Favorable effects of hydroxychloroquine on serum low density lipid in patients with systemic lupus erythematosus: A systematic review and meta-analysis
Abstract
Aims
Hydroxychloroquine (HCQ) has shown to have significant immunomodulatory effects in the treatment of systemic lupus erythematosus (SLE). Current studies show favorable effects of HCQ on traditional cardiac risk factors in patients with SLE. This review examined the effects of HCQ on serum low-density lipoprotein (LDL) level in patients with SLE.
Methods
A systematic search of seven major literature search databases from their inception until 3 April, 2017 identified nine studies. Random-effects pooled mean difference with corresponding 95% confidence intervals (CI) were estimated. Heterogeneity was measured by I2. Publication bias was assessed by visual inspection of funnel plots. Sensitivity analysis examined whether HCQ effect on serum total cholesterol level was similar to the main analysis. The Grading of Recommendations Assessment, Development, and Evaluation system was used to assess the overall quality of evidence.
Results
Pooled study participants were 559 patients from eight observation studies (two before-after studies; six case-control studies) examining the effects of HCQ on serum LDL. Pooled study participants' characteristics were as follows: mean age 45.719, female 95.262%, and prednisone use 58.366%. HCQ reduced mean LDL levels by 24.397 mg/dL (95% CI 8.921–39.872; P = 0.002). The number of studies identifying statin use was too few to perform meta-regression analysis of statin use. Heterogeneity was extensive (I2 = 94.739%). Symmetrical funnel plot visualized no evidence of publication bias.
Conclusion
HCQ was associated with serum LDL level reduction by mean 24.397 mg/dL in patients with SLE. Future prospective studies are need to fully characterize the treatment effect.
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disorder that leads to inflammation and tissue damage involving multiple organ systems. It is a chronic inflammatory disease that is poorly understood, with an increased frequency in women of childbearing age and non-White populations.1 The etiology of the disease includes hormonal, genetic and environmental factors.2-5 Immune cutaneous findings, joint pain, and fatigue are the most common initial findings, and with more advanced disease, renal, pulmonary, cardiovascular, and central nervous systems are affected.6, 7
SLE continues to be a large and growing public health problem. The incidence and prevalence of SLE vary. Based on recent population-based registries it is estimated that the incidence of lupus across different countries in North America, Europe and South America vary from as low as one to as high as 8.77 per 100 000.8 The Center for Disease Control (CDC) estimates the prevalence of adults with SLE in the US at 322 000 based on the National Arthritis Data Workgroup.9 In addition, patients with SLE have an increased susceptibility toward many life-threatening complications that include infections, malignancy and diseases of the circulatory systems compared with the general population.10 Heart disease contributes to an alarming proportion of mortality in this patient population.10, 11 Lee et al. found that patients with SLE have a 2.6-fold higher mortality rate compared to an age-matched and sex-matched general population.12
Indeed, it is known that patients with SLE have a propensity for accelerated coronary artery disease.13, 14 Yet these patients have been difficult to risk stratify, and the current cardiac risk scores only take into account traditional risk factors for atherosclerotic disease such as hypertension, diabetes mellitus, and smoking.15 Szabo et al. reported that accelerated atherosclerosis is also related to non-traditional, disease-related factors of SLE.16 According to a 3-year international inception cohort study of patients with SLE, the prevalence of coronary artery disease has been noted to be as high as 60% after 3 years of disease burden.17 Borba et al. observed that SLE patients had a lipid profile abnormality which was aggravated by disease activity.18 In the current era of rheumatic disease management, there is an increased interest in analyzing the effects of disease-modifying medications on traditional cardiac risk factors. Given that hydroxychloroquine (HCQ) has shown to have significant disease activity lowering effects in the treatment of SLE, there has been an increased interest on its effects on cardiac risk factors.19-21 Current studies show favorable effects of HCQ on traditional cardiac risk factors in rheumatic diseases. Favorable effects that have been noted include the following: less atherogenic lipid profiles, reduced risk of diabetes, and antithrombotic properties.22-27 Using the systematic review and meta-analysis approach, we aim to examine the effects of HCQ on lipid profiles, specifically, low density lipoprotein (LDL) of patients with SLE.
Methods
Search strategy
We searched relevant English articles in seven databases that included PubMed, EMBASE, ISI Web of Science, Medline/Ovid, Google Scholar, Cumulative Index to Nursing and Allied Health Literature (CINAHL), and Cochrane Database of Systematic Review, from their inception to 3 April, 2017. In these seven databases, the following combinations of terms were used as follows in order to search the studies: (i) (lupus OR systemic lupus erythematosus) AND; (ii) (lipid OR LDL OR cholesterol) AND; (iii) (hydroxychloroquine OR chloroquine OR chloroquine sulfate). We restricted language to English and age of 18 years or older. Supplement presented PubMed search strategy.
Study selection and data extraction
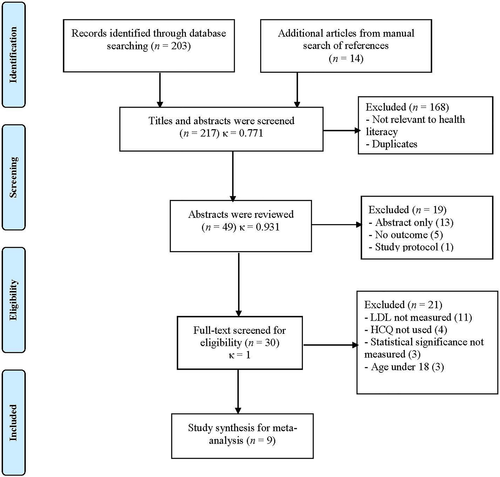
Two authors (XL and JY) independently screened titles and abstracts. After an independent review, data from each study were entered into a standard sheet. For all phases, researchers resolved any disagreements through discussions or second opinions from two additional authors (HB and AY). Kappa statistics were computed to assess the inter-rater reliability of the screening phases. We also searched through the articles from the bibliographies of the retrieved studies and review articles. When actual data were not presented in certain studies, researchers (XL and JY) directly contacted the corresponding authors to obtain missing data. As the selected studies were already published elsewhere, this study was exempted from the institutional review board approval. The study selection process was based on accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)28 statement, presented in Figure 1.
Quality assessment
We used the Grading of Recommendations Assessment, Development, and Evaluation (GRADE)29 system to assess overall quality of evidence for each outcome. The overall quality of evidence took into consideration the following five domains: risk of bias, consistency, directness, precision and publication bias. The GRADE system can be used for rating the quality of evidence (high, moderate, low and very low). The gradePRO® software was used to present the quality of evidence.30 Meta-analysis from observational studies starts from low quality of evidence. The quality of evidence may decrease when there is serious limitation of any of the five domains. Publication bias was assessed by visual inspection of funnel plots.
Data synthesis and statistical analysis
We assessed within and between study variations and heterogeneity using Cochran's Q-statistics. The heterogeneity test was used to assess the null hypothesis that all studies were evaluating the same effect. Because a significant Q-static (Q = 133.057; P < 0.001) indicated significant heterogeneity across studies,31 the random-effect model with corresponding 95% confidence intervals (CI) was estimated.32 Between-study heterogeneity was assessed using the I2 static values of 50%, representing extensive statistical inconsistency.33 When mean value or standard deviation is not available and/or reported, we used the formula estimating this information published elsewhere.34, 35 All analyses were performed in Comprehensive Meta-Analysis version 3 (Biostat Inc., Englewood, NJ, USA, 2014). A two-sided P < 0.05 was considered statistically significant. Meta-regression analysis was performed to confirm other variables would be predictors of serum LDL level. Subgroup analysis was performed to examine effects of the study type and location.
Results
Effect of HCQ on serum LDL
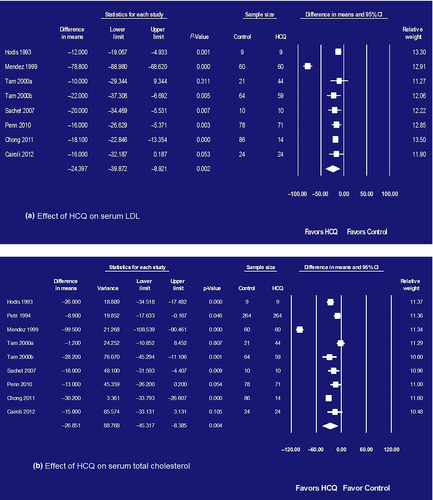
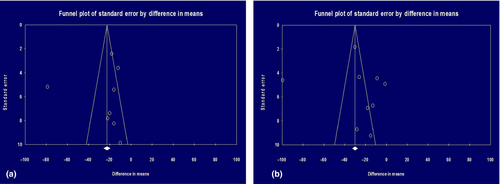
Pooled study participants were 559 patients from eight observation studies (two before-after studies;36, 37 six case-control studies38-43) examining the effects of HCQ on LDL as shown in Table 1 (left column). Pooled study participants' characteristics were as follows: mean age 45.719 and standard deviation 11.893, female 95.262% and prednisone use 58.366%. HCQ reduced mean LDL levels by 24.397 mg/dL (95% CI 8.921–39.872; P = 0.002) as shown in Figure 2a. Heterogeneity was extensive (I2 = 94.739%). Symmetrical funnel plot visualized no evidence of publication bias in Figure 3a.
| Studies | Study participants | Study site | Serum LDL level (mg/dL) | Serum total cholesterol level (mg/dL) | ||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | P-value | Mean ± SD | Mean ± SD | P-value | |||
| Before-after design (three studies, n = 348) | ||||||||
| Before HCQ | After HCQ | Before HCQ | After HCQ | |||||
| Petri 199419 | 264 | USA | — | — | — | 200 ± 46 | 191.1 ± 55.9 | 0.009 |
| Mendez 199936 | 60 | USA | 183.7 ± 31.2 | 104.9 ± 25.4 | < 0.001 | 279.4 ± 27.5 | 179.9 ± 22.8 | < 0.001 |
| Cairoli 201237 | 24 | Uruguay | 117 ± 31.3 | 101 ± 26.2 | 0.02 | 198 ± 33.7 | 183 ± 30.3 | 0.02 |
| Case-control design (six studies, n = 465) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Control | Case | Control | Case | |||||
| Hodis 199338 | 18 | USA | 97 ± 9 | 85 ± 6 | Not significant | 180 ± 11 | 154 ± 7 | Not significant |
| Tam 2000a39 | 65 | Hong Kong | 95.4 ± 28.9 | 105.4 ± 40.5 | Not significant | 177.6 ± 28.9 | 176.4 ± 10.8 | Not significant |
| Tam 2000b40 | 123 | Canada | 138.4 ± 42.5 | 116.4 ± 44.1 | 0.007 | 225.8 ± 47.6 | 197.6 ± 49.1 | 0.002 |
| Sachet 200741 | 20 | Brazil | 108 ± 17 | 88 ± 16 | < 0.001 | 174 ± 15 | 156 ± 16 | < 0.001 |
| Penn 201042 | 149 | USA | 118 ± 34 | 102 ± 32 | 0.004 | 196 ± 42 | 183 ± 40 | 0.06 |
| Chong 201143 | 100 | Hong Kong | 116.2 ± 3.9 | 98.1 ± 20.8 | 0.045 | 208.4 ± 5.0 | 178.3 ± 12.0 | 0.025 |
- LDL, low-density lipoprotein; SD, standard deviation; HCQ, hydroxychloroquine.
Effect of HCQ on serum total cholesterol
A total of 823 patients were analyzed from nine observation studies (three before-after studies;19, 36, 37 six case-control studies38-43) examining the effects of HCQ on total cholesterol as shown in Table 1 (right column). HCQ reduced mean total cholesterol by 26.851 mg/dL (95% CI 8.385–45.317; P = 0.004) as shown in Figure 2b. Heterogeneity was extensive (Q = 296.632; P < 0.001; I2 = 94.739%). Symmetrical funnel plot visualized no evidence of publication bias in Figure 3b.
Quality of evidence
Table 2 presents the GRADE quality of evidence of two outcomes, serum LDL and total cholesterol. The quality of evidence was very low in both serum LDL and total cholesterol because of extensive heterogeneity.
| Outcomes | Control vs. HCQ mean difference with 95% CI | 95% CIs and P-values | No of studies and study types | Quality of the evidence (GRADE) | Comments | |
|---|---|---|---|---|---|---|
| Control, n = 352 | HCQ, n = 291 | |||||
| Serum LDL (mg/dl) | Mean LDL 130.23 mg/dL | Mean LDL 24.39 lower than control | 8.92 lower to 39.87 lower in HCQ group and P = 0.002 | Eight observational studies (two before-after; six case-control studies) |
⨁◯◯◯ Very low |
Random effect I2 = 94.73 |
| Control, n = 616 | HCQ, n = 555 | |||||
| (Total cholesterol mg/dL) | Mean total cholesterol 209.48 mg/dL | Mean total cholesterol 26.85 lower than control | 8.38 lower to 45.31 lower in HCQ group and P = 0.004 | Nine observational studies (three before-after; six case-control studies) |
⨁◯◯◯ Very low |
Random effect I2 = 97.30 |
- GRADE, Grading of Recommendations Assessment, Development, and Evaluation; LDL, low density lipid; CI, confidence intervals; HCQ, hydrolochloroquine.
Meta-regression and subgroup analysis
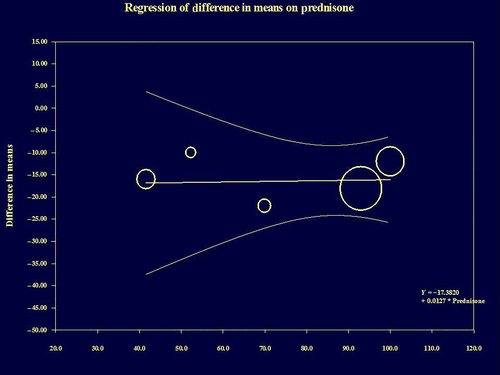
Meta-regression analysis found there was absence of LDL predictors among age (P = 0.216), gender (P = 0.880) and prednisone use (P = 0.895). Figure 4 presents meta-regression analysis of prednisone use (from five studies).38-40, 42, 43 The results of meta-regression analysis were confirmed even at sensitivity analysis using a second-order for age, gender and prednisone use. The number of studies identifying statin use was too low to perform meta-regression analysis. Subgroup analysis confirmed consistency of HCQ's favorable effects on serum LDL across study type and location. HCQ reduced mean LDL levels by 16.492 mg/dL (95% CI 13.062–19.922; P < 0.001) in case-control studies and by 61.002 mg/dL (95% CI 53.384–61.002; P < 0.001) in before-after studies. HCQ reduced mean LDL levels by 29.651 mg/dL (95% CI 24.556–34.747; P < 0.001) in the US studies and by 18.034 mg/dL (95% CI 13.950–22.119; P < 0.001) in international studies. Because Mendez et al. presented the most favorable effects of HCQ on serum LDL and total cholesterol, we performed analysis after excluding the Mendez et al. study.36 HCQ's favorable effects on LDL and total cholesterol were unchanged: mean reduction in LDL, 16.741 mg/dL (95% CI, 13.115–19.826 mg/dL, P < 0.001); mean reduction in total cholesterol, 17.638 mg/dL (95% CI, 8.663–26.612. P < 0.001).
Discussion
To our best knowledge, the current meta-analysis is the first quantitively measured report of HCQ's favorable effect on serum LDL in patients with SLE. Anti-malarials have long been the cornerstone of controlling disease activity in patients with SLE.20, 21 Recently, there has been an increased interest in the effect of HCQ on lipid profiles. Wallace et al.22 were the first to make note of these effects in the 1990s. In 1996, Petri et al.19 confirmed the cholesterol lowering properties of HCQ using a longitudinal database of the Baltimore Lupus Cohort. This brings light to the question of whether the atherogenic profile seen in patients with SLE is due to the traditional cardiac risk factors such as dyslipidemia or from the effects of autoimmunity, which contribute to an increase in cytokine levels, dysfunctional lipids, and oxidative stress.44 Or rather, it could be due to a combination of both the former and latter.15, 16
The effects of HCQ in contributing to this less atherogenic profile have been postulated through different mechanisms. It has been suggested through rat hepatocyte isolates that chloroquine affects the cholesterol biosynthetic pathway outside of the cytosolic acetyl-coA.45 This pathway involves a halt in the synthesis of an intermediate in cholesterol biosynthesis, lanoesterol, by inhibiting the enzymatic step catalyzed by 2,3-oxidosqualene-lanosterol cyclase.45
In addition, the upregulation of LDL receptors has been shown through an in vivo study, contributing to lowered serum LDL levels.46 And more recently in an in vitro experiment, HCQ has been shown to target endosomal nicotinamide adenine dinucleotide phosphate oxidase the enzymatic activity of which is involved in many inflammatory and prothrombotic signaling pathways.47
Although there is not a general consensus about the exact mechanism of action, the effects of HCQ on lipid profiles in lupus patients is apparent based on our meta-analysis. Of note, there were conflicting reports of HCQ's effect on the serum LDL level in patients with mild SLE. Tam et al.39 reported that HCQ had no significant effect on the serum LDL in this population. Caroli et al.37 presented significant improvement in lipoprotein after 3 months of therapy that became more stable after 12 months, with all 24 patients having a SLE Disease Activity Index (SLEDAI) score < 5 at the entry of the study, which favors the effect of the HCQ on the cholesterol pathway. However, it must be noted that given the heterogeneity of our findings, the small sample sizes in each study and the inability to adjust for confounding factors, such as statin usage, limit our conclusions. There were limited study designs (before-after and case-control) and lack of dose-response relationship analyses between HCQ and serum LDL that require future well-designed studies that could fully characterize HCQ's effects on serum LDL.
Despite the above limitations, we still do believe that the clinical implications of HCQ leading to lipid lowering effects should be highlighted in preventative health care for this specific population. Current cardiovascular risk models need to be adjusted for patients with lupus and other autoimmune disorders. Our current systematic review demonstrates the favorable effect of HCQ on both serum LDL and total cholesterol levels.
Author contributions
Study conception and design: Babary, Ayatollahi, Chen, Yoo. Data acquisition, analysis, and interpretation: Liu, Doo, Uppaluru, Kulaga, Yoo. Draft preparation and critical revision: Babary, Ayatollahi, Kwak, Modjinou, Olec, Yoo. All authors agreed to the final version draft.




