Hypertrophic pachymeningitis is a characteristic manifestation of granulomatosis with polyangiitis: A retrospective study of anti-neutrophil cytoplasmic antibody-associated vasculitis
Abstract
Aim
To elucidate the characteristics of patients with hypertrophic pachymeningitis (HP) in a population with anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV).
Methods
We retrospectively investigated the clinical records of 39 patients who were diagnosed with AAV. To determine the characteristics of HP in AAV, the epidemiological and clinical data from patients with HP were statistically compared with those from patients without HP.
Results
Of 39 patients with AAV, seven (17.9%) had associated HP. All patients with HP were classified as having granulomatosis with polyangiitis (GPA), whereas only five of 32 patients without HP were diagnosed as having GPA (P < 0.0001). The frequencies of myeloperoxidase (MPO)-ANCA and proteinase 3-ANCA positivity in patients with HP were equivalent, while MPO-ANCA positivity was obviously dominant in patients without HP. HP occurred as the initial clinical episode of AAV in three patients (7.7% of all AAV). Frequent significant characteristics of patients with HP were headache, cranial neuropathy and paranasal involvement (P < 0.05), and histopathological findings from paranasal involvement were useful for the diagnosis of GPA in some patients with HP. Combination therapy of corticosteroid and an immunosuppressant, such as methotrexate, cyclophosphamide or rituximab, was effective for achieving remission and improving radiographic findings of HP.
Conclusion
AAV is a common cause of HP; epidemiological features of AAV patients with HP are different from those of patients without HP. Additionally, HP impacts diagnosis because it may be an initial clinical sign of AAV.
Introduction
Hypertrophic pachymeningitis (HP) is recognized as an inflammatory disorder causing focal and diffuse thickening of the dura mater. The thickened intracranial and/or spinal dura mater is the cause of several neurological disorders such as chronic headache, seizure, cranial neuropathies, paralysis and ataxia. It is realized that intracranial infections, neoplastic diseases and some autoimmune disorders such as sarcoidosis and immunoglobulin G4 (IgG4)-related disease contribute to the development of HP, although cases of ‘idiopathic HP’ where etiology is uncertain still exist.1, 2 Considering the etiology of HP, which is associated with autoimmune disorders, anti-neutrophil cytoplasmic antibody (ANCA)-related HP is the most frequently recognized.2, 3 ANCA is implicated in small vessel vasculitis, which is designated as ANCA-associated vasculitis (AAV), and is distinct from immune complex-mediated vasculitis.4 AAV is categorized as microscopic polyangiitis (MPA), granulomatosis with polyangiitis (GPA), and eosinophilic granulomatosis with polyangiitis (EGPA) based on the phenotypes of ANCA, the histopathological findings of granulomatosis and/or necrotizing vasculitis formation, and clinical findings such as prior asthma, existence of respiratory tract lesions, and/or urinary tract involvement.4, 5 AAV develops not only in a single organ, such as ‘pulmonary-limited type’ or ‘renal-limited type’,6-8 but also into multi-organ disorders targeting the skin, respiratory tract, kidneys, cardiovascular and gastrointestinal systems, with general symptoms including fever, arthralgia or myalgia. The nervous system is also a common target of AAV, and furthermore, this is sometimes life-threatening and the cause of serious handicap. Disorders of the peripheral nervous system are dominant compared with those of the central nervous system (CNS)9-11 and central nervous involvement occurs in approximately 10% or more of patients with GPA and EGPA.11, 12 Regarding central nervous involvement in GPA, HP is the most frequent disorder compared with other involvements such as ischemic or hemorrhagic changes.13 On the other hand, the epidemiologic and clinical characteristics of patients with HP in the whole population of AAV are still uncertain.
Therefore, in order to clarify the characteristics of AAV-related HP, we retrospectively demonstrated the epidemiologic and clinical features of patients with HP compared with those of patients without HP in AAV.
Materials and methods
Patients and study design
We reviewed the clinical records of patients with AAV who were admitted to our hospital from March 2007 to December 2015. According to the definitive criteria of the Chapel Hill Consensus Conference (CHCC)4 and the consensus algorithm proposed by the European Medicines Agency (EMA algorithm),14 we recruited 39 Japanese patients who were diagnosed with definitive AAV (18 men and 21 women, 61 ± 13 years [range 21‒88 years]). Among them, 24 (61.5%), 12 (30.8%) and three (7.7%) patients were classified as having MPA, GPA and EGPA, respectively. In addition, we detected patients with associated HP. HP was diagnosed by magnetic resonance imaging (MRI) with a 1.5 Tesla scanner (Siemens AG, Erlangen, Germany), which demonstrated thickening of the dura mater with gadolinium (Gd)-enhancement on MRI T1-weighted imaging. We excluded patients with intracranial hypotension, malignancy, infection and other autoimmune disorders, which cause thickening and/or abnormal Gd-enhancement of the dura mater according to systemic assessment on admission to our hospital. The case of one patient has been previously reported in the Japanese literature.15 In order to investigate the epidemiological and clinical characteristics of HP associated with AAV, patients with HP were differentiated from those without HP as independent groups comprising ‘the HP group’ and ‘the non-HP group’, respectively. We extracted the demographic and clinical findings from the clinical records at initial admission to our hospital as the baseline in this study. HP was initially diagnosed at the first visit to our hospital in all patients, except for one who came to our hospital because of exacerbation of HP. This study was approved by the Local Ethics Committee in Shinshu University. Informed consent was obtained from all participants included in this study.
Data collection and assessment
To determine the demographic and clinical characteristics of the two groups, we obtained information about gender, age, serum levels of C-reactive protein (CRP), positivity of myeloperoxidase anti-neutrophil cytoplasmic antibody (MPO-ANCA), and proteinase 3 (PR3)-ANCA. Disease activity and symptoms of AAV were evaluated according to the Birmingham Vasculitis Activity Score (BVAS).16 The symptoms associated with the involved organs were indicated according to nine categories that are shown in the BVAS evaluation system as follows: (1) general symptoms; (2) cutaneous symptoms; (3) mucous membrane and eyes; (4) ear, nose and throat (ENT); (5) chest; (6) cardiovascular; (7) gastrointestinal; (8) renal; and (9) nervous systems. The examination of cerebrospinal fluid (CSF), including protein, cell count and IgG index, was performed in all patients with HP.
Statistical analysis
All results are presented as the mean ± SD, and two-sided P-values of <0.05 were considered statistically significant. The Mann-Whitney U-test and Chi-square test of independence were employed for comparison between patients with HP and those without HP. The Wilcoxon signed-rank test was performed to compare data before and after treatment in patients with HP.
Results
Comparison of demographic characteristics between the HP and non-HP groups
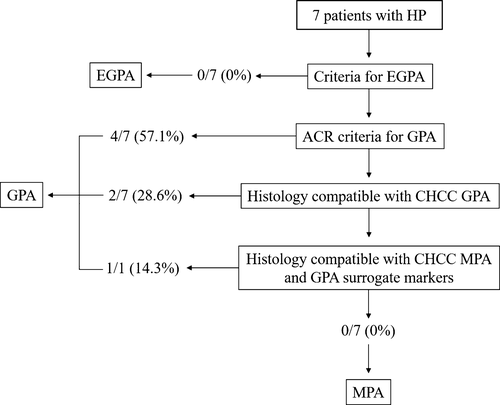
Of 39 patients with AAV who were recruited in this study, seven (17.9%) presented with HP (the HP group). Table 1 shows the comparison of epidemiologic and clinical characteristics between the HP and non-HP groups. In the analysis of AAV classification according to the EMA algorithm, all patients were classified as having GPA in the HP group (Fig. 1), whereas only five patients (15.6%) were diagnosed as having GPA in the non-HP group (P < 0.0001). Neither MPA nor EGPA were classified in the HP group, while 24 patients (75.0%) were diagnosed as having MPA in the non-HP group (P < 0.0001). With regard to the types of ANCA, frequencies of MPO- and PR3-ANCA positivity were equivalent in the HP group, although the frequency of MPO-ANCA was higher than that of PR3-ANCA in the non-HP group. Serum levels of CRP and BVAS were not significantly different between the two groups. In the analysis of categorized organ-related symptoms, ENT was significantly more frequent in the HP group than in the non-HP group (P = 0.008). Frequencies of headache and cranial neuropathy were significantly higher in the HP group (85.7% vs. 6.3%, 71.4% vs. 15.6%, [P < 0.0001, P = 0.007], respectively) (Table 2). On the other hand, none of the patients in the HP group had mononeuritis multiplex, which was significantly present in the non-HP group (39.4%, P = 0.043).
| With HP (n = 7) | Without HP (n = 32) | P-valuea | |
|---|---|---|---|
| Male: female | 3: 4 | 15: 17 | 0.591 |
| Age, years | 67 ± 10 | 60 ± 13 | 0.180 |
| Classification of AAV | |||
| MPA (%) | 0 | 24 (75.0) | < 0.0001 |
| GPA (%) | 7 (100) | 5 (15.6) | < 0.0001 |
| EGPA (%) | 0 | 3 (9.4) | 0.502 |
| MPO-ANCA positive (%) | 3 (42.9) | 24 (75.0) | 0.114 |
| PR3-ANCA positive (%) | 3 (42.9) | 4 (12.5) | 0.094 |
| CRP, mg/dL | 6.8 ± 6.6 | 7.6 ± 6.2 | 0.884 |
| BVAS | 13.7 ± 6.0 | 19.9 ± 9.1 | 0.081 |
| Symptoms | |||
| General symptoms (%) | 4 (57.1) | 23 (71.9) | 0.917 |
| Cutaneous (%) | 1 (14.3) | 11 (34.4) | 0.289 |
| Mucous membrane and eyes (%) | 2 (28.6) | 4 (12.5) | 0.290 |
| ENT (%) | 6 (85.7) | 9 (28.1) | 0.008 |
| Chest (%) | 4 (57.1) | 18 (56.3) | 0.952 |
| Cardiovascular (%) | 0 | 3 (9.4) | 0.543 |
| Gastrointestinal (%) | 0 | 4 (12.5) | 0.437 |
| Renal (%) | 3 (42.9) | 17 (53.1) | 0.225 |
| Nervous system (%) | 7 (100) | 17 (53.1) | 0.060 |
- ANCA, anti-neutrophil cytoplasmic antibody; AAV, ANCA-associated vasculitis; CRP, C-reactive protein; MPA, microscopic polyangiitis; GPA, granulomatosis with polyangiitis; MPO-ANCA, myeloperoxidase ANCA; PR3-ANCA, proteinase 3 ANCA; EGPA, eosinophilic granulomatosis with polyangiitis; BVAS, the Birmingham Vasculitis Activity Score; ENT, ear, nose and throat.
- The variable data are shown as mean ± SD (standard deviation).
- a Data between two groups were compared by using Mann-Whitney U-test and Chi-square for independence test.
- P < 0.05 was regarded as statistically significant.
| With HP (n = 7) | Without HP (n = 32) | P-valuea | |
|---|---|---|---|
| Headache (%) | 6 (85.7) | 2 (6.3) | < 0.0001 |
| Seizure (%) | 2 (28.6) | 1 (3.1) | 0.077 |
| Consciousness disturbance (%) | 1 (14.3) | 2 (6.3) | 0.467 |
| Cerebrovascular event (%) | 0 | 4 (12.5) | 0.764 |
| Cranial neuropathy, total (%) | 5 (71.4) | 5 (15.6) | 0.007 |
| I (%) | 0 | 1 (3.1) | 0.397 |
| II (%) | 1 (14.3) | 1 (3.1) | 0.331 |
| V (%) | 0 | 1 (3.1) | 0.397 |
| VII (%) | 0 | 1 (3.1) | 0.397 |
| VIII (%) | 3 (42.9) | 3 (9.4) | 0.059 |
| IX, X (%) | 1 (14.3) | 0 | 0.179 |
| Sensory peripheral neuropathy (%) | 1 (14.3) | 13 (39.4) | 0.237 |
| Mononeuritis multiplex (%) | 0 | 13 (39.4) | 0.043 |
- AAV, anti-neutrophil cytoplasmic antibody-associated vasculitis; HP, hypertrophic pachymeningitis.
- a Data between two groups were compared by using the Chi-square for independence test.
- P < 0.05 was regarded as statistically significant.
In the analysis of all patients who were classified as having GPA, headache was a significantly common symptom in the HP group despite none of patients in the non-HP group exhibiting this symptom (P = 0.008); nevertheless frequency of ENT symptoms and cranial neuropathy was not significantly different between the two groups (P = 0.583 and P = 0.311, respectively). In fact, five (100%) and two (40%) of five patients in the non-HP group demonstrated paranasal sinus involvement and cranial polyneuropathy, respectively. Overall, patients with GPA in the non-HP group indicated no other disorders in the nervous system, except for cranial neuropathy. Consequently, total frequency of disorders in the nervous system was significantly higher in the HP group than in the non-HP group (P = 0.045). Except for the nervous system, there were no other statistically significant differences between the two groups in GPA (data not shown).
Clinical characteristics of patients with HP
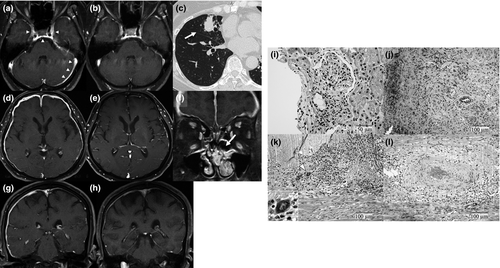
The mean time to diagnosis of HP since the first onset of AAV was 4.9 ± 6.8 years; however, three of seven patients (42.9%) indicated HP as the first manifestation of AAV (Table 3). Six patients with HP who indicated ‘ENT symptoms’ had involvement of the paranasal sinus, which was selected as the biopsied site for the histopathological diagnosis of AAV in three of them. Other biopsied sites were selected as the beneficial sites for the diagnosis according to the examination at first onset of AAV as follows: kidney (n = 2), ulcerative tongue tissue (n = 1) and dura mater (n = 1). Six patients were diagnosed as having GPA according to the histopathological findings on the basis of American College of Rheumatology or CHCC criteria, and another was classified as having GPA according to the histology of necrotizing glomerulonephritis as well as the surrogate marker of a pulmonary nodular lesion that had remained for more than 1 month (Figs 1, 2). With respect to the distribution of thickened dura mater, Gd-enhanced lesions on the convexity, basicranium, and tentorium cerebelli were seen in 5 (71.5%), 4 (57.1%), and 3 (42.9%) patients, respectively (Table 4). None of the patients had dural Gd-enhanced lesions of the spine. In the analysis of CSF findings, all seven patients indicated elevation of protein levels (> 45 mg/dL, mean 79.4 ± 97.0 mg/dL). Four (57.1%) had mild pleocytosis (> 5 cells/μL, mean 9.4 ± 9.3 cells/μL) and an elevated IgG index (> 0.75, mean 0.92 ± 0.37).
| Characteristics | |
|---|---|
| Disease durationa | 4.9 ± 6.8 (0–17) |
| HP as initial episode of AAV (%) | 3 (42.9) |
| Biopsied site | |
| Nasal cavity (%) | 3 (42.9) |
| Kidney (%) | 2 (28.6) |
| Tongue (%) | 1 (14.3) |
| Dura mater (%) | 1 (14.3) |
| Chief symptom at initial AAV diagnosis | |
| General symptoms (%) | 5 (71.4) |
| Cutaneous (purpura) (%) | 1 (14.3) |
| Mucous membrane and eyes (%) | 1 (14.3) |
| Mouth ulcer (%) | 1 (14.3) |
| Scleritis (%) | 1 (14.3) |
| ENT (%) | 6 (85.7) |
| Bloody nasal discharge (%) | 2 (28.6) |
| Paranasal sinus involvement (%) | 6 (85.7) |
| Chest (nodules) (%) | 2 (28.6) |
| Renal (hematuria) (%) | 2 (28.6) |
| Nervous system (%) | 3 (42.9) |
| Headache (%) | 3 (42.9) |
| Seizure (%) | 1 (14.3) |
| Cranial neuropathy (%) | 3 (42.9) |
| Sensory peripheral neuropathy (%) | 1 (14.3) |
- AAV, anti-neutrophil cytoplasmic antibody-associated vasculitis; HP, hypertrophic pachymeningitis; ENT, ear, nose and throat.
- a Between AAV onset and HP occurrence (years, mean ± SD)
| Characteristics | ||
|---|---|---|
| Lesions with thickened dura matter | ||
| Convexity (%) | 5 (71.4) | |
| Basicranium (%) | 4 (57.1) | |
| Tentorium cerebelli (%) | 3 (42.9) | |
| Spine (%) | 0 | |
| Cerebral spinal fluid (CSF) findings | ||
| Protein, mg/dL, mean ± SD | 79.4 ± 97.0 | |
| Protein > 45 mg/dL (%) | 7 (100) | |
| Cell count, cells/μL, mean ± SD | 9.4 ± 9.3 | |
| Cell count > 5 cells/μL (%) | 4 (57.1) | |
| IgG index, mean ± SD | 0.92 ± 0.37 | |
| IgG index > 0.75 (%) | 4 (57.1) | |
| Treatment | Prior to HP onset | After HP diagnosis |
|---|---|---|
| mPSL (%) | 0 | 4 (57.1) |
| PSL (%) | 4 (57.1) | 7 (100) |
| CPA (%) | 2 (28.6) | 1 (14.3) |
| AZA (%) | 2 (28.6) | 1 (14.3) |
| CsA (%) | 1 (14.3) | 0 |
| MTX (%) | 1 (14.3) | 4 (57.1) |
| MZR (%) | 1 (14.3) | 1 (14.3) |
| RTX (%) | 0 | 1 (14.3) |
| Anticonvulsant (%) | 0 | 2 (28.6) |
| Outcome | ||
|---|---|---|
| Symptom | ||
| Remission (%) | 7 (100) | |
| Thickened dura matter | ||
| Improve (%) | 6 (85.7) | |
| No change (%) | 1 (14.3) | |
- HP, hypertrophic pachymeningitis; mPSL, methylprednisolone (1g x 3 consecutive days); PSL, prednisolone; CPA, cyclophosphamide; AZA, azathioprine; CsA, cyclosporine A; MTX, methotrexate; MZR, mizoribine; RTX, rituximab.
Headache and seizure disappeared in all patients after the induction of treatment for HP, and MRI findings of Gd-enhanced dura mater were also improved in six (85.7%) patients (Table 4). Cranial neuropathy also improved in all patients, except for one whose deafness partially remained. All patients had maintained remission without deterioration of thickened dura mater during the observation period (3.2 ± 1.6 years), and BVAS was significantly decreased 6 months after therapy (4.1 ± 2.9, P = 0.017). Methylprednisolone pulse therapy (1 g daily × 3 consecutive days) was administered in four patients, and prednisolone (PSL) (42 ± 17 mg daily) followed in all patients. Methotrexate (MTX) or cyclophosphamide (CPA) was newly administered in five patients, whereas azathioprine or mizoribine was continued in two patients. One patient needed the administration of rituximab (RTX) because of resistance to high doses of corticosteroid with MTX. Anticonvulsants were also administered in two patients for suppressing seizures, together with immunosuppressive therapies described earlier.
Discussion
The present study demonstrated that approximately 18% of patients had HP in the whole population of AAV, and all were classified as having GPA. The geographical studies of AAV indicated the predominance of MPA and MPO-ANCA positivity as epidemiologically specific to Japan, whereas GPA and PR3-ANCA positivities are dominant in European countries.9, 17-19 According to the results in the present study, this Japanese specificity is consistent with the epidemiology of patients without HP; however, the epidemiology of patients with HP is similar to that of patients in European countries. With regard to the pattern of ANCA positivity, frequency of PR3-ANCA was equal to that of MPO-ANCA in patients with HP, although the predominance of MPO-ANCA has been demonstrated in the Japanese population with GPA.20, 21 In the cohort of ANCA-related HP, all patients with PR3-ANCA positivity and 82% of those with MPO-ANCA positivity were classified as having GPA, even though neither MPA nor EGPA were shown.3 Accordingly, HP occurrence may be strongly implicated in the classification of GPA, while some cases of HP which were diagnosed as MPA have been reported.22, 23 The mechanisms of GPA-related disorders of the CNS have been proposed as follows: (1) granulomatosis tissue may directly extend to the intracranial nervous system from adjacent lesions in the orbit or paranasal cavity; (2) granulomatosis tissue may transfer to the intracranial nervous system from the respiratory tract; and (3) vasculitis may affect intracranial vessels.11, 24 Granulomatous inflammation with giant cells was found in the biopsied dura mater from one patient in our study. The pathological study of the involved dura mater in GPA revealed that the most common finding was necrotizing granulomatosis,24 although vasculitis could also be seen in ANCA-related HP.3 Regarding the pathological description of GPA, the predominance of granulomatosis is obvious in the respiratory tract lesions, while small-vessel vasculitis principally concerns the pathogenesis of renal involvement.8, 25, 26 Considering these indications, existence of granulomatous lesions may be required for the formation of HP in AAV. In fact, all patients with HP in the present study significantly indicated paranasal sinus involvement since the early phases of the illness, and a pulmonary nodular lesion was detected in one patient without paranasal sinus involvement. On the other hand, one patient was classified as having GPA according to the surrogate marker of pulmonary nodular lesions at the diagnosis of HP even though the renal biopsy revealed necrotizing glomerulonephritis, investigated at the onset of AAV, suggesting that the appearance of granulomatous lesions may be the key factor in the formation of HP.
Of 39 patients with AAV in the present study, three (7.7%) were initially diagnosed as having AAV when HP occurred as the first clinical episode of AAV, and furthermore, they coincided with 43% of all patients with HP. The present study, together with previous descriptions,2, 3 suggests that HP may occur as the first involvement in approximately half of patients with AAV-related HP who frequently present with headache and/or cranial neuropathy as a common symptom, in addition to ENT involvement. In this regard, we should consider the previous description that the disease severity eventually developed into a systemic type of AAV in 18% of patients with MPO-ANCA positivity and 50% of those with PR3-ANCA positivity, even though they had been categorized as ‘CNS-limited type’ (i.e., HP without other visceral disorders), at the disease onset.3 In order to prevent the progression of disease by promptly initiating appropriate therapy, it is required to make a definitive diagnosis according to the clinical features of HP associated with AAV.
AAV is generally recognized as a refractory autoimmune disorder, which involves frequent relapses and resistance to conventional treatments.27 As a minimum, combination therapy with corticosteroid and an immunosuppressive agent is recommended as the consensus therapeutic strategy for AAV,28 in which CPA or RTX are desired in patients who have life-threatening organ involvement, and MTX is also available in cases without life-threatening organ involvement.29 RTX was actually effective for one patient who suffered from intractable HP which was resistant to other conventional immunosuppressive agents, and the efficacy of RTX has also been reported in some cases of refractory HP associated with AAV.30-32 Additionally, favorable outcomes were achieved with the combination of MTX and PSL in patients who indicated HP as initial and/or limited involvement without another severe visceral disorder, suggesting the concomitant use of MTX may be useful for improving HP associated with AAV unless other life-threatening organ impairment is involved. Considering not only our favorable results but also the inferiority of PSL monotherapy as previously described,3 the therapeutic strategy combining PSL and an immunosuppressant should be required as the initial therapy for all patients with HP in AAV.
To our knowledge, this is the first study that has described the epidemiological analyses of HP in the whole population of AAV. We conclude that HP impacts the diagnosis of AAV because HP could be an initial episode as well as associated with frequent existence of granulomatous lesions in the respiratory tract. However, in the present study we recruited patients from a single institution and retrospectively performed the analyses of small size samples. In a further study, we need a larger number of patients in order to determine the definitive specificities of HP associated with AAV.
Author contributions
All authors substantially conceived and made the design of this study. Y. Shimojima, D. Kishida, A. Hineno, M. Yazaki and Y. Sekijima recruited and analyzed clinical data. All authors developed the structure and argument for this study. Y. Shimojima prepared the draft of this manuscript. Y. Shimojima and S. Ikeda contributed to revising the manuscript. All authors revised and approved of the final manuscript.
Acknowledgements
We greatly appreciate the efforts of all the members of the Department of Medicine (Neurology & Rheumatology), the members concerned in the Department of Neurosurgery, and Otorhinolaryngology, Shinshu University School of Medicine. This study was supported by a grant from the Ministry of Health, Labour, and Welfare of Japan.
Conflicts of interest
The authors declare that they have no financial or personal conflicts of interest.




