APLAR rheumatoid arthritis treatment recommendations
Abstract
Aims
Rheumatoid arthritis is a chronic inflammatory condition that affects approximately 1% of the world's population. There are a wide number of guidelines and recommendations available to support the treatment of rheumatoid arthritis; however, the evidence used for these guidelines is predominantly based on studies in Caucasian subjects and may not be relevant for rheumatoid arthritis patients in the Asia-Pacific region. Therefore, the Asia Pacific League of Associations for Rheumatology established a Steering Committee in 2013 to address this issue.
Materials and methods
The AGREE II instrument and the ADAPTE Collaboration framework were applied to systematically identify, appraise, synthesize, and adapt international rheumatoid arthritis guidelines for use in the Asia-Pacific region.
Results
Forty rheumatoid arthritis treatment recommendations, based on evidence and expert opinion, were drafted and are presented in this report.
Conclusion
The Asia Pacific of Associations for Rheumatology rheumatoid arthritis treatment recommendations are intended to serve as a reference for best practice management of rheumatoid arthritis in Asia-Pacific, focusing on local issues to ensure the delivery of basic care for these patients, and to improve their outcomes. In addition, the document will serve as a reference for national rheumatology associations in Asia-Pacific for developing guidelines in their respective countries.
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disease of unknown etiology that affects approximately 1% of the global population.1-3 The disease is characterized by inflammation, pain, stiffness and progressive joint destruction leading to high rates of morbidity and mortality in the affected individuals.1-3 Furthermore, RA is associated with productivity losses and increased financial burden, increased psychological distress, depression and, consequently, significantly decreased health-related quality of life.4-6
Disease-modifying antirheumatic drugs (DMARDs) form the cornerstone of RA treatment. These agents have the capacity to modify the disease process by reducing or reversing signs and symptoms, disability, impairment of quality of life, inability to work, and progression of joint damage.7 Early and aggressive treatment with DMARDs has been shown to be effective in altering the clinical course of RA, and slowing or stopping the radiographic progression. DMARDs are broadly classified into conventional DMARDs (cDMARDs) including synthetic chemical agents such as methotrexate, sulfasalazine and leflunomide, and biological DMARDs (bDMARDs), including: tumor necrosis factor (TNF) inhibitors (adalimumab, certolizumab pegol, etanercept, golimumab and infliximab); the T-cell costimulation inhibitor, abatacept; the anti-B cell agent, rituximab; the interleukin (IL)-6 receptor (IL-6R)-blocking monoclonal antibody, tocilizumab; as well as the IL-1 inhibitor, anakinra. Recently, tofacitinib, a Janus kinase (JAK) inhibitor, has also been shown to have disease-modifying effects in RA.
Need for RA recommendations in the Asia-Pacific (AP) region
As the evidence used in most international RA treatment guidelines is obtained predominantly from studies in Caucasian subjects, these guidelines may not be relevant for RA patients in the AP countries. Data show that there is an increased prevalence of certain infections (e.g., tuberculosis [TB], hepatitis B and C infection, Epstein–Barr virus infection)8, 9 and malignancies (e.g., T-cell and natural killer-cell lymphomas,10 stomach cancer11) in the AP region. Thus, a Steering Committee under the auspice of the Asia Pacific League of Associations for Rheumatology (APLAR) was formed in 2013 to formulate AP region-specific treatment recommendations for RA that address AP-specific issues.
However, the AP region has vast intra-regional diversity in terms of ethnicities, socioeconomic structures and health resources; these characteristics also differ from those in Western countries. Furthermore, the availability and dosage of medications vary across AP countries. Thus, it is difficult to develop RA treatment recommendations that will be appropriate for all AP countries. Owing to a shortage of rheumatologists, RA patients in the region are also often managed by general practitioners and allied health practitioners. Consequently, the treatment practices are not standardized and vary widely, even within countries. Furthermore, there are limited data from the AP region to endorse evidence-based recommendations that may be considered more appropriate in some countries in the region. Nevertheless, this Steering Committee aimed to develop recommendations that will be as evidence-based as possible and define the best practices for managing RA in the AP region.
In addition, the Steering Committee also made recommendations based on expert opinion and consensus so that countries with limited resources may be able to achieve the minimum essential standard of care for their RA patients.
Target audience and contents
The intended target audience for this document includes rheumatologists and all practitioners who manage RA. It focuses predominantly on recommendations for the pharmacological treatment of RA. The document includes 40 recommendations across the following RA treatment domains: general RA treatment strategies; role of non-steroidal anti-inflammatory drugs (NSAIDs), including: cyclooxygenase-2 (COX-2) inhibitors; role of corticosteroids; role of conventional DMARDs (cDMARDS); and role of bDMARD agents. Specific key questions across these key domains were identified and recommendation statements developed accordingly (Table 1).
| A. General RA treatment strategies |
|
| B. Role of NSAIDs including COX-2 inhibitors |
|
| C. Role of cDMARDs |
|
| D. Role of corticosteroid agents |
|
| E. Role of bDMARD agents |
|
| F. What is the role of complementary/unproven therapies in the treatment of RA? |
- AP, Asia Pacific; bDMARD, biological DMARD; cDMARD, conventional DMARD; COX-2 inhibitors, cyclooxygenase-2 inhibitors; DMARD, disease-modifying antirheumatic drug; NSAID, non-steroidal anti-inflammatory drug; RA, rheumatoid arthritis; TB, tuberculosis.
This document does not include recommendations for the diagnosis of RA, patient referral policies or management of comorbid conditions. Furthermore, costs were not embedded in the discussion of the recommendations, as formal cost-effectiveness analyses were not performed.
Funding and conflict of interest
The process of developing the APLAR RA treatment recommendations was funded by APLAR and was also supported by unrestricted educational grants from the following pharmaceutical companies: AbbVie, Janssen, Pfizer, Roche and UCB. Relevant disclosures for the Steering Committee members, including industry funding, consultancies and commercial interests, are included at the end of this article.
Objectives
The primary objective was to develop a document that would serve as a reference for best RA management practices in the AP region, focusing on local issues in the region. In addition, the document would also serve as a reference for national rheumatology associations in the region for developing RA guidelines in respective countries.
Materials and methods
The ADAPTE framework was used to systematically identify, appraise, synthesize, and adapt international RA guidelines for use in the AP region. This was done by following the steps outlined in the ADAPTE manual and toolkit, and helped to expedite the process of recommendation development.12, 13
Assembly of the APLAR RA Recommendations Steering Committee
A Steering Committee was formed by inviting 22 members of APLAR representing 12 countries from the AP region, as well as one RA patient for developing this set of recommendations. The APLAR members were rheumatology experts who had served on numerous RA research projects and decision-making panels both internationally and in their respective countries. All members of the Steering Committee attended the meetings, contributed to discussions and were actively involved in every phase of recommendation development. No representative of pharmaceutical companies was involved in any part of recommendation development.
Scope of the recommendations
Members of the Steering Committee developed key questions pertaining to RA treatment in the AP region. These questions, developed during a face-to-face meeting, addressed various domains of RA treatment, as described earlier (Table 1).
Search criteria
The studies included were clinical practice guidelines and consensus statements with recommendations for adult RA populations, and published in English between January 2000 and December 2013. Studies that provided evidence from the AP region to support recommendation of RA treatment practices specific to the region were also included. Non-English articles were considered, provided a member of the Steering Committee could translate them into English. Articles were excluded if they did not address the key questions or were deemed to be of poor methodological quality by a validated guidelines quality appraisal instrument.14, 15
Search strategy
A systematic search was performed according to the inclusion–exclusion criteria described above in Medline, EMBASE, Google Scholar and SCOPUS. The search terms included RA, specific drug names for cDMARDs and bDAMRDs, NSAIDs, corticosteroids, AP, guidelines, consensus statements and recommendations. All search results were reviewed by two independent members. The steps involved in the systematic search are shown in Figure 1.
Appraisal of guideline quality
The quality of each guideline was assessed using a validated questionnaire, the Appraisal of Guidelines, Research and Evaluation (AGREE) instrument.15 This instrument includes 23 questions that are organized into six domains: (i) scope and purpose; (ii) stakeholder involvement; (iii) rigor of development; (iv) clarity of presentation; (v) applicability; and (vi) editorial independence. Each of the 23 items targets various aspects of practice guideline quality. Each guideline was independently assessed by two reviewers to formulate a single-item overall assessment as ‘Recommend’, ‘Recommend with modifications’ or ‘Not recommend’.
Grading evidence
Each guideline had a different system for grading evidence. To reconcile these differences, we translated each guideline's evidence grading system into a simplified system as suggested by the Scottish Intercollegiate Guideline Network (SIGN) to assign a level of evidence and strength of recommendation for each recommendation.16
Evidence synthesis
We prepared a table of included guidelines containing descriptive characteristics, including guideline developer, country, year, summary of recommendations and AGREE assessment, for each subsection of the guideline. This was followed by development of evidence tables for each question; these included guideline characteristics, recommendations, summary of guideline assessment (AGREE assessment) and supporting evidence (Table 2).
| Levels of evidence | Strength of recommendation |
|---|---|
| I. Meta-analyses, systematic reviews of RCTs, or individual RCT | A. Strong recommendation: Direct level I evidence |
|
II. Meta-analyses, systematic reviews of observational studies (cohort/case control studies), or individual observational studies OR RCT subgroup/post-hoc analyses |
B. Moderate recommendation: Direct level II evidence or extrapolated level I evidence |
| III. Non-analytical studies, e.g., case reports, case series | C. Weak recommendation: Direct level III evidence or extrapolated level II evidence |
|
IV. Expert opinion OR Recommendations are not linked to evidence |
D. Consensus recommendation: Expert opinion based on very limited evidence |
- RCT, randomized controlled trial.
Development of recommendations
Members of the Steering Committee summarized recommendations and supporting evidence from international guidelines to address each key question. A recommendation for the AP region was developed by adapting and rewording the existing recommendation. An emphasis was placed on recent guidelines with strong methodological quality. Supporting evidence from randomized controlled trials (RCTs) and observational studies referenced by the guideline was reviewed by members in detail. All members participated in developing the wording of recommendations. Consensus was achieved by using the Delphi technique whereby members had an opportunity to cast a vote anonymously, without getting swayed by opinions of fellow members; disagreements were resolved through discussions and multiple rounds of voting. Statements were included as recommendations provided more than 80% of the members participated in the polling and more than 50% of the members voted in favor of the outcome. Setting the acceptance margin to 70% resulted in exclusion of many questions considered important during meeting discussions. Thus, for the purpose of this recommendation document, a majority was determined by more than 50% of votes.
Extended review
Draft recommendations developed by the group were sent to Josef Smolen and Vibeke Strand for review and comments. The draft recommendations were also presented in an open forum during the 2014 APLAR Congress to seek opinions and suggestions from participants. Feedback from the respondents was used to finalize the recommendations and inform supporting text. This document was developed in accordance with the principles outlined by the AGREE II instrument and the ADAPTE collaboration. The recommendations were also sent for review and official endorsement by APLAR.
Results
Each recommendation is presented with a level of evidence and strength (Table 3) and accompanied with supporting text which is structured as follows.
| Recommendations | Level | Strength |
|---|---|---|
| Section 1 – General RA treatment strategies | ||
| 1. RA treatment should be aimed at maintaining physical functioning and good quality of life through achieving a state of sustained remission, or low disease activity when remission may not be an achievable target. | II | B |
| 2. Treatment of RA is a shared decision between the clinician and patient, and should be started once diagnosed. | I | A |
| 3. The choice of treatment is based on the findings of active disease and/or poor prognosis and comorbidities. | II | B |
| 4. Poor prognostic factors include positivity for ACPA or RF, increased ESR or CRP, radiological evidence of erosion or progression of erosions. | II | B |
| 5. All patients with recently diagnosed RA or active disease should be monitored for disease activity every 1 to 3 months. | I | A |
| 6. A suitable and practical standardized measure of disease activity should be routinely performed to assess patients’ response to treatment. | I | A |
| 7. Safety monitoring while patients are on bDMARD therapy is likewise recommended. | II | B |
| 8. All patients should be assessed clinically at presentation for extra-articular disease manifestations, comorbidities, and infections such as TB and hepatitis (II). Information on vaccination status and special situations such as pregnancy and lactation should be obtained (II). | II | B |
| 9. If patients show persistent remission for 6 months, treatment with corticosteroids and NSAIDs may be tapered, with the aim of eventually stopping these treatments. | II | B |
| 10. If a patient is in sustained remission for more than 6 to 12 months after discontinuation of NSAIDs, corticosteroids and bDMARDs, then a gradual reduction in cDMARDs can be attempted with caution, as a shared decision between the patient and physician. | IV | D |
| Section 2 – Role of NSAIDs (including COX-2 inhibitors) | ||
| 11. NSAIDs and COX-2 inhibitors should be used at the lowest effective dose for the shortest possible period of time. | IV | D |
| Section 3 – Role of corticosteroids | ||
| 12. Oral corticosteroid monotherapy is not recommended. | IV | D |
| 13. Oral corticosteroids can be considered to control active RA in combination with cDMARDs. | I | A |
| 14. In early RA, the addition of low-dose corticosteroids (prednisolone ≤ 7.5 mg/day) to cDMARDs leads to a reduction in radiographic progression. | I | A |
| 15. Corticosteroids should be used in the lowest possible dose and tapered as rapidly as clinically feasible. | IV | D |
| Section 4 – Role of conventional DMARDs | ||
| 16. Treatment with cDMARDs as monotherapy or in combination should be started as soon as the diagnosis of RA is made. | I | A |
| 17. Methotrexate is the first-line cDMARD for RA patients, and is considered as the “anchor drug”. | I | A |
| 18. Patients who cannot tolerate methotrexate may receive other cDMARDs such as leflunomide, sulfasalazine and hydroxychloroquine as first-line treatments. | I | A |
| Bucillamine, iguratimod, cyclosporin, azathioprine, IM gold or tacrolimus may also be considered in some AP countries. | I | B |
| 19. Pretreatment investigations: complete blood count, liver function and renal function tests, viral hepatitis serology and chest radiograph should be ordered prior to initiating methotrexate therapy. | II | B |
| 20. Combination cDMARD therapy should be considered in active RA patients, particularly those with poor prognostic factors. | I | B |
| 21. Combination cDMARD therapy should include methotrexate as the anchor drug unless methotrexate is contraindicated. | II | B |
| 22. Triple therapy with cDMARDs is an effective option in patients who show inadequate response to methotrexate monotherapy. | II | B |
| 23. Patients should be assessed every 1 to 3 months after the initial treatment or change of regimen until the disease is stabilized, in remission or in low disease activity state. | I | A |
| 24. Patients who have been stabilized or are in remission or low disease activity can be monitored every 3 to 6 months. | IV | D |
| 25. Definition of treatment failure: Inadequate response with cDMARDs is defined as failure to achieve remission or low disease activity after a therapeutic trial of at least two standard cDMARDs in combination at optimal doses for 6 months (I). One of the failed cDMARDs must be methotrexate unless methotrexate is contraindicated (I). | I | A |
| Section 5 – Role of bDMARDs | ||
| 26. A bDMARD can be prescribed in patients who have inadequate response or intolerance to cDMARDs. | I | A |
| 27. Early bDMARD use can be considered in patients who have active disease with poor prognostic factors. | IV | D |
| 28. Prior to starting treatment with bDMARDs, history regarding active or current infections, comorbidities including tumors and malignancies, vaccinations, pregnancy, and possible contraindications should be obtained in all patients. | I | A |
| 29. All patients should be screened for TB, and HBV and HCV infections before initiating bDMARD therapy. | I | A |
| 30. Live vaccines should be given at least 4 weeks prior to bDMARD administration. | III–IV | C–D |
| 31. Monotherapy or combination with methotrexate/cDMARDs: bDMARDs are most effective when combined with methotrexate. | I | A |
| 32. In patients with RA who are candidates for bDMARD therapy, the therapeutic options include TNF antagonists, abatacept, rituximab and tocilizumab. | I | A |
| 33. Patients who fail to achieve remission or low disease activity after 6 months of bDMARD therapy are recommended to switch to another bDMARD agent. | III | C |
| 34. Dose reduction | ||
| In patients who have achieved remission, a reduction in treatment should be considered. | I | A |
| If the patient remains in extended remission (> 12 months), tapering of bDMARDs can be considered. | II | B |
| 35. Infectious complications: TB | ||
| Screening for TB is recommended prior to starting bDMARD therapy. | II | B |
| All patients with latent TB infection should receive prophylactic anti-TB therapy. | II | B |
| Patients with active TB infection need to be adequately treated before consideration of bDMARD treatment. | III | C |
| 36. Infectious complications: hepatitis | ||
| Patients should be screened for HBV and HCV infections prior to the commencement of bDMARDs. | IV | D |
| bDMARDs should be avoided in patients with active or untreated chronic HBV infection and active HCV infection. | III | C |
| 37. Active infections | ||
| Active infections are contraindications for bDMARDs. | I | A |
| When an infection is suspected, based on clinical judgement, the bDMARD agent should be stopped and the patient must be treated appropriately. | IV | D |
| 38. Pregnancy and lactation while on bDMARDs should only be considered after thorough assessment of benefits and risks. | IV | D |
| 39. Vaccination | ||
| Administration of all vaccines, if indicated, should, ideally, be undertaken at least 4 weeks before starting a bDMARD. | III–IV | C–D |
| Concurrent administration of live, attenuated vaccines is an absolute contraindication for patients being treated with bDMARDs. | IV | D |
| Section 6 – Role of tofacitinib | ||
| 40. Tofacitinib may be considered if a bDMARD has failed | II | B |
- ACPA, anti-citrullinated peptide antibodies; AP, Asia-Pacific; bDMARD, biological DMARD; COX-2 inhibitors, cyclooxygenase-2 inhibitors; cDMARD, conventional DMARD; CRP, C-reactive protein; DMARD, disease-modifying antirheumatic drug; ESR, erythrocyte sedimentation rate; HBV, hepatitis B virus; HCV, hepatitis C virus; IM, intramuscular; NSAID, non-steroidal anti-inflammatory drug; RA, rheumatoid arthritis; RF, rheumatoid factor; TB, tuberculosis; TNF, tumor necrosis factor.
Supporting evidence
Description of the source guidelines used for adaptation
Summary of evidence linked to recommendation statement
Summary of original evidence presented in source guidelines.
Special comment/recommendation for the AP region
A special comment/expert opinion that is relevant for the region.
Recommendations
A summary of the recommendations is presented in Table 3. It should be noted that the recommendations are stratified into different sections relevant to the different stages and groups of drugs used in the treatment of RA. These sections may thus be referenced separately and individually. An algorithm summarizing the recommendations for treatment of patients with RA in the AP region is presented in Figure 2.
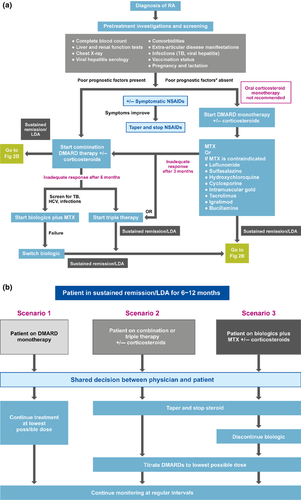
Section 1 – General RA treatment strategies
Recommendation 1
RA treatment should be aimed at maintaining physical functioning and good quality of life through achieving a state of sustained remission, or low disease activity when remission may not be an achievable target. (Level II; Strength B)
Supporting evidence
Summary of evidence linked to recommendation statement
It was noted that, in every patient, treatment should be aimed at reaching a target of remission17-24 or low disease activity,17-20, 24 as soon as possible25 and measuring disease activity using objective parameters such as Disease Activity Score (DAS), DAS28 (DAS of 28 joints), Simplified Disease Activity Index (SDAI) and Clinical Disease Activity Index (CDAI).26, 27 Consequently, this will halt joint damage, prevent disability and improve quality of life.28, 29 It should be noted that DAS28 < 2.6 is regarded by the Food and Drug Administration (FDA) as a cut-off point for low disease activity, whereas remission is probably better defined according to the American College of Rheumatology–European League Against Rheumatism (ACR–EULAR) criteria based on SDAI, CDAI or the Boolean criteria.30
Special comment/recommendation for the AP region
In the AP region, there may be some situations in which remission or even low disease activity may not be possible. For example, many AP patients first present to their clinician with advanced disease and often severe joint deformity. For these patients to achieve remission, the use of bDMARDs is often required but these agents are not affordable for most of these patients. Furthermore, many patients are engaged in work involving physical labor which may aggravate the signs and symptoms of RA, rendering a low disease activity state non-achievable. Thus, for many RA patients in the region, we recommend counselling to ensure compliance, and an agreement on a treatment target, maintaining symptom control and work ability, to be reached between the clinician and the patient.
Recommendation 2
Treatment of RA is a shared decision between the clinician and patient, and should be started once diagnosed. (Level I; Strength A)
Supporting evidence
References 17, 19-25, 27, 29, 31, 32 and 33.
Summary of evidence linked to recommendation statement
Successful management of patients with RA depends upon empowering the patients with the knowledge about the chronic and fluctuating course of the disease, treatment goal31 and, particularly, about the possible evolution and prognosis,24 including side effects, costs of the drugs and the continuous need of physiotherapy.22 Offering verbal and written information to people with RA will improve their understanding of the condition and its management, and counter any misconceptions they may have.32
Early treatment could improve the outcome of RA.23, 29 Treatment with traditional DMARDs with or without low dose glucorticoids should be considered as soon as the diagnosis of RA is made17, 24, 25, 32 to increase clinical response and decrease radiographic progression.
Special comment/recommendation for the AP region
Early treatment of RA in this region may not be possible in some countries with poor economic status due to an inefficient referral system and a shortage of rheumatologists. The Steering Committee would, therefore, like to reinforce that this recommendation is aimed not only for rheumatologists but any clinicians who participate in the care of patients with RA. For many countries in the AP region, improving the knowledge of general clinicians of RA and its treatment is of utmost importance.
Recommendation 3
The choice of treatment is based on the findings of active disease and/or poor prognosis and comorbidities. (Level II; Strength B)
Supporting evidence
References 17, 18, 21-24 and 27-29.
Summary of evidence linked to recommendation statement
The presence of poor prognostic features should be assessed at baseline and considered when making treatment decisions. These include high disease activity state, (high number of swollen and tender joints, elevated erythrocyte sedimentation rate [ESR] or C-reactive protein [CRP]), rheumatoid factor (RF) positivity, anti-cyclic citrullinated peptide antibody (ACPA) positivity, and early presence of joint damage,17, 18, 21-24, 28, 29 extra-articular features (e.g., presence of rheumatoid nodules, vasculitis, Felty's syndrome) and functional limitation as measured by the Health Assessment Questionnaire Disability Index (HAQ-DI).18, 23, 28
Special comment/recommendation for the AP region
Most RA patients in the AP region present late with it and many would have developed erosive disease. Basing radiological erosions solely as an indication for aggressive treatment may lead to over-treatment for some patients. Therefore, other markers of high disease activity and poor prognosis may be more appropriate indications for intensive DMARD treatment.
Recommendation 4
Poor prognostic factors include positivity for ACPA or RF, increased ESR or CRP, radiological evidence of erosion or progression of erosions. (Level II; Strength B)
Supporting evidence
References 17, 18, 21-24, 28, 29 and 34.
Summary of evidence linked to recommendation statement
Refer to Recommendation 3 above.
Special comment/recommendation for the AP region
In addition to comments made under Recommendation 3 above, clinicians should be aware of the widespread misuse of corticosteroid agents, many of which may be purchased over the counter or from a quack in the AP region, which may mask some of these poor prognostic factors.
Recommendation 5
All patients with recently diagnosed RA or active disease should be monitored for disease activity every 1 to 3 months. (Level I; Strength A)
Supporting evidence
References 18-20, 22, 24, 28, 32, 34-37, 33.
Summary of evidence linked to recommendation statement
At the beginning of RA treatment, especially in patients with high/moderate disease activity, the patient should preferably be assessed every month.19, 20, 24 The frequency of monitoring in patients with active RA should be 1 to 3 months,22, 28, 32, 34-37, 33 aiming to achieve remission by 6 months33 when the interval for monitoring may be between 3 to 6 months, depending on the degree of disease activity.18
Special comment/recommendation for the AP region
Lack of resources in many countries in the AP region is a major obstacle for frequent monitoring of RA disease activity and treatment. Rheumatologists may need the assistance of general clinicians and allied health workers in the monitoring process, as well as a patient self-reporting system. The development of a good allied health worker and patient education program is of utmost importance in the overall management of patients with RA in this region.
Recommendation 6
A suitable and practical standardized measure of disease activity should be routinely performed to assess patients’ response to treatment. (Level I; Strength A)
Supporting evidence
References 17-19, 21-24, 26-28, 32, 34-36 and 38-42.
Summary of evidence linked to recommendation statement
Most guidelines recommend the use of a validated composite score such as DAS28, SDAI and CDAI for measuring disease activity before starting treatment, and for monitoring disease activity and drug response after starting treatment.17, 18, 21-24, 26-28, 32, 35, 36, 38-42 In addition, some guidelines recommend using the Health Assessment Questionnaire (HAQ) for evaluating the functional impact of the disease.21, 32, 36, 41 The French guidelines also recommend anteroposterior X-rays of the hands, wrist and forefeet to monitor the course of RA; this may be done every 6 months until the end of the first year, then once every year until end of the third year, and thereafter every 2 to 4 years.42
Special comment/recommendation for the AP region
Regular measurement of serum CRP may not be affordable in many AP countries. Rheumatologists from these countries are recommended to use ESR instead; otherwise, clinical activity measurement without ESR, for example, CDAI, should be used in patient monitoring.
Recommendation 7
All patients should be assessed clinically at presentation for extra-articular disease manifestations, comorbidities and infections, such as TB and hepatitis (Level II; Strength B). Information on vaccination status and special situations such as pregnancy and lactation should be obtained (Level II; Strength B)
Supporting evidence
References 18, 22-24, 29, 31, 34, 35, 37, 33, 38 and 43-59.
Summary of evidence linked to recommendation statement
Given that RA is a systemic disease accompanied with not only joint dysfunction but also other comorbidities (such as cardiovascular disease and osteoporosis), extra-articular manifestations (such as pericarditis, pleuritis and vasculitis), and an increased risk of infections,32 it is recommended to evaluate and screen patients for these conditions before initiating treatment with DMARDs, NSAIDs or corticosteroids.18, 22-24, 29, 31, 34, 35, 37, 33, 38, 43-59 As most drugs used in the treatment of RA are contraindicated during pregnancy and breastfeeding, it is recommended to rule out these situations before initiating treatment.24, 31, 34, 35, 37, 33, 38, 48, 52-54, 58 Before starting cDMARDs or bDMARDs, patients’ vaccination status should be assessed and updated, and all killed, recombinant and live attenuated vaccinations undertaken.18, 24, 31, 37, 33, 38, 46, 53, 54, 56
Special comment/recommendation for the AP region
No region-specific comments.
Recommendation 8
Safety monitoring while patients are on bDMARD therapy is likewise recommended. (Level II; Strength B)
Supporting evidence
References 18, 22, 23, 32, 34, 35, 37, 43-47 and 60.
Summary of evidence linked to recommendation statement
Treatment with bDMARDs, particularly TNF inhibitors, is associated with an increased risk of certain infections, including TB, as well as that of non-Hodgkin's lymphoma and congestive heart failure.43, 44 Thus, many guidelines recommend screening for active and latent TB before starting bDMARDs,18, 22, 23, 34-37, 43-47 and performing baseline hepatitis serology as well as other investigations, including a complete blood picture, liver function tests, serum urea and creatinine levels and antinuclear antibody status, and to rule out presence of active and latent infections and other comorbidities. Furthermore, continuous monitoring for these conditions and side effects is also emphasized during bDMARD therapy.18, 22, 23, 32, 34, 35, 37, 43-47, 60
Special comment/recommendation for the AP region
It is especially important to monitor for the emergence of infective complications. Clinicians should be on the alert for not only TB and hepatitis infections but all other opportunistic infections, including fungal and parasitic infections.
Recommendation 9
Once the patient has improved symptomatically, treatment with corticosteroids and NSAIDs may be tapered, with the aim of eventually stopping these treatments. (Level II; Strength B)
Supporting evidence
References 17, 21, 22, 24-26, 28, 29, 32, 55, 57, 61 and 62.
Summary of evidence linked to recommendation statement
While NSAIDs in combination with DMARDs are effective in controlling pain and inflammation in RA, long-term use is not recommended as these drugs are associated with significant gastrointestinal, cardiovascular and renal adverse effects.21, 22, 24, 55, 57, 61 Furthermore, these agents have not demonstrated any efficacy in modifying the disease course.57 Corticosteroids, in combination with DMARDs, are effective in limiting disease progression, and are particularly effective in decreasing inflammation in patients with flares and improving symptom control in those with early disease.59 However, given the toxicity with long-term use, it is recommended to taper therapy as symptoms improve and discontinue completely once patients achieve remission.17, 21, 22, 24-26, 28, 29, 32, 55, 57, 61, 62
Special comment/recommendation for the AP region
Clinicians should be aware of over-the-counter and quack-provided corticosteroid preparations, and their use must be discouraged.
Recommendation 10
If a patient is in sustained remission for more than 6 to 12 months after discontinuation of NSAIDs, corticosteroids and bDMARDs, then a gradual reduction in cDMARDs can be attempted with caution, as a shared decision between the patient and physician. (Level IV; Strength D)
Supporting evidence
References 17, 21, 22, 25, 26, 28, 32 and 55.
Summary of evidence linked to recommendation statement
Duration of remission is required for more than 1 year in the Indian 2008 guidelines,22 6–12 months in the 2013 Brazilian guidelines26 and over 6 months in the German 2013 guidelines.25 The suggested sequence of drug withdrawal is NSAID, corticosteroid, bDMARD and then cDMARD.26, 28 The reduction in treatment should be performed cautiously, gradually and in a stepwise manner. A shared decision must be reached between the patient and physician in this regard.17, 25, 28 According to the BeST study, sustained remission was defined as DAS < 1.6 for more than 6 months. According to the Indian guidelines, a minimum maintenance dose will be required for an indefinite period.22 The British Society for Rheumatology and British Health Professionals in Rheumatology (BSR-BHPR) guidelines warn about the frequent association of withdrawal with flare and disease progression.55 The 2013 Brazilian guidelines describe withdrawal of cDMARD in exceptional situations.26
Special comment/recommendation for the AP region
Because of the poor affordability of bDMARDs for most patients in this region, patients may rely more on cDMARDs for disease control. Thus, we recommend primarily cautious dose reduction but not complete cessation of cDMARDs for the majority of patients.
Section 2 – Role of NSAIDs (including cyclo-oxygenase-2 inhibitors)
Recommendation 11
NSAIDs and COX-2 inhibitors should be used at the lowest effective dose for the shortest possible period of time. (Level IV; Strength D)
Supporting evidence
Summary of evidence linked to recommendation
The National Institute for Health and Care Excellence (NICE) 2009 guidelines reviewed data from several studies to show that NSAIDs and COX-2 inhibitors were effective in treating the symptoms of RA and recommended that oral NSAIDs/COX-2 inhibitors be used in the lowest effective dose over the shortest period of time; it is advisable to use NSAIDs as needed.32 However, no optimum dose was proposed. The BSR 2006 and 2009 guidelines emphasized that long-term use of NSAIDs should be at the lowest effective dose and are best avoided because of the associated risks.29, 55
NSAIDS and COX-2 inhibitors, undoubtedly, are efficacious in controlling RA symptoms, but their gastrointestinal, cardiovascular and renal adverse effects are a matter of serious concern. A balanced approach should be undertaken whereby the benefits are weighed against the possible adverse effects.
Special comment/recommendation for the AP region
In the AP region, there is a general lack of awareness among patients and clinicians of the adverse effects associated with long-term NSAID use. Since NSAID use can mask symptoms by reducing inflammation,32, 61 it can obscure RA progression and often delays referral from general practitioners. Availability of NSAIDs over the counter in many Asian countries also poses a significant challenge. Clearly, therefore, educating the public and allied healthcare workers is an important priority concern in this region.
Section 3 – Role of corticosteroids
Recommendation 12
Oral corticosteroid monotherapy is not recommended. (Level IV; Strength D)
Supporting evidence
References 17, 21, 22, 25, 28, 32, 57, 62 and 63.
Summary of evidence linked to recommendation statement
Advice from a rheumatologist must be obtained before treatment initiation with oral corticosteroids.62 The Latin American guidelines do not recommend corticosteroids as a sole disease-modifying agent.57 The EULAR 2013 guidelines state that monotherapy is not specifically recommended and should only be used in exceptional cases when all other DMARDs are contraindicated.17 Other guidelines advise corticosteroid usage in combination with DMARDs.25, 62
Special comment/recommendation for the AP region
The use of corticosteroids in Asia poses particular risks. There is a widespread practice of self-medication as a result of ineffective control of the sales of prescription medications, leading to inappropriate use of corticosteroid agents.57 Misuse of corticosteroids among clinicians in some AP countries is also widespread. It is, therefore, recommended that patients with suspected RA be assessed by a practitioner experienced in dealing with RA and not be put on corticosteroid monotherapy without a firm indication.
Recommendation 13
Oral corticosteroids can be considered to control active RA in combination with cDMARDs. (Level I; Strength A)
Supporting evidence
References 17, 21, 22, 25, 28, 32, 57, 62 and 63.
Summary of evidence linked to recommendation
Low-dose corticosteroids can be considered as part of the initial treatment strategy (in combination with one or more cDMARDs) for up to 6 months, but should be tapered as rapidly as clinically feasible.17 Short-term treatment with corticosteroids should be offered for managing flares in patients with recent-onset or established disease, to rapidly decrease inflammation.28, 32
For polyarticular flares, or at first presentation of the disease, intramuscular/intra-articular, or short oral courses of corticosteroids can decrease symptoms while waiting for other slower-acting drugs to take effect; this is described as “bridging therapy”.17, 22, 23, 25, 32, 57, 62, 63 Intra-articular injections are extremely useful for treating a flare in one or only a few joints.22, 28, 32 Injection into the same joint should not be repeated before 3 months, and it is advised that no more than three injections be administered per joint per year.22
For severe extra-articular manifestations, intravenous corticosteroids can save critical organs (e.g., eyes in scleritis) or, occasionally, even life-threatening complications (e.g., severe serositis or vasculitis); however, corticosteroids should be used in combination with immunosuppressive agents such as cyclophosphamide.32
In people with established RA, only continue long-term treatment with corticosteroids when: (a) the long-term complications of corticosteroid therapy have been fully discussed; and (b) all other treatment options (including bDMARDs) have been offered.32
Special comment/recommendation for the AP region
No region-specific comments.
Recommendation 14
In early RA, the addition of low-dose corticosteroids (prednisolone ≤ 7.5 mg/day) to cDMARDs leads to a reduction in radiographic progression. (Level I; Strength A)
Supporting evidence
References 17, 21, 22, 25, 28, 32, 57, 62 and 63.
Summary of evidence linked to recommendation
The EULAR 2010, Latin American and NICE guidelines discuss the evidence that low-dose prednisolone (< 7.5 mg daily) in patients with early RA does reduce radiographic progression over 2 years.32, 57, 63 According to the European Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT) 2007 guidelines, corticosteroids are “probably effective in slowing radiographic progression in early and established RA”.21
Special comment/recommendation for the AP region
No region-specific comments.
Recommendation 15
Corticosteroids should be used in the lowest possible dose and tapered as rapidly as clinically feasible. (Level IV; Strength D)
Supporting evidence
References 17, 21, 22, 25, 28, 32, 57, 62 and 63.
Summary of evidence linked to recommendation statement
It is recommended to keep the requirement for continuing corticosteroid treatment under constant review, and titrate the dose against therapeutic response, risk of under-treatment and development of adverse events.17 However, the panel could not recommend an optimal tapering strategy based on the existing evidence. The Canadian 2012 and German 2013 guidelines recommend that corticosteroids should be used at the lowest possible dose and tapered as rapidly as possible.25, 28 In contrast, the EULAR 2010 guidelines recommend slow tapering to avoid clinical relapses.63
Special comment/recommendation for the Asia-Pacific region
This recommendation is particularly relevant in the AP region due to the widespread misuse of corticosteroids in some countries as stated above.
Section 4 – Role of cDMARDs
Recommendation 16
Treatment with cDMARDs as monotherapy or in combination should be started as soon as the diagnosis of RA is made. (Level I; Strength A)
Supporting evidence
References 17, 18, 21-26, 28, 29 and 57.
Summary of evidence linked to recommendation statement
Most of the guidelines recommend starting cDMARDs as soon as possible once the diagnosis of RA is confirmed;17, 23, 25, 28, 29, 57 treatment should not be delayed by more than 3 months.23 The ACR–EULAR 2010 classification criteria should be used to confirm diagnosis of RA and facilitate early introduction of effective therapy in RA. In patients with undifferentiated arthritis, the use of cDMARDs can be considered,26 but in patients at risk of developing persistent and/or erosive arthritis, treatment with cDMARDs should be started as early as possible even if they do not fulfil the diagnostic criteria.21 In patients with early RA, cDMARD monotherapy is recommended in low and moderate disease activity, or high disease activity without poor prognostic markers.
Special comment/recommendation for the AP region
Delay in initiating cDMARD treatment for patients with RA in the AP region remains a major concern, primarily due to delay in the diagnosis of the underlying condition. Much work is needed to enhance public and clinician awareness of RA and its treatment.
Recommendation 17
Methotrexate is the first-line cDMARD for RA patients, and is considered as the “anchor drug”. (Level I; Strength A)
Supporting evidence
Summary of evidence linked to recommendation statement
Methotrexate is the preferred cDMARD with respect to efficacy and safety and should be the first cDMARD used in RA unless contraindicated;17, 24-26, 28 it is described as the “anchor drug”21, 22, 27 or “drug of choice”.24, 57 DMARD-naive patients should be started on methotrexate monotherapy, and treatment should be given for a duration of no less than 3 months at the maximally tolerated dose.23 Choice of the first agent is based on the risk : benefit ratio with hydroxychloroquine an option in disease perceived as mild, and methotrexate or sulfasalazine in diseases adjudged moderate-to-severe, or likely to progress.29
Special comment/recommendation for the AP region
Methotrexate used to be a taboo and thought to be excessively hepatotoxic in many AP countries. However, the efficacy and safety of this agent is now well established and methotrexate should be used unless contraindicated or there is poor tolerance.
Recommendation 18
Patients who cannot tolerate methotrexate may receive other cDMARDs such as leflunomide, sulfasalazine and hydroxychloroquine as first-line treatment (Level I; Strength A). Bucillamine, iguratimod, cyclosporin, azathioprine, intramuscular gold or tacrolimus may also be considered in some AP countries. (Level I; Strength B)
Supporting evidence
References 17, 23-26, 57 and 64-68.
Summary of evidence linked to recommendation statement
Patients without poor prognostic factors (i.e., with no erosions, are RF-negative, with low CRP levels, or with low disease activity) or those who cannot tolerate methotrexate may receive other cDMARDs, such as leflunomide, sulfasalazine, hydroxychloroquine or injectable gold.23 The antimalarials hydroxychloroquine and chloroquine are less effective and should be reserved for mild disease forms and diseases with low erosive potential.24
Special comment/recommendation for the AP region
In many Asian countries, chloroquine may be recommended and is preferred to hydroxychloroquine or other cDMARDs due to its low treatment cost and high availability. In some AP countries, cDMARDs such as bucillamine and iguratimod, which are not available in Western countries, are widely used. Though these drugs have not been thoroughly evaluated outside their countries of origin, they have undergone rigorous testing locally and may also be the cDMARDs of choice for RA in the respective countries. For example, the efficacy of bucillamine can be judged within 3 months in moderately active RA patients either before or after methotrexate treatment.64 Iguratimod is non-inferior in active RA patients when compared to methotrexate65 or salazosulfapyridine.66 Tacrolimus improves RA symptoms in patients with RA inadequately controlled with at least one prior cDMARD67 and is well tolerated.68
Recommendation 19
Pre-treatment investigations: complete blood count, liver function and renal function tests, viral hepatitis serology and chest radiograph should be ordered prior to initiating methotrexate therapy (Level II; Strength B).
Supporting evidence
Summary of evidence linked to recommendation statement
Pre-treatment investigations prior to initiating methotrexate should include complete blood count (CBC), liver function test (LFT) and renal function test (RFT), hepatitis B and C serology and chest X-ray.22, 23, 57 Other guidelines also consider screening for risk factors for cardiovascular diseases and osteoporosis,23 and human immunodeficiency virus (HIV) in high-risk patients.28 The pre-treatment investigations for other cDMARDs are: eye examination (funduscopy and perimetry) for antimalarials; and CBC, RFT and LFT for leflunomide, sulfasalazine, cyclosporine, and azathioprine. Blood pressure and serum creatinine should also be measured prior to initiating leflunomide and cyclosporine.22
Special comment/recommendation for the AP region
The high prevalence of hepatitis and TB in the AP region provides the rationale for recommending the pre-treatment investigations described above before starting methotrexate treatment.
Recommendation 20
Combination cDMARD therapy should be considered in patients with active RA, particularly those with poor prognostic factors. (Level I; Strength B)
Supporting evidence
References 17, 18, 23-25 and 28.
Summary of evidence linked to recommendation statement
If, after 3 months of cDMARD monotherapy (in patients without poor prognostic features), a patient deteriorates from low to moderate/high disease activity, then methotrexate, hydroxychloroquine or leflunomide should be added.18 A combination of cDMARDs (including double or triple therapy) may be considered in patients with very serious disease and poor prognostic factors.17, 18, 23-25, 28 Patients with suboptimal treatment response should receive step-up therapy with combination therapy of methotrexate plus another agent (leflunomide, sulfasalazine, hydroxychloroquine).23 Initial combination therapy with cDMARDs may also be considered, particularly in patients with poor prognostic features, moderate-to-high disease activity, and patients with recent-onset disease.
Special comment/recommendation for the AP region
Long-term treatment compliance with mono- or combination cDMARD therapy is a challenge in many AP countries. This may be related to the cost of treatment, anxiety about drug side effects and the need for regular monitoring. Patients should be educated about the disease and the need for uninterrupted treatment. Clinicians should explore this possibility and patients should also be reassured of the safety of the prescribed drugs, and encouraged to have adequate trials of cDMARD monotherapy before escalation to combination cDMARD therapy.
Recommendation 21
Combination cDMARD therapy should include methotrexate as the anchor drug unless methotrexate is contraindicated. (Level II; Strength B)
Supporting evidence
References 17, 18, 21-26, 28, 32 and 57.
Summary of evidence linked to recommendation statement
Most guidelines recommend methotrexate as the anchor drug unless it is contraindicated.17, 18, 21-24, 26, 28, 57 Although direct comparisons between different regimens are lacking, combination cDMARD therapies, including methotrexate, have been proven to be superior to cDMARD monotherapy. Other combinations without methotrexate are not sufficiently evaluated. On the basis of guideline review, the panel recommends methotrexate combination therapy in patients who have inadequate response to methotrexate monotherapy.
The most commonly used cDMARDs in combination with methotrexate are hydroxychloroquine, sulfasalazine or their concurrent use. Leflunomide has also been studied in association with methotrexate. However, methotrexate combined with leflunomide should be cautiously used because of higher toxicity.69, 70 Methotrexate-based combination therapies with azathioprine, cyclosporine A and intramuscular gold have been evaluated in at least one randomized controlled trial. The details of the regimens are listed in the ACR 2008, NICE 2009 and EULAR guidelines and a meta-analysis by Katchamart et al.32, 69, 71-73
Special comment/recommendation for the AP region
As described above, cDMARDs available for treatment of RA extend to bucillamine, iguratimod and tacrolimus in certain AP countries. However, the use of these drugs combined with methotrexate or as an anchor drug in combination regimens has not been extensively studied and requires further evaluation.
Recommendation 22
Triple therapy with cDMARDs is an effective option in patients who show inadequate response to methotrexate monotherapy. (Level II; Strength B)
Supporting evidence
References 17, 18, 21-26, 28, 32 and 57.
Summary of evidence linked to recommendation statement
All the reviewed guidelines recommend changing regimen in patients who fail methotrexate monotherapy.17, 18, 21-26, 28, 32, 57 The options include switching among cDMARDs and switching from cDMARDs to bDMARD agents. Combination cDMARD therapy can be an effective alternative to bDMARD therapy. In methotrexate-inadequate responders, methotrexate combination with one or two kinds of other cDMARDs was proven to be superior to methotrexate monotherapy.73 More recent data showed that triple therapy with cDMARDs had similar efficacy with bDMARD therapy.74-76 Although most recommendations did not specify the mandatory application of triple cDMARDs after methotrexate failure, current evidence suggests that triple therapy with cDMARDs may be able to substitute for bDMARD therapy.
The TACIT trial, which was published since the current consensus was reached, showed combination methotrexate and leflunomide was an equally effective regimen as methotrexate plus a TNF inhibitor.77 This combination may also be considered as an alternative for triple combination cDMARDs.
Special comment/recommendation for the AP region
Because of the high cost and limited availability of bDMARDs in many AP countries, the panel recommends triple cDMARD therapy as an effective option for patients with inadequate response to methotrexate.
Recommendation 23
Patients should be assessed every 1 to 3 months after the initial treatment or change of regimen until the disease is stabilized, in remission or in low disease activity state. (Level I; Strength A)
Supporting evidence
References 17-19, 21, 24-26, 28, 32 and 72.
Summary of evidence linked to recommendation statement
One systematic review of trials on strategy-driven treatment approaches in RA concluded that intensive steering (treatment target, follow up method) and intensive medication strategies in early active RA produces a better clinical outcome, improved physical function and less structural damage.78
Early-stage active RA can be monitored monthly.19, 24, 26, 31 Radiographs of joints are recommended as frequently as every 6 to 12 months during the first few years21, 28 or annually19 to estimate potential progression of joint damage.
Special comment/recommendation for the AP region
The shortage of rheumatologists in many AP countries represents a major hurdle to the frequent monitoring of RA patients. As indicated earlier, an effective educational program for patients and other healthcare providers, including general practitioners and allied health workers, is urgently needed in many AP countries.
Recommendation 24
Patients who have been stabilized or are in remission or low disease activity can be monitored every 3 to 6 months. (Level IV; Strength D)
Supporting evidence
References 17, 19, 24, 26, 28, 32 and 55.
Summary of evidence linked to recommendation statement
Most guidelines agree that monitoring can be less frequent once the treatment target has been stabilized. However, the suggested monitoring intervals are variable and may only represent the compromise of expert opinion (IV, D). According to the Canadian guidelines, patients in remission can be monitored at longer intervals without further specification.28 The Brazilian guidelines suggest monitoring every 3 months for controlled disease.25, 26 The treat-to-target guidelines consider less frequent monitoring such as every 3 to 6 months for patients in sustained, low disease activity or remission.19 The EULAR 2013 guidelines propose monitoring every 6 to 12 months once the treatment target has been stabilized.17 The BSR-BHPR 2009 and NICE guidelines recommend annual review.32, 55
Special comment/recommendation for the AP region
No region-specific comments.
Recommendation 25
Definition of treatment failure: inadequate response with cDMARDs is defined as failure to achieve remission or low disease activity after a therapeutic trial of at least two standard cDMARDs in combination at optimal doses for 6 months (Level I; Strength A). One of the failed cDMARDs must be methotrexate unless methotrexate is contraindicated. (Level I; Strength A)
Supporting evidence
References 17, 18, 21-26, 28, 32, 34, 35, 52 and 57.
Summary of evidence linked to recommendation statement
Six guidelines define treatment target as remission or low disease activity; thus, any other status would be inadequate response/treatment failure.18, 24-26, 28, 57 Three guidelines define target as remission.17, 21, 23 The required treatment usually includes at least methotrexate monotherapy unless not tolerated;18, 28, 34, 35 or in combination with another cDMARD.17, 18, 22-24, 26, 28, 32, 48, 52 The treatment duration, at a standard target dose, may be at least 3 months18, 23, 28, 34, 35, 48, 57 or 6 months.17, 22, 23, 26, 31, 52
Special comment/recommendation for the AP region
No region-specific comments.
Section 5 – Role of bDMARDs
Recommendation 26
A bDMARD can be prescribed in patients who have inadequate response or intolerance to cDMARDs. (Level I; Strength A)
Supporting evidence
References 17, 18, 22, 23, 25, 26, 28, 31, 34-36, 33, 38, 39, 48-51, 53, 54, 56-58 and 79.
Summary of evidence linked to recommendation statement
The BSR 2010 guidelines recommend starting a TNF inhibitor in those who have active RA and have inadequate response to cDMARDs.39 Inadequate response is defined as DAS28 > 3.2 with ≥ 3 tender and swollen joint counts, and being treated with at least two cDMARDs (one should be methotrexate unless contraindicated) in combination over a 6-month period, and at a standard dose for at least 2 months, unless a significant toxicity occurs and limits the dose and duration of treatment. Rituximab (anti-CD20) should be prescribed in a patient who has inadequate response or intolerance to at least one TNF inhibitor,58 and still has active disease defined by DAS28 ≥ 3.2 or SDAI > 11 or similar indices. Tocilizumab (anti-IL-6) is recommended for those who have moderate or severe disease activity, according to a validated composite measure, and have had inadequate response or intolerance to at least one cDMARD or TNF inhibitor. Abatacept (CTLA-4Ig) is recommended for those who have moderate or severe disease activity, according to a validated composite measure, and have had inadequate response or intolerance to at least one cDMARD or TNF inhibitor.
Special comment/recommendation for the AP region
As has been stated above, as a result of the high cost and limited availability of bDMARDs in many AP countries, triple cDMARD combination therapy may be considered for patients in whom bDMARDs are indicated.
Recommendation 27
Early bDMARD use can be considered in patients who have active disease with poor prognostic factors. (Level IV; Strength D)
Supporting evidence
Reference 18.
Summary of evidence linked to recommendation statement
In 2012, the ACR suggested that TNF inhibitors, with or without methotrexate, may be considered in patients with early RA (duration < 6 months) and high disease activity and poor prognostic features. This recommendation is based on expert opinion as the data on the use of TNF inhibitors in early RA patients with active disease and poor prognostic factors are limited.18
Special comment/recommendation for the AP region
Once again, for countries with poor socioeconomic status and where the reimbursement system is inadequate, combination cDMARD therapies, including triple therapy, may be considered for these patients instead.
Recommendation 28
Prior to starting treatment with bDMARDs, history regarding active or current infections, comorbidities including tumors and malignancies, vaccinations, pregnancy, and possible contraindications should be obtained in all patients. (Level I; Strength A)
Supporting evidence
References 18, 21, 22, 24, 26, 31, 35, 33, 38, 46, 48-50, 53, 54, 56 and 57.
Summary of evidence linked to recommendation statement
Reports on the risk of serious infections and the development of malignancies in RA patients receiving bDMARD therapy, particularly TNF inhibitors, showed conflicting results. A meta-analysis showed the use of TNF inhibitors is associated with increased risk of serious infections,80, 81 but another meta-analysis could not confirm this finding.82
A meta-analysis of randomized controlled trials found that the point estimate of malignancy risk was higher in etanercept-treated patients than in controls,83 but two recent meta-analyses failed to confirm these findings.84, 85
Regarding pregnancy, data from the BSR Biologics Register found that the incidence of spontaneous loss among those who were prior exposed to TNF inhibitors was higher than in controls (17% vs. 10%), but without risk of significant congenital abnormalities. Nevertheless, they recommended that pregnancy be avoided during anti-TNF therapy.86
A study on the effect of high-dose TNF inhibitor (infliximab) showed a detrimental effect on congestive heart failure;87 however, two recent studies could not confirm this finding.88, 89
Rituximab may be used in RA patients with lymphoma46 and should be avoided in those with hypogammaglobulinemia or low CD4 counts.56 Tocilizumab should be avoided in those with a history of bowel perforation.24, 33, 48
Special comment/recommendation for the AP region
It is of particular importance that clinicians have a high level of alertness of pre-existing infectious diseases, including uncommon infections, and other comorbidities in AP patients receiving bDMARDs because of differences in the pattern and frequency of occurrence of these conditions in this region.
Recommendation 29
All patients should be screened for TB, and hepatitis B and C virus infections before initiating bDMARD therapy. (Level I; Strength A)
Supporting evidence
References 18, 22-24, 28, 31, 34, 33, 38, 43, 46, 48-50, 52-54 and 56-58.
Summary of evidence linked to recommendation statement
Overall, 22 studies recommended screening for infections that included TB, HBV and HCV infections prior to starting bDMARD therapy.18, 22-24, 28, 31, 34, 33, 38, 43, 46, 48-50, 52-54, 56-58 In Japan, testing for beta D-glucan is also recommended prior to initiating TNF inhibitors and tocilizumab (anti-IL-6).48, 49
Special comment/recommendation for the AP region
The AP region is endemic for TB. Therefore, active and latent TB should be evaluated and treated properly, according to each individual country guideline, prior to commencement of bDMARDs.
Recommendation 30
Live vaccines should be given at least 4 weeks prior to administration of bDMARD agents. (Level III–IV; Strength C–D)
Supporting Evidence
References 18, 22-24, 31, 34, 35, 37, 33, 38, 43, 46, 48, 52, 55, 56 and 58.
Summary of evidence linked to recommendation statement
Patients who are immunocompromised are at risk of disseminated infection with live attenuated vaccines. All guidelines reviewed emphasized that concurrent administration of live attenuated vaccines was not recommended in patients receiving bDMARDs. Most guidelines agree that live attenuated vaccines should be, ideally, given 4 weeks prior to initiation of bDMARDs but the level of evidence for this remained low (Level IV).
Patients already on bDMARDs who require live vaccines should only receive it 6 months after the last infusion of infliximab or 23 weeks after the last dose of etanercept.22, 46, 52 There was no guidance on how long to discontinue other bDMARD agents, although the Canadian guidelines recommend that the timing for withholding/restarting therapy should be based on the pharmacokinetic properties of the agent used.
In the rare event when a live vaccine may be required urgently in a patient on bDMARDs (e.g., outbreak or urgent travel), discussion about the risks and benefits should be undertaken with the patient in consultation with an infectious disease physician.
Special comment/recommendation for the AP region
No region-specific comments.
Recommendation 31
Monotherapy or combination with methotrexate/cDMARDs: bDMARDs are most effective when combined with methotrexate. (Level I; Strength A)
Supporting evidence
References 17-19, 21, 23-26, 28, 29, 31, 32, 35, 36, 33, 38, 48, 51, 52, 56 and 57.
Summary of evidence linked to recommendation statement
The guidelines reviewed recommend the use of methotrexate in combination with bDMARD agents. This combination is associated with significant benefits in terms of control of disease activity, improved function and quality of life as well as retardation of radiographic progression.17, 32 Methotrexate is also believed to reduce development of human anti-chimeric antibody (HACA) in patients receiving infliximab.51 In the CONCERTO trial that enrolled early RA patients receiving adalimumab plus methotrexate, an increasing trend of efficacy was observed with increased doses of methotrexate. However, the efficacy of the 10 mg weekly dose of methotrexate was equivalent to the 20 mg weekly dose.17
In cases where methotrexate is contraindicated, several guidelines mention that alternative cDMARDs such as leflunomide, sulfasalazine, azathioprine and cyclosporine can be combined with bDMARD agents.36, 56 In addition, adalimumab, etanercept and certolizumab have been approved as monotherapy18, 25, 38, 56 and tocilizumab has, likewise, been used as monotherapy for patients with RA.17, 23, 25, 33, 48
Special comment/recommendation for the AP region
Please refer to recommendations 18 and 21.
Recommendation 32
In patients with RA who are candidates for bDMARD therapy, the therapeutic options include TNF inhibitors, abatacept, tocilizumab and rituximab. (Level I; Strength A)
Supporting evidence
References 17-19, 21, 23-26, 28, 29, 31, 32, 35, 36, 33, 38, 48, 51, 52, 56 and 57.
Summary of evidence linked to recommendation statement
In the ACR 2012 guidelines, TNF inhibitors may be used as initial treatment for patients with early RA (< 6 months’ duration) who present with high disease activity and poor prognostic factors).18 TNF inhibitors provide the most robust efficacy and safety among all bDMARD agents for RA. However, recent data have shown other bDMARD agents such as abatacept and tocilizumab have sufficient and comparable efficacy and safety data and can be considered as first-line bDMARD agents.17, 25 The use of rituximab as first-line bDMARD therapy has been described in certain circumstances, such as in patients with history of lymphoma, demyelinating disease or TB which precludes the use of other bDMARD agents.17, 56
Special comment/recommendation for the AP region
Patient preference and cost of treatment should always be considered when choosing a particular bDMARD. Several countries may have problems in sustaining the use of bDMARDs due to non-reimbursement.
Recommendation 33
Patients who fail to achieve remission or low disease activity after 6 months of bDMARD therapy are recommended to switch to another bDMARD agent. (Level III; Strength C)
Supporting evidence
References 17, 18, 23-25, 28, 29, 31, 34, 36, 33, 38, 44, 52-54, 57 and 58.
Summary of evidence linked to recommendation statement
In the era of treat-to-target in RA, a significant delay should not be allowed before switching therapy in patients who have not achieved an adequate response. Most guidelines suggest a timeline of 12 weeks. The ACR guidelines separated the duration of failure with a TNF inhibitor (3 months) and a non-TNF inhibitor bDMARD (6 months) as it was felt that the response to treatment to non-TNF inhibitor bDMARDs may take longer.18
Observational studies have suggested that switching to a second TNF inhibitor after failure of the first may be a feasible option. There are no controlled trials to compare switching to a second TNF inhibitor or a bDMARD agent of another mode of action, so the choice should be based on a shared decision between the patient and the physician based on patient's preferences and characteristics. The EULAR recommendations emphasize that no preference is stated.17
Two guidelines specifically looked at switching to another bDMARD after failure of rituximab.31, 58 Early studies suggested a numerical but not statistically significant increase in infections with the use of TNF inhibitors after rituximab but further follow up showed that there was no difference in incidence of infection. Data from registries suggest that use of abatacept after rituximab is also safe, but further data are needed.
Special comment/recommendation for the AP region
Biosimilars may be available in certain AP countries. They may be considered because of their comparatively lower cost. However, the use of these agents must also be based on available evidence. This will be discussed in a subsequent APLAR statement.
Recommendation 34
Dose reduction: in patients who have achieved remission, a reduction in treatment should be considered. (Level I; Strength A). If the patient remains in extended remission (> 12 months), tapering of bDMARDs can be considered. (Level II; Strength B)
Supporting evidence
References 21, 28, 29, 34-37, 44 and 52.
Summary of evidence linked to recommendation statement
Of the 10 guidelines which discussed tapering of treatment in the case of sustained remission, several recommend that corticosteroids and/or NSAIDs should be tapered first.28, 29, 34-37, 44, 52 Some, but not all, recommend tapering of bDMARDs in patients with sustained remission.21, 29, 35, 37, 44, 52 The issue of withdrawal after remission is reached remains inadequately researched and is stated as an item in the ‘research agenda’ in the EULAR 2013 guidelines.17
Special comment/recommendation for the AP region
As has been highlighted earlier, long-term bDMARDs are not often affordable by most patients in the AP region. They often request their clinician to taper or even stop their treatment early. While awaiting further data to become available, the Steering Committee recommends that tapering should only be considered if a patient has remained in disease remission for at least 12 months.
Recommendation 35
Infectious complications: TB
Screening for TB is recommended prior to starting bDMARD therapy. (Level II; Strength B) All patients with latent TB infection (LTBI) should receive prophylactic anti-TB therapy. (Level II; Strength B) Patients with active TB infection need to be adequately treated before consideration of bDMARD treatment. (Level III; Strength C)
Supporting evidence
References 18, 22-24, 28, 35, 37, 43, 45-47 and 48-52.
Summary of evidence linked to recommendation statement
The most common criteria for “LTBI” included a weal > 10 mm (different guidelines/consensus/recommendations suggested different cut-off values for tuberculin skin testing [TST]; the Hong Kong recommendations used 10 mm induration for immunocompetent patients and 5 mm for patients who are significantly immunocompromised such as those with HIV infection; the French 2003 TNFi and Latin American 2006 guidelines also suggested 10 mm; the Japanese 2007 guidelines suggested 20 mm; the French 2007, Portuguese 2011 and Brazilian 2012 guidelines used 5 mm; the Philippine 2006 guidelines suggested 8 mm) or a blister in response to a TST done more than 10 years after the last Bacille-Calmette Guerin (BCG) vaccination, with no history of correct treatment for active TB, or residual radiographic TB lesions > 1 cm in size with no certainty.49
TNF inhibitors, particularly the monoclonal antibodies, have been found to be associated with increased incidence of TB.90 Prophylaxis with anti-TB drugs with an accepted regimen, such as isoniazid 300 mg/day is recommended at least 1 month prior to starting TNF inhibitors and other bDMARD agents and be maintained for 9 months in RA patients with occult TB infection.
Special comment/recommendation for the AP region
The AP region has a high prevalence of active TB infections. Thus, the proportion of patients at risk for TB infection and re-infection during bDMARD therapy is higher when compared with Europe and North America.
The high cost and limited availability of interferon-gamma release assay (IGRA) and the lack of timely access to infectious disease specialists may be barriers to the accurate diagnosis of latent TB infection. Nevertheless, a chest X-ray and TST are the minimum requirements for screening for LTBI.
The common practice of administration of the BCG vaccine across many AP countries may lead to a high false-positive rate for TST. Thus, many AP countries have set a higher cut-off value for LTBI screening purposes. Clinicians are therefore recommended to refer to their national guidelines for their respective criteria for LTBI based on TST results. Where such guidelines are unavailable, the committee recommends using the development of a weal > 10 mm following TST as the cut-off value for LTB1 warranting the use of prophylactic anti-TB antibiotics prior to bDMARD treatment.
The issue of TB infections and RA treatment will be dealt with in a subsequent APLAR recommendation report.
Recommendation 36
Infectious complications: Hepatitis
Patients should be screened for HBV and HCV infections prior to the commencement of bDMARDs. (Level IV; Strength D) bDMARDs should be avoided in patients with active or untreated chronic HBV infection and active HCV infection. (Level III; Strength C)
Supporting evidence
References 18, 22-24, 31, 35, 37, 33, 43, 48, 51, 52, 56 and 58 addressed the topic of hepatitis-related complications associated with bDMARD use, but no evidence was identified to support this statement.
Summary of evidence linked to recommendation
Although there was some evidence, most is in the form of case reports, some of which are contradictory.
Special comment/recommendation for the AP region
Patients with evidence of chronic viral hepatitis B infection, including serum positivity for hepatitis B surface antigen or anti-hepatitis core antibodies, should be given appropriate anti-viral treatment while on bDMARD therapies. The cost of anti-viral treatment plus the need for regular monitoring, including blood hepatitis B DNA detection, will further lower the affordability of bDMARDs for many RA patients in this region. While appreciating the very high cost of anti-HCV treatments, they should be considered in HCV-infected patients prior to dDMARDs.
The timely access to infectious disease and hepatology specialists is not always possible in many AP countries. Rheumatologists need to stay vigilant of these infections when starting bDMARDs for these patients.
Recommendation 37
Active infections are contraindications for bDMARDs. (Level I; Strength A) When an infection is suspected, based on clinical judgement, the bDMARD agent should be stopped and the patient must be treated appropriately. (Level IV; Strength D)
Supporting evidence
References 25, 26, 35, 37, 38, 43, 48-52 and 57.
Summary of evidence linked to recommendation
Of the 48 RA management recommendation reports, 11 discussed non-TB, non-hepatitis infections.25, 26, 35, 37, 43, 48-52, 57 Active infections as contraindications for bDMARDs were discussed in four recommendations.35, 38, 48 Besides, past history of serious infections in the last 6 months and history of pneumocystis pneumonia (PCP) were identified as contraindications for bDMARDs in four guidelines.48-50
Compared with the general population, patients with RA have an increased risk of infection, which is nearly twice as high as that observed in matched non-RA controls.91, 92 When considering serious infections, a trend toward an increased frequency compared to cDMARDs has been noted regarding monoclonal anti-TNF antibodies,93-95 with a significant increase reported in two previous studies with infliximab and adalimumab.96, 97 These findings are consistent with the results of a meta-analysis published in 2006 that included all randomized controlled trials performed with infliximab and adalimumab.80
Special comment/recommendation for the AP region
While there is no prevalence data from the AP region of several transmissible diseases such as leprosy, malaria, Chagas’ disease, schistosomiasis, yellow fever, dengue fever, filariasis and helminthic infections, we recommend physicians to remain alert regarding these conditions so as to allow timely diagnosis and appropriate treatment.
Recommendation 38
Pregnancy and lactation while on bDMARDs should only be considered after thorough assessment of benefits and risks. (Level IV; Strength D)
Supporting evidence
References 22, 24, 31, 37, 33, 38, 43, 48, 51, 52, 57 and 58.
Summary of evidence linked to recommendation
There is an inherent inadequacy of safety data on bDMARD agents during pregnancy because pregnant women as a group are excluded from the majority of premarketing clinical trials and safety studies for ethical reasons. However, most recommendations stated that pregnancy and lactation should be avoided while on bDMARDs and effective contraception is strongly recommended to prevent pregnancy in women with childbearing potential until more safety data are available.
Special comment/recommendation for the AP region
Earlier, the Steering Committee suggested the consideration of triple combination cDMARDs as an alternative to bDMARDs because of cost and availability concerns. It should be noted that cDMARDs are as potentially, or more, toxic than bDMARDs.98
Recommendation 39
Vaccination: administration of all vaccines, if indicated, should, ideally, be undertaken at least 4 weeks before starting a bDMARD. (Level III–IV; Strength C–D) Concurrent administration of live, attenuated vaccines is an absolute contraindication for patients being treated with bDMARDs. (Level IV; Strength D)
Supporting evidence
References 18, 22-24, 31, 34, 35, 37, 33, 38, 43, 46, 48, 52, 55, 56, 58.
Summary of evidence linked to recommendation
Overall, there is a lack of data on the safety of vaccination during treatment with most bDMARDs. Furthermore, there are not many studies on the efficacy of vaccines during treatment with bDMARDs; a study on pneumococcal vaccination suggested that patients on TNF inhibitors may not respond adequately to vaccination.99 Thus, the recommendation of the Steering Committee in this regard aligns with that of most guidelines and is predominantly based on expert opinion.
Special comment/recommendation for the AP region
No region-specific comments.
Section 6 – Role of tofacitinib
Recommendation 40: Tofacitinib may be considered if a bDMARD has failed. (Level II; Strength B).
Supporting evidence
This recommendation is made not based on a review of the treatment recommendations/guidelines that the Steering Committee had initially identified. However, the role of tofacitinib was included in the EULAR RA treatment recommendations 2013 update.17
Summary of evidence linked to recommendation statement
Tofacitinib, a JAK inhibitor, has been under investigation as a therapy for RA. It is not a bDMARD or a cDMARD. In a number of placebo-controlled studies, tofacitinib, used either as a monotherapy or in combination with a cDMARD, primarily methotrexate, was associated with reductions in signs and symptoms of RA and improvement in physical function in patients with active disease despite previous treatment with cDMARDs.100-102 In another study of active disease-patients receiving background methotrexate, tofacitinib was found to be significantly superior to placebo and was numerically similar to adalimumab in efficacy.103 Furthermore, in a 6-month, double-blind, parallel-group phase 3 study involving 266 patients who were TNF inhibitor refractory, tofacitinib use was associated with meaningful improvements in signs and symptoms of RA and physical function.104 However, the efficacy of this agent in other bDMARD refractory RA patients has not been reported.
Special comment/recommendation for the AP region
While there is evidence that tofacitinib is useful in active RA disease patients who have failed cDMARDs, and probably those who are refractory to TNF inhibitors, more data is needed to support its use as a first-line DMARD or after a patient has failed a bDMARD, specifically the non-TNF inhibitors. While we await long-term data on the efficacy and side-effect profiles, particularly infectious disease complications, of tofacitinib, the Steering Committee felt it is not yet ready to recommend its use in DMARD-naïve patients. The other consideration is to do with the possible high financial cost of tofacitinib which is significantly higher than that of all cDMARDs. This is an important consideration for many AP countries where there are financial constraints in obtaining access to expensive medications. Our decision to recommend its use in patients who have failed a bDMARD is based on an extrapolation of the data reported by Burmester et al.104 The Steering Committee encourages national societies to include tofacitinib in their national drug formulary.
Discussion
These are the first RA treatment recommendations developed by APLAR. The AP region is unique in many ways. It accounts for 61% of the world population with diverse ethnic, cultural and socioeconomic variations.105 The prevalence, clinical manifestations, response to treatment and outcome of RA patients in many Asian countries have been suggested to differ from those reported in the West. Importantly, rheumatology is still a developing medical subspecialty in the region with a severe shortage of trained rheumatologists in many countries.106 Not only are many RA patients in the AP region managed by non-rheumatologists, the lack of resources also means RA clinical studies are sparse and that a big gap exists in the evidence-based management of RA in this region.
The primary aim of these treatment recommendations is to provide clinicians with an evidence-based reference for the treatment of RA in the AP region. The Steering Committee was comprised of rheumatologists from 12 AP countries representing a diverse spectrum of cultures and ethnicities, health economic statuses and practises of rheumatology in the region. A patient with RA was also invited to join the Committee so as to include patients’ perspectives on the treatment of their condition in the recommendations. Because of the nature of the recommendation drafting process which required members to communicate in English, a patient from Hong Kong who was literate in the language was invited to join the Committee.
The Steering Committee did not perform a systematic review of original articles to inform recommendations. Instead, the recommendations were developed using the ADAPTE framework12, 13 appraising all international RA practice guidelines and recommendations through to December 2013. The ADAPTE Collaboration is an international collaboration of researchers, guideline developers and guideline implementers who aim to promote the development and use of clinical practice guidelines through the adaptation of existing guidelines. Having first established the scope and purpose of the current RA treatment recommendations, the Steering Committee conducted a thorough search for guidelines and relevant documentations that have been previously published. Each of these articles was then assessed for guideline quality, currency, content, consistency and acceptability/applicability for the AP region using the AGREE instrument.15 Selected previous guidelines were rigorously and systematically reviewed and discussed by members of the Steering Committee before they were customized to create the current adapted recommendations. The generic adaptation process of ADAPTE has been shown to be valid and of high quality.12, 13
Based on the key questions that the Steering Committee had identified regarding the treatment of RA in the AP region, 40 recommendations concerning the general RA treatment strategies, and the role of NSAIDs, corticosteroids, cDMARDs, bDMARDs and tofacitinib have been made. Specific issues relating to the safety and monitoring of cDMARDs and bDMARDs are not covered in the current report. These include screening and prophylactic treatment for latent TB, management of patients who are chronic viral hepatitis B/C carriers and precautions with other regional prevalent infectious diseases. These will be covered separately in a later instalment of the recommendations.
The role of tofacitinib, a JAK inhibitor, in RA was not originally included as one of the key questions. However, midway through the development of these recommendations, tofacitinib became available in a number of AP countries and a recommendation on the use of this agent was included in the EULAR RA treatment recommendations 2013 update. The Steering Committee therefore felt it would be appropriate to include the role of tofacitinib in RA treatment in the current recommendations.
Realizing that many Asian countries are under-resourced to allow them to adhere strictly to the recommendations made in this report, the Steering Committee added comments based on expert opinions and consensus following each recommendation in the hope that minimum care requirement is achieved for all RA patients in these countries. In addition, it is hoped that some of these region-specific comments, which may lack full evidence, may form the basis of a research agenda, which is by no means exhaustive, for clinicians in the AP region (Table 4).
|
I. General RA treatment strategies Longitudinal studies of the socioeconomic cost of RA Prevalence of use of complementary, over-the-counter and self-medications for RA Setting a realistic treatment target for patients from countries with low resources Evaluation of the role of non-rheumatologists and allied health workers in RA management |
|
II. Role of NSAIDs The use of NSAIDs and their short- and long-term side effects (much of this has already been carried out previously) |
|
III. Role of corticosteroids The prevalence of corticosteroid abuse Evaluation of the effects of long-term low-dose corticosteroids |
|
IV. Role of cDMARDs Do Asian patients tolerate methotrexate less well? Efficacy of combination cDMARDs involving agents such as bucillamine, iguratimod, intramuscular gold or tacrolimus Effects of cDMARDs on TB reactivation and novel infections Effects of cDMARDs on chronic viral hepatitis carriers Comparison of the efficacy of triple cDMARD combination and biologic agents |
|
V. Role of bDMARDs Effects of bDMARDs on TB reactivation and novel infections Effects of bDMARDs on chronic viral hepatitis carriers The frequency and occurrence of infective complications, including opportunistic infections associated with bDMARD use The frequency and occurrence of malignant conditions associated with bDMARD use Dose titration of bDMARDs |
|
VI. Role of tofacitinib The efficacy and long- and short-term side-effect profile of tofacitinib The efficacy of tofacitinib in bDMARD failure patients The possible role of tofacitinib in bDMARD-naïve patients |
- bDMARD, biological DMARD; cDMARD, conventional DMARD; DMARD, disease-modifying antirheumatic drug; NSAID, non-steroidal anti-inflammatory drug; RA, rheumatoid arthritis; TB, tuberculosis.
It should be noted that the current report does not include recommendations for physical and occupational treatment, and alternative/complementary therapies which are widely practised by many patients in the AP region. This is partly because evidence on the role of these treatment modalities is sparse. Furthermore, in many AP countries, the physical and occupational therapy disciplines are poorly developed. However, where physical and other non-pharmacological therapies are available, clinicians should advise RA patients about their roles in protecting normal joint function. For example, the BSR 2005 guidelines mentioned that patients should be informed about alternative techniques of effective pain management, including transcutaneous nerve stimulation (TENS) and behavioral approaches.60 Others have also found that physical therapy and exercise are beneficial in RA patients.57 Where resources are limited and patients are unable to afford the cost of procedures such as TENS, patients may be advised to exercise at home, as leisure time physical activity has also been shown to be beneficial and should be encouraged.22
Conclusion
APLAR has developed a set of general recommendations for the best practise management of RA in the AP region. We are also hopeful that some of these recommendations, where evidence for this region may be lacking, may stimulate clinicians to embark on research studies to resolve them. Specific issues such as the prevention of reactivation or novel TB and viral hepatitis infections will be addressed in a second instalment.
Acknowledgements
We would like to thank the Asia Pacific League of Associations for Rheumatology (APLAR) for funding this project, Ms King Chu Chan for serving as a patient representative on the Steering Committee, Dr Josef Smolen and Dr Vibeke Strand for reviewing the recommendations, and Dr Pradnya Kulkarni and Mr Chris Facey of MIMS (Hong Kong) Limited for providing medical writing support.
Funding
APLAR and unrestricted educational grants from the following pharmaceutical companies: AbbVie, Janssen, Pfizer, Roche and UCB.
Conflict of interest
All authors have disclosed any conflicts of interest. The individual declarations are summarized below. CSL has received unrestricted educational grants from AbbVie, Janssen-Cilag, Pfizer, Roche and UCB, and personal fees from Abbvie, Bristol-Myers Squibb, Celltrion, GlaxoSmithKline, Pfizer, Roche, Sanofi-Aventis and UCB; AH has received grants from Roche, and personal fees from Abbvie and Pfizer; MK has received personal fees from AbbVie, Astellas, Bristol-Myers Squibb, Chugai, Eisai, Janssen, Ono, Pfizer, Santen and Tanabe-Mitsubishi; JJL has received personal fees from Janssen, Pfizer and Roche; WL has received personal fees from Janssen, Pfizer, Roche and Sanofi-Aventis, and investigator fees from Janssen, Pfizer and Roche; PN has received research grants and personal fees from Pfizer; PS has received research grants from Pfizer. All other authors declare no conflict.
Contributions
All authors were involved in performing the literature search, appraisal of guideline quality, grading of evidence, evidence synthesis, development of recommendations, and writing and proofreading of recommendations.




