 | .0080 | .009445488 | .1552 | |
| Gout | .0007 | .0530 | 5.103485e−35 | .9853 | |
| Estradiol | SUA | .1263 | .3357 | .2841 | .5257 |
| Urate | .5401 | .2503 | .6917 | .2868 | |
| Gout | .9793 | .3151 | .0063 | .2837 | |
| Malignant neoplasm of breast HER-negative | SUA | .0985 | .7174 | .9301 | .8625 |
| Urate | .0015 | .8603 | .5598 | .6421 | |
| Gout | .7128 | .4345 | .4312 | .3743 | |
| Malignant neoplasm of breast HER-positive | SUA | .0571 | .7794 | .1749 | .4345 |
| Urate | .0273 | .1020 | .4667 | .1883 | |
| Gout | .8125 | .6501 | .8165 | .6932 | |
- Abbreviations: HER, human epidermal growth factor receptor; IVW, inverse variance weighted; MR, Mendelian randomization; pval, p-value; SUA, serum uric acid; Tes, testosterone.
We explored the causal relationships with SUA, urate, and gout using genetically predicted HER-negative breast malignancy. Our findings indicate no causal association between HER-negative breast malignancy and SUA levels (p for IVW: .0985). Employing the IVW method, HER-negative breast malignancy appeared to increase the risk of urate levels (IVW, OR: 1.21, 95% CI: 1.08–1.36, p: .0015), with no pleiotropy (p: .6421) and heterogeneity (Q-pval [Qpval]: .5598). Refer to Figure S7 for the scatterplot and forest plot. No correlation was identified between HER-negative breast malignancy and gout. However, through our analysis of HER-positive breast malignancy, we observed a correlation with urate levels (IVW, OR: 1.08, 95% CI: 1.01–1.15, p: .0273, Figure S8), devoid of pleiotropy (p = .1883) and heterogeneity (Qpval: .4667). In this bidirectional two-sample MR study, our research thoroughly confirmed the causal relationship between genetically inferred testosterone levels and SUA, urate, and gout. However, no causal associations were observed for genetically predicted estradiol levels (IVW method, p for SUA: .1263, p for urate: .5401, p for gout: .9793). This study did not find evidence of a genetically predicted estradiol protective effect on gout-related factors. Additionally, we employed data on the malignant neoplasm of the breast (HER-positive and HER-negative). This revealed a notable association with urate levels (IVW method, p for HER-positive: .0273, p for HER-negative: .0015) but not with SUA or an escalated risk of gout (Table 1). Elevated SUA due to uricase deficiency increased the growth and metastasis of breast cancer cells in mice.17 In this investigation, MR analysis was utilized to examine the association between urate levels and breast cancer, encompassing both HER-positive and HER-negative subtypes. This study presented novel insights, as the causal relationship in this context has not been previously reported. The MR analysis provided evidence that breast cancer increases urate levels. The discrepancies between prior results and our findings might arise from diverse analytical approaches. Observational or retrospective studies can be muddled by various confounders, undermining causal inference.
In contrast, our application of the MR methodology, leveraging genetic variations, circumvented many potential confounders and offered a more robust assessment of causal relationships. Genetic predictors indicated a causal relationship between both HER-positive and HER-negative malignant neoplasms of the breast and serum urate levels. However, no causal association was observed between these neoplasms and gout. The reverse MR analysis negated the potential influence of reverse causation.
From a genetic perspective, we found that lower total testosterone levels in men may increase SUA levels, urate levels, and risk of gout. However, our findings do not establish a genetically significant causal link between estradiol and SUA. Moreover, we found that breast cancer increased the risk of elevated urate levels, which may be one of the disease burdens of concern for breast cancer patients and had never been reported.
AUTHOR CONTRIBUTIONS
YSR and YQJ designed the study and authored the manuscript. HFP provided references and revised the manuscript. HWJ advised on the study's methodology and technology. All authors contributed and approved the final version.
ACKNOWLEDGMENTS
The authors thanked all participants in the study.
FUNDING INFORMATION
This work was supported by the Henan Provincial Science and Technology R&D Joint Fund (Key Program) (225200810054), Henan Province Science and Technology R&D Program Joint Fund Project (Applied PR Category) (232103810049) and the Medical Science and Technology Research and Development Plan Major Project Jointly Constructed by the Henan Province and Ministerial Departments in China (No. SBGJ202301010).
CONFLICT OF INTEREST STATEMENT
All authors declared no conflict of interest.
ETHICS STATEMENT
The data used in this study were obtained from published ethically approved studies.
Open Research
DATA AVAILABILITY STATEMENT
Data are available on request from the authors.




