Real-world effectiveness and persistence of golimumab as second-line anti-TNFα drug in rheumatoid arthritis, psoriatic arthritis, and axial spondyloarthritis patients in Italy: GO-BEYOND, a 12-month prospective observational study
Plain Language Summary
A high proportion of patients may fail a first-line anti-TNF drug, necessitating the switch to another anti-TNF treatment. After 12 months of GLM treatment, 80% of RA patients achieved low disease activity (LDA), 37.1% with PsA achieved minimal disease activity and 55.3% with axSpA achieved LDA while persistence at 12 months in all patients was 77.7%. In this 1-year analysis of the GO-BEYOND study in Italy, GLM had a favorable benefit: risk profile and high retention rate in patients with PsA, RA and axSpA.
Dear Sir,
Tumor necrosis factor-alpha (TNFα) inhibitors have substantially improved the management of rheumatoid arthritis (RA), psoriatic arthritis (PsA) and axial spondyloarthritis (axSpA).1 However, data from real-life studies reveal that as many as half of patients interrupt or stop first-line anti-TNF agents.2
European Guidelines recommend that if one anti-TNF fails, patients with RA may receive a second anti-TNF or another drug with a different mode of action.3 Current approved anti-TNF inhibitors for the treatment of RA, PsA, and axSpA include adalimumab, infliximab, etanercept, certolizumab pegol, and golimumab (GLM).
Data from randomized controlled trials have shown that GLM is effective for the treatment of RA,4 PsA,5and axSpA,6 with ~70% maintaining treatment through 5 years.7 In GO-AFTER, a phase-III trial,8 GLM was effective and safe in RA patients who had failed one or more anti-TNF drugs.8 However, limited data are available from real-life studies in RA as well as PsA and axSpA.9-14
Previously, we evaluated the effectiveness of GLM as a second anti-TNF drug in patients with RA, axSpA, or PsA up to 6 months.15 This analysis of the GO-BEYOND study evaluated the effectiveness and retention rate up to 12 months.
1 METHODS
1.1 Patients and study design
Patients diagnosed with RA, PsA, or axSpA who initiated GLM after first-line anti-TNFα inhibitor failure participated in this study from 2017 to 2019. All patients received a 50 mg (100 mg in patients ≥100 kg) monthly dose of GLM subcutaneously (as specified in the Summary of Product Characteristics).6 Visits were performed at baseline, 3, 6, and 12 months. The characteristics of patients as well as inclusion and exclusion criteria have been described in detail elsewhere.15 In this study, the effectiveness and persistence of GLM, in addition to QoL, was evaluated up to 12 months. Ethics committee approval from all participating centers and written informed consent was obtained from every patient, in compliance with the Legislative Decree 196/2003 and in accordance with the 1975 Declaration of Helsinki.
1.2 Outcome measures
The following outcomes were assessed. Low disease activity of Disease Activity Score in 28 joints (LDA; DAS28-CRP ≤3.2) and remission (DAS28-CRP ≤2.6) for RA, minimal disease activity (MDA) for PsA, LDA according to Ankylosing Spondylitis Disease Activity Score based on C-reactive protein (ASDAS-CRP <2.1), and the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) 50 (50% improvement in baseline BASDAI) and The Assessment of SpondyloArthritis International Society Health Index (ASAS HI) were measured in axSpA patients. The EULAR response criteria were used to evaluate the change in DAS28-CRP from baseline and level of DAS28-CRP at 12 months in patients with RA and PsA. In all patients, health-related quality of life (HRQoL) was assessed using the EuroQoL 5-Dimension 5-Level (EQ-5D-5L) questionnaire. Reasons for GLM discontinuation were also recorded.
1.3 Statistical analysis
Percentages for effectiveness outcomes were calculated together with the corresponding 95% confidence interval (CI) computed using the Clopper–Pearson method. Differences in disease activity scores at baseline versus 12 months were assessed using the paired t-test or the paired-sample sign test, as appropriate, after checking for normal distribution. Differences at baseline and 12 months in the proportion of patients with problems across the five domains of the EQ-5D-5L were tested using the McNemar test. GLM persistence rate at 12 months was estimated using Kaplan–Meier analysis. Analysis was performed using SPSS statistical software (SPSS, Chicago, IL, USA).
2 RESULTS
2.1 Baseline characteristics
A total of 194 patients with RA (N = 39; 20.1%), PsA (N = 91; 46.9%), and axSpA (N = 64; 32.9%) were included in the GO-BEYOND study. Baseline clinical characteristics have been previously reported15 and are briefly summarized in Table S1.
2.2 Clinical response in RA patients
In RA, 80% (95% CI: 56.3%–94.3%) of the patients achieved at least LDA at 12 months of GLM treatment, with 60% (95% CI: 36.1%–80.9%) achieving complete remission (based on DAS28-CRP), while a good/moderate EULAR response was observed in 88.2% (95% CI: 63.6%–98.5%) of patients. Mean DAS28-CRP, SJC, TJC, and PGA significantly decreased at 12 months compared to baseline values (Table 1).
| RA patients (n = 23a) | PsA patients (n = 69a) | axSpA patients (n = 45a) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12 months | p-Value | Baseline | 12 months | p-Value | Baseline | 12 months | p-Value | |
| Disease activity measures | |||||||||
| DAS28-CRP | 4.2 ± 0.9 | 2.6 ± 1 | <.001 | 3.9 ± 1 | 2.4 ± 1 | <.001 | — | — | — |
| CRP (mg/L) | 12.6 ± 23.1 | 6.9 ± 11.1 | .36 | 5.5 ± 8.8 | 2.5 ± 2.7 | .19 | 5.8 ± 9.2 | 3 ± 4.2 | .23 |
| ESR (mm/h) | 24.5 ± 23.7 | 25.6 ± 22.2 | .77 | 21.4 ± 20.6 | 15.4 ± 13.7 | .13 | 16.9 ± 15 | 12.5 ± 9.8 | .09 |
| SJC (28 joints) | 2.2 ± 1.9 | 0.8 ± 2.1 | .001 | 2.2 ± 3.7 | 0.4 ± 1.1 | <.001 | — | — | — |
| TJC (28 joints) | 6.7 ± 5.1 | 1.8 ± 2.6 | <.001 | 5.4 ± 4.5 | 1.9 ± 4.2 | <.001 | — | — | — |
| PASI | — | — | — | 4 ± 11.7 | 0.5 ± 1.1 | .003 | — | — | — |
| PGA | 64.7 ± 19.3 | 30.5 ± 23.6 | <.001 | 61.9 ± 20.1 | 37.4 ± 25.3 | <.001 | 62.4 ± 21.8 | 38.9 ± 27.5 | <.001 |
| ASDAS-CRP | — | — | — | — | — | — | 2.8 ± 1.1 | 1.9 ± 1 | <.001 |
| BASDAI | — | — | — | — | — | — | 5.8 ± 2.3 | 4 ± 2.4 | .004 |
| ASAS-HI | — | — | — | — | — | — | 10 ± 4.2 | 8.1 ± 4.6 | .001 |
| QoL by EQ-5D-5L | |||||||||
| Domain (% patients with some problems) | |||||||||
| 1. Mobility | 16 (72.7) | 13 (59.1) | .38 | 43 (81.1) | 32 (60.4) | .013 | 35 (83.3) | 29 (69) | .18 |
| 2. Self-care | 14 (63.6) | 10 (45.5) | .22 | 33 (62.3) | 31 (58.5) | .82 | 28 (66.7) | 18 (42.9) | .006 |
| 3. Usual activities | 18 (81.8) | 15 (68.2) | .38 | 47 (88.7) | 38 (71.7) | .012 | 37 (88.1) | 32 (76.2) | .063 |
| 4. Pain/discomfort | 21 (95.5) | 17 (77.3) | .13 | 51 (96.2) | 44 (83) | .016 | 41 (97.6) | 36 (85.7) | .063 |
| 5. Anxiety/depression | 13 (59.1) | 16 (72.7) | .38 | 34 (64.2) | 31 (58.5) | .63 | 26 (61.9) | 23 (54.8) | .51 |
| Health today | 54.5 ± 13 | 66.2 ± 19 | .027 | 52.7 ± 18.7 | 64.7 ± 20.4 | .001 | 52.7 ± 24.2 | 61.6 ± 20 | .039 |
| EQ-5D-5L index | 0.73 ± 0.21 | 0.79 ± 0.14 | .027 | 0.73 ± 0.12 | 0.79 ± 0.13 | .002 | 0.73 ± 0.13 | 0.8 ± 0.12 | .001 |
| EQ-VAS | 60.9 ± 14.5 | 57.6 ± 29.1 | .65 | 53 ± 20.4 | 51.1 ± 27.2 | .68 | 52.4 ± 25.5 | 51 ± 25.6 | .79 |
- Note: Statistically significant differences compared to baseline values are represented by p-values in bold text. Data are presented as frequencies (number and %) or as mean ± standard deviation.
- Abbreviations: ASAS-HI, Assessment of SpondyloArthritis International Society Health Index; ASDAS-CRP, Ankylosing Spondylitis Disease Activity Score based on C-reactive protein; AxSpA, axial spondyloarthritis; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; CRP, C-reactive protein; EQ-5D-5L index, EuroQoL 5-Dimension 5-Level (index); EQ-VAS, EuroQol Visual Analogue Scale; ESR, erythrocyte sedimentation rate; PASI, psoriasis area and severity index; PGA, patient global assessment; PsA, psoriatic arthritis; RA, rheumatoid arthritis; RF, rheumatoid factor; SJC, swollen joint count based on 28 joints; TJC, tender joint count based on 28 joints.
- a The number of patients shown represent the number of patients who completed 12 months of GLM treatment. Data were not available/missing in a small number of patients for some of the variables examined.
2.3 Clinical response in PsA patients
MDA was achieved in 37.1% (95% CI: 25.2%–50.3%), and DAS28-CRP-based disease remission was achieved in 72.7% (95% CI: 59%–83.9%) of PsA patients at 12 months. A good/moderate EULAR response was achieved in 78.4% (95% CI: 61.8%–90.2%) of patients. A significant improvement was observed for DAS28-CRP, SJC, TJC, PASI, and PGA at 12 months (Table 1).
2.4 Clinical response in axSpA patients
In axSpA patients, 55.3% (95% CI: 38.3%–71.4%) achieved at least LDA and 23.7% (95% CI: 11.4%–40.2%) achieved remission according to ASDAS-CRP at 12 months. BASDAI 50 was achieved in 27.3% (95% CI: 15%–42.8%) of patients. Mean ASDAS-CRP score, BASDAI, and ASAS-HI as well as PGA were significantly improved at 12 months (Table 1).
2.5 QoL assessment
QoL scores improved (i.e., increased) for the five EQ-5D-5L domains from baseline to 12 months in the three patient groups, with greater improvement observed in the PsA group (Table 1). Mean scores of patients' “health today” and EQ-5D-5L index were observed to significantly increase from baseline to 12 months in all patient groups (Table 1).
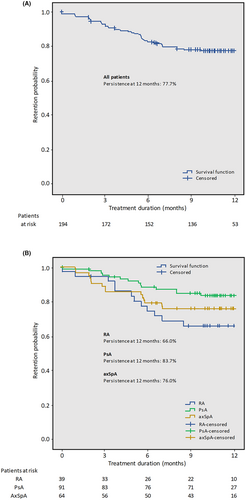
2.6 Persistence
The 12-month persistence rate for all patients was 77.7% (95% CI: 70.9%–83.0%; Figure 1A). The persistence rates for RA, PsA, and axSpA patients were 66% (95% CI. 47.9%–79.1%), 83.7% (95% CI: 74%–90%), and 76% (95% CI: 63.3%–84.8%), respectively (Figure 1B).
2.7 Reasons for GLM treatment interruption
Reasons for GLM interruption are presented in Table S2. Over the 12-month treatment period, 57 patients (29.4%) interrupted the study: 41 (21.1%) discontinued due to definitive interruption of GLM treatment, 14 (7.2%) were lost to follow-up, 1 (0.5%) interrupted due to lack of compliance, and 1 (0.5%) for an unspecified reason.
3 DISCUSSION
Results from this 1-year GO-BEYOND study confirm and extend our previous 6-month results.15 GLM as second-line anti-TNFα for RA, PsA, and axSpA displayed a favorable benefit: risk profile, with approximately 80% of patients maintaining treatment up to 12 months. Our results corroborate with other recent real-life studies performed in European countries.9-14
Alegre-Sancho et al. found a significant decrease in DAS28 in PsA, and BASDAI in axSpA patients on GLM after first anti-TNF drug failure.14 In the GO-BEYOND study performed in Turkey, the persistence of GLM and change in disease activity measures over 2 years in RA and axSpA patients after GLM was evaluated.9 Although their study was limited by the small number of RA patients who were biologic experienced (N = 7), DAS28-CRP decreased from 4.8 to 2.1 at 12 months, similar to our cohort.9 In patients with axSpA previously treated with an anti-TNF drug (N = 28), an approximately threefold reduction was seen for ASDAS (baseline vs. 12 months; 3.08 vs. 1.18) and BASDAI (4.3 vs. 1.6).9 The smaller improvement observed in the present study may be due to older age of axSpA patients (51 vs. 40 years) and higher CRP levels, and ASDAS and BASDAI scores at baseline.9
Results from the Italian GISEA registry also corroborate with our findings.13 A similar reduction in DAS28 (from 4.9 ± 1.2 at baseline to 3.1 ± 1.2 at 12 months) in the subgroup of RA patients who were inadequate responders to one biologic (N = 94) was observed. Yet, a lower proportion of RA patients achieved a good EULAR response in the GISEA cohort (69% vs. 88.2%). In GISEA, 57% of patients with axSpA achieved BASDAI 50 at 12 months while 67% achieved LDA and 36% were in remission according to ASDAS.13 These differences may be attributed to the older age (51 vs. 46 years) and higher frequency of comorbidities (76.6% vs. 41%) in the GO-BEYOND Italy versus GISEA cohort.
A post-hoc analysis of the prospective GO-NICE study in Germany evaluated the effectiveness of GLM by line of treatment in patients with RA, PsA and AS.12 In patients with RA (N = 104) given GLM as second-line biologic, DAS28 decreased from 4.9 ± 1.3 to 3.4 ± 1.6 and 41.3% were in remission at 12 months. The Psoriatic Arthritis Response Criteria in patients with PsA showed a 46.3% improvement at 12 months and BASDAI score decreased from 4.9 ± 2 to 3.0 ± 2.2 in AS patients. Similar results were also seen in a post-hoc analysis of the prospective GO-PRACTICE study in France in second-line biologic patients with axSpA, where BASDAI decreased to a similar extent (5.7 to 3.5) at 12 months.11 Our 12-month results in terms of DAS28 and BASDAI improvement in patients with RA and axSpA, respectively, corroborate with results from GO-NICE and GO-PRACTICE.
The overall persistence rate in our study was 77.7%, with slightly higher persistence seen in PsA (83.7%) than in axSpA (76%) and RA patients (66%).
In the study by Alegre-Sancho et al., the probability of persistence after 1 year was 80%14 and retention rates were 57.1% in biologic-experienced RA and 80.4% in anti-TNF experienced axSpA patients in the GO-BEYOND study in Turkey.9 In the GISEA registry, persistence of second-line GLM in RA, PsA, and SpA patients ranged from 70% (in RA patients) to about 85% in axSpA patients. In GO-NICE,12 the 2-year retention rate was 45.5% (1 year results were not reported) and in GO-PRACTICE,11 the 12-month retention rate was 57.2%.
These generally favorable retention rates with GLM as second-line anti-TNF may be associated to the once-monthly, self-administered regimen and good tolerability profile.
Lower retention rates observed in RA patients may be attributed to the different role of TNF inhibition in this disease.9, 13 It is also well documented that female gender frequently emerges as a predictor of anti-TNF discontinuation,16 and in the present study, the proportion of females was highest in the RA group (74.4%) compared to PsA (51.6%) and axSpA (53.1%).
Results from the present study confirm findings of earlier studies on the effectiveness and persistence of GLM given as second-line anti-TNF in patients with RA, PsA, and axSpA and extend our previous 6-month results15 up to 1 year.
AUTHOR CONTRIBUTIONS
S.D., A.M.G., and C.B. were involved in the design of the study and data analysis. All other authors were involved in the enrolment of patients and data collection and contributed to drafting the paper/revising it critically for intellectual content. All authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGMENTS
We thank Yghea (Ecol Studio S.p.A. Bologna, Italy), the Contract Research Organization involved in the organization and conduct of the trial and Colin Gerard Egan (CE Medical Writing SRLS, Pisa, Italy) for his support in medical writing, both funded by MSD Italia.
FUNDING INFORMATION
This study was funded by MSD Italia S.r.l. (Rome, Italy). A.M.G., C.B., and T.D. are employees of MSD Italia and were involved in the design of the study and analysis of the data.
CONFLICT OF INTEREST STATEMENT
S.D. declares consulting and speaking fees from AbbVie, Amgen, Bristol Myers Squibb, Janssen, Lilly, MSD Italia, Novartis, Pfizer, and UCB. E.T. declares consulting and speaking fees from AbbVie, Alfasigma, Amgen, Bristol, Galapagos, Novartis, Janssen, and Pfizer. F. Cantatore declares consulting and speaking fees from AbbVie, Bristol Myers Squibb, and Galapagos. O.V. has received payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing or educational events, or participation in advisory boards from Eli Lilly, AbbVie, and Fresenius Kabi. A.C. has received honoraria for consultations or presentations or research grants from AbbVie, Alfasigma, Biogen, BMS, Celgene, Galapagos, Eli Lilly, Janssen, MSD Italia, Novartis, Pfizer, Sanofi Genzyme, and UCB. F.I. has received speaker or consultant fees from AbbVie, BMS, Celgene, Pfizer, Roche, Novartis, and MSD Italia. All other authors have no conflict of interest to declare.
ETHICS STATEMENT
The study (protocol number: MK-8259-6415) was approved by the Regional Ethics Committee for Clinical Studies from the Tuscany Region (Sezione, Area Vasta Sud Est) on March 20, 2017. Local ethics committee approval was obtained from all the participating centers.
INFORMED CONSENT STATEMENT
All patients provided written informed consent in accordance with existing applicable laws (DL 196/2003) and the study was conducted in accordance with the Declaration of Helsinki.
Open Research
DATA AVAILABILITY STATEMENT
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information. The datasets generated during or analyzed during the current study are not publicly available. All data used in this study were anonymized to respect the privacy of patients in line with the applicable laws and regulations.




