A prospective, randomized, double-blind, multicentre, parallel-group, active controlled study to compare efficacy and safety of biosimilar adalimumab (Exemptia; ZRC-3197) and adalimumab (Humira) in patients with rheumatoid arthritis
Abstract
Aim
In this study, efficacy, tolerability and safety of biosimilar adalimumab (Exemptia; Zydus Cadila) was compared with reference adalimumab (Humira; AbbVie) in patients with moderate to severe rheumatoid arthritis (RA).
Method
In this multicentre, prospective, randomized, double-blind, active controlled parallel arm study, 120 patients with moderate to severe RA were given 40 mg of either test adalimumab (Exemptia) or reference adalimumab (Humira) by subcutaneous route every other week for 12 weeks. The primary endpoint was proportion of responders in two tretament groups by American College of Rheumatology 20 (ACR20) at week 12. The secondary endpoints were change in Disease Activity Score of 28 joints – C-reactive protein (DAS28-CRP) and proportion of patients with an ACR50 and ACR70 response in two treatment groups at week 12. Safety outcomes were also assessed.
Results
After 12 weeks, patients treated every other week with test adalimumab (Zydus Cadila) had statistically similar response rates as compared to reference adalimumab (AbbVie): ACR20 (82% vs. 79.2%; P > 0.7); ACR50 (46%, vs. 43.4%; P > 0.7); ACR70 (14% vs. 15.1%; P > 0.8). The change in DAS28-CRP score was −2.1 ± 1.09 and −2.1 ± 1.21, in test and reference products, respectively. It was statistically significant compared to baseline, but not significantly different between the two products. Three serious adverse events and no death was reported during the study. Both adalimumab preparations were safe and well tolerated in this study.
Conclusion
The results demonstrated biosimilarity with respect to efficacy, tolerability and safety of test adalimumab (Exemptia) and reference adalimumab (Humira) in patients with moderate to severe RA.
Introduction
Rheumatoid arthritis (RA) is a chronic, progressive, debilitating autoimmune disease that occurs in approximately 1% of adults, in a female : male ratio of 2.5 : 1.0.1-3 It is characterized by chronic inflammation of the synovium, which over time results in damage to the joints, leading to pain and disability. The cause of RA is still unknown, although the contribution of infection and genetic susceptibility have long been suspected. The damage caused by RA is believed to be irreversible. Thus it is of paramount importance to offer immediate and effective treatment to RA patients to slow or halt disease progression.4
The primary goals in the treatment of RA are: prevention or control of structural damage to joints; prevention or reversal of disability; pain relief; and improvement in quality of life. The ultimate therapeutic goal is to achieve disease remission.5 At present, there is no complete cure for the disease.
Symptomatic treatment of RA with non-steroidal anti-inflammatory drugs (NSAIDs) may relieve pain, but does not stop disease progression.6 Most patients are now treated with one or more disease-modifying anti-rheumatic drugs (DMARDs), with methotrexate (MTX) being the most commonly used agent. DMARDs have the ability to slow or halt the underlying disease process. Although they may stabilise disease in many patients, DMARD therapy rarely leads to complete remission and can be associated with significant side-effects.7 At least one-third of patients with advanced RA do not have a long-term therapeutic option.
The first biological therapies to become established in RA treatment were antagonists of the proinflammatory cytokine, tumour necrosis factor (TNF). TNF inhibitors, usually in combination with MTX, have become established as the standard therapy for patients who have failed previous DMARD treatment.
TNF-α is a potent proinflammatory cytokine that plays a critical part in the progression of inflammatory synovitis, articular matrix degradation in RA and immune responses.
Cadila Healthcare Limited, the Zydus group, has developed a biosimilar of TNF-α blocker, adalimumab (Exemptia) which is a fully human monoclonal immunoglobulin G1 (IgG1) antibody produced recombinantly by Chinese hamster ovary (CHO) cells. It is a 1330 amino acid containing glycoprotein consisting of two copies of heavy- and two copies of light-chains in heterodimeric form with a molecular weight of ≈148 kDa.
Adalimumab marketed as HUMIRA by AbbVie (North Chicago, IL, USA) is approved (2002) by the US Food and Drug Administration for the treatment of RA. The present study was designed to evaluate and compare the efficacy, tolerability and safety of biosimilar adalimumab (Exemptia) as a test product with innovator's adalimumab (Humira) as a reference product in patients with RA.
Research Design and Methods
Study design and participants
This was a multicentre, prospective, randomized, double-blind, active controlled parallel arm study designed to evaluate the efficacy, tolerability and safety of test adalimumab (Exemptia; ZRC-3197) in comparison with innovator's adalimumab as a reference product (HUMIRA by Abbott, North Chicago, IL 60064, USA) in patients with RA. In this study 120 subjects were enrolled at 11 investigational sites across India to receive double-blindedly either test adalimumab or reference adalimumab. The study was conducted from 20 November 2013 to 8 July 2014.
The inclusion criteria for the study were: adult subjects of either gender in age group of ≥ 18 years and ≤ 65 years; history of RA, as defined by the American College of Rheumatology (2010 ACR/European League Against Rheumatism) Classification,8, 9 for at least 6 months; moderate to severe active seropositive disease; history of treatment with MTX 10–25 mg per week for at least 12 weeks with the last 4 weeks at the stable dose before screening; subjects who were able and willing to give written informed consent and comply with the requirements of the study protocol. All subjects were evaluated for tuberculosis using Gold Quantiferon and chest X-ray (if not done in the last 3 months) during the screening visit to rule out tuberculosis. Female patients of childbearing potential had to have a negative pregnancy test at the time of screening and agreed to use adequate contraception throughout the study period.
The exclusion criteria of the study were: subjects with significant systemic manifestations of RA; breastfeeding female; rheumatic autoimmune disease other than RA; history of diagnosis of juvenile idiopathic arthritis (JIA) (also known as juvenile rheumatoid arthritis [JRA]) and/or RA before age of 16 years; history of inflammatory arthritis other than RA (e.g., inflammatory bowel disease [IBD], systemic lupus erythematous [SLE] or psoriatic arthritis); any surgical procedure, including bone/joint surgery or planned surgery within 8 weeks prior to screening or during the study period; functional Class IV as defined by the ACR classification of functional status in RA; history of use of DMARDs other than MTX within 4 weeks prior to randomization (8 weeks prior for leflunomide); treatment with any investigational agent within 4 weeks of screening or 5 half-lives of the investigational drug (whichever was longer); preceding treatment with any TNF antagonist, including adalimumab; use of intra-articular or parenteral corticosteroids within 4 weeks prior to screening visit (however, inhaled corticosteroids for stable medical conditions were allowed); receipt of a vaccine within 4 weeks prior to enrolment visit; history of severe allergic or anaphylactic reactions to latex; history of primary or secondary immunodeficiency; evidence of significant uncontrolled concomitant diseases such as cardiovascular disease, nervous system, renal, hepatic, endocrine, gastrointestinal, or pulmonary disease, including any pulmonary or other condition that would preclude subject participation; known active bacterial, viral, fungal, mycobacterial or other infection (including tuberculosis or atypical mycobacterial disease, but excluding fungal infections of nail beds); history of travel to areas endemic for mycoses, such as histoplasmosis, coccidioidomycosis or blastomycosis; history of recurrent significant infection or any significant episode of infection requiring hospitalization or treatment with intravenous antibiotics within 4 weeks of screening or oral antibiotics within 2 weeks prior to screening; history of cancer, including solid tumors and hematologic malignancies (except basal cell and squamous cell carcinoma of the skin that have been excised and cured); lack of peripheral venous access; history of chronic daily use of narcotic analgesics; history of alcohol, drug, or chemical abuse within 6 months prior to screening; positive hepatitis B surface antigen or antibodies to hepatitis C; history of significant cytopenias or other bone marrow disorders. Subjects were excluded if serum creatinine > 1.4 mg/dL for women or 1.6 mg/dL for men, aspartate aminotransferase (AST) or alanine aminotransferase (ALT) > 2.5 times upper limit of normal (ULN), platelet count < 100 000/μL, hemoglobin < 8.0 g/dL and neutrophil < 1.5 × 103/μL.
The study was Good Clinical Practice (GCP) compliant and was initiated after obtaining approvals from the Drug Controller General of India and Independent/Institutional Ethics Committee (IEC) of each investigational site, and registration of the trial with Clinical Trial Registry of India (Phase III/CTRI/2013/10/004040). Written informed consent was obtained from each participant before the initiation of any study-related investigation.
Procedure
This 12-week multicentre, prospective, randomized, double-blind, active controlled parallel arm study was preceded by a screening visit (Visit 0). During this visit patients were screened for eligibility to participate in the study. The eligible subjects were enrolled in the study at Visit 1. Subjects were randomly assigned in a 1 : 1 ratio to test adalimumab and reference adalimumab. The statistical analysis system (SAS ver. 9.3: SAS Institute Inc., Cary, NC, USA) software was used to generate randomization in blocks of two. The treatment was administered double-blinded fashion and treatment was assigned to patient enrolment number. The blinding was maintained till the final database was closed. Baseline characteristics and clinical evaluations were recorded.
A total of six visits were scheduled during this study including: screening visit; following screening enrolment Visit 1 (Day 1); Visit 2, Day 15 ± 1; Visit 3, Day 28 ± 3; Visit 4, Day 56 ± 3; and Visit 5, Day 84 ± 3. At the time of scheduled visits, all subjects received the injection by trained nursing staff under the supervision of the investigator or his designated nominee. However, when the injections were to be taken at home (Days 42 and 70), the subject was asked to receive the injection under the supervision of a trained nurse/physician, if the patient could not self-administer the injection by his/her own. The assessments were performed by the investigators.
Patients were clinically examined and assessed periodically for the efficacy and safety parameters. If further investigations were required in case of any adverse events, investigators were advised to assess the adverse events and take necessary action, if required.
The primary efficacy endpoint was proportion of patients with an ACR20 response in both the treatment groups at week 12. ACR core components were assessed: tender joint count and swollen joint count; patient assessments of pain; disease activity and disability (Disability Index of the Health Assessment Questionnaire [HAQ]);10 investigators' global assessment of disease activity; erythrocyte sedimentation rate (ESR); and C-reactive protein (CRP).11, 12
The secondary efficacy endpoints were change in Disease Activity Score of 28 joints – CRP (DAS28-CRP),13 proportion of patient with an ACR50 response and proportion of patients with an ACR70 response in both the treatment groups at week 12. The immunogencity was assessed as percentage of patients who develop detectable anti-drug antibodies on Day 1, Day 28 and Day 84 (week 12). In addition safety and tolerability of test adalimumab (Exemptia) was assessed in patients with RA.
Safety parameters evaluated in this study included general and systemic clinical examination (cardiovascular system, respiratory system, gastro-intestinal system, central nervous system, etc.), laboratory investigations (complete blood count, liver function tests, renal function tests) and assessment of all adverse events.
Statistical analysis
Sample size was estimated based on an assumed ACR20 response rate of 55% at week 1214 and margin of 28.5% with 80% power and two-sided 5% level of significance.
Considering a dropout rate of 20%, 60 subjects in each treatment group were enrolled in the study.
The demographic and baseline characteristics were summarized by treatment group. For continuous measurements such as age, the mean, median, standard deviation and range were tabulated. For categorical measurements such as gender, the frequencies were computed.
The primary efficacy variable was proportion of patients with an ACR20 response in both the treatment groups at week 12. Comparison among treatment groups at week 12 was done using 95% confidence intervals (95% CIs) and Pearson's Chi-square test.14
Secondary efficacy variable was proportion of patient with an ACR50 and ACR70 response in both the treatment groups at week 12. Comparison among treatment groups at week 12 was performed using 95% CIs and Pearson's Chi-square test.14
Another secondary variable was change in DAS 28-CRP at week 12. Analysis of covariance (ancova) model was used to compare the change in DAS 28-CRP values of treatment groups. The ancova model included treatment as fixed effect and baseline score as covariate. Change was estimated using the least-square means derived from the ancova model. Comparison between each treatment groups was made using the difference in least-square means and P-values from the ancova model.
For safety analysis, the frequency tabulations of abnormal clinical laboratory findings were performed.
Results
In this study 162 subjects were screened at 11 investigational sites in India, of which, 120 subjects were enrolled, 60 subjects in each group, namely test adalimumab (Exemptia) and reference adalimumab (Humira). A total 116 subjects completed the study and four subjects dropout or withdrew from the study. Two subjects were withdrawn due to adverse events, one subject withdrew consent for participation in the study and one subject was lost during the follow-up visits. A total 103 subjects were qualified for per protocol analysis and 119 subjects qualified for intent-to-treat (ITT) analysis. Subject disposition during the study is presented in Table 1 and Consolidated Standards for Reporting Trials (CONSORT) flow chart is presented in Figure 1.
| Adalimumab (test) | Adalimumab (reference) | Total | |
|---|---|---|---|
| No. of subjects randomized | 60 | 60 | 120 |
| No. of subjects dropped out/withdrawn | 3 | 1 | 4 |
| Due to adverse event† | 2 | 0 | 2 |
| Lost to follow-up | 0 | 1 | 1 |
| Subject's voluntary withdraw | 1 | 0 | 1 |
| No. of subjects completed | 57 | 59 | 116 |
| No. of subjects analyzed for safety | 60 | 60 | 120 |
| No. of subjects analyzed for ITT | 60 | 59 | 119 |
| No. of subjects analyzed for PP | 50 | 53 | 103 |
- †Subject EGR111 and Subject EGB057. ITT, intention-to-treat; PP, per protocol.
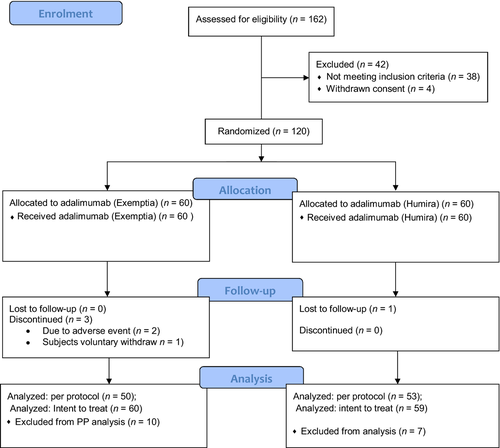
The mean age of patients was 45 ± 10.95 years, with the range among groups of 19–65 years. The groups were similar in age distribution, with a majority of female subjects. All other subject characteristics were balanced in both study groups at baseline. The demographic characteristic of the safety population is presented in Table 2.
| Variables | Adalimumab (test) (N = 60) | Adalimumab (reference) (N = 60) | P-value† |
|---|---|---|---|
| Gender n (%) | |||
| Male | 9 (15.0%) | 12 (20.0%) | 0.47 |
| Female | 51 (85.0%) | 48 (80.0%) | |
| Age (years) | |||
| Mean ± SD | 45 ± 11.06 | 45 ± 10.92 | 0.84 |
| Median | 45.0 | 45.0 | |
| Min, Max | 19.0, 64.0 | 24.0, 65.0 | |
| Race n (%) | |||
| Asian | 60 (100.0%) | 60 (100.0%) | NA |
| Height (cm) | |||
| Mean ± SD | 154.1 ± 7.87 | 155.3 ± 7.11 | 0.38 |
| Median | 152.0 | 155.5 | |
| Min, max | 141.0, 177.0 | 139.0, 171.0 | |
| Weight (kg) | |||
| Mean ± SD | 55.2 ± 10.51 | 55.9 ± 11.79 | 0.72 |
| Median | 53.7 | 56.0 | |
| Min, Max | 33.0, 87.0 | 33.8, 90.0 | |
| Duration of disease (years) | |||
| Mean ± SD | 3.3 ± 4.19 | 4.0 ± 4.98 | 0.41 |
| Median | 1.9 | 2.1 | |
| Min, max | 0.0, 23.0 | 0.0, 27.1 | |
| MTX dose | |||
| < 15 mg | 19 (31.7%) | 17 (28.3%) | 0.69 |
| = 15 mg | 35 (58.3%) | 32 (53.3%) | 0.58 |
| > 15 mg | 6 (10.0%) | 10 (16.7%) | 0.28 |
| Anti-cyclic citrullinated peptide antibodies | |||
| Positive n (%) | 57 (95.0%) | 55 (91.7%) | 0.54 |
| Mean ± SD | 290.6 ± 182.93 | 276.1 ± 181.79 | |
| Median | 320.8 | 266.6 | |
| Min, max | 7.0, 500.0 | 7.0, 500.0 | |
| Rheumatoid factor | |||
| Positive n (%) | 57 (95.0%) | 60 (100.0%) | 0.67 |
| Mean ± SD | 104.8 ± 57.51 | 101.7 ± 56.21 | |
| Median | 115.6 | 98.5 | |
| Min, max | 8.9, 189.5 | 14.2, 186.2 | |
| DAS28-CRP | |||
| Mean ± SD | 5.9 ± 0.94 | 6.0 ± 0.78 | 0.57 |
| Median | 5.7 | 5.9 | |
| Min, max | 3.9, 8.4 | 4.4, 8.3 | |
| DAS28-ESR | |||
| Mean ± SD | 6.9 ± 0.74 | 6.9 ± 0.72 | 0.78 |
| Median | 6.9 | 6.8 | |
| Min, max | 5.6, 8.5 | 5.6, 8.2 | |
- †P-values for categorical variables are calculated with Chi-square test and for continuous variables P-values are calculated with analysis of variance. CRP, C-reactive protein; DAS28, Disease Activity Score of 28 joints; ESR, erythrocyte sedimentation rate; MTX, methotrexate; NA, not available.
The ACR response was significantly improved for each group at each evaluation throughout the study. After 12 weeks of test adalimumab (Exemptia) treatment, 82% of patients had an ACR20, 46% had an ACR50 and 14% had an ACR70; whereas in reference adalimumab (Humira) treatment group after 12 weeks of treatment, 79.2% of patient had an ACR20, 43.4% had an ACR50 and 15.1% had an ACR70 response (Table 3). There was no statistically significant difference in ACR20, ACR50 and ACR70 among the two treatments groups.
| Adalimumab (test) | Adalimumab (reference) | 95% CI | P-value† | P-value‡ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total subjects | Response | % | Total subjects | Response | % | Lower (%) | Upper (%) | |||
| ACR20 | ||||||||||
| ITT | 60 | 47 | 78.33 | 59 | 47 | 79.66 | −15.29 | 12.63 | 0.86 | 1.00 |
| PP | 50 | 41 | 82.00 | 53 | 42 | 79.25 | −11.99 | 17.50 | 0.72 | 0.81 |
| ACR50 | ||||||||||
| ITT | 60 | 26 | 43.33 | 59 | 26 | 44.07 | −17.37 | 15.90 | 0.94 | 1.00 |
| PP | 50 | 23 | 46.00 | 53 | 23 | 43.40 | −15.46 | 20.67 | 0.79 | 0.84 |
| ACR70 | ||||||||||
| ITT | 60 | 8 | 13.33 | 59 | 9 | 15.25 | −14.16 | 10.31 | 0.76 | 0.80 |
| PP | 50 | 7 | 14.00 | 53 | 8 | 15.09 | −14.46 | 12.28 | 0.88 | 1.00 |
- †P-values are calculated from Pearson's Chi-square test. ‡P-values are calculated from Fisher's exact test. ACR20, ACR50 and ACR70 responders: ≥ 20%, ≥ 50% and ≥ 70%, respectively, improvement in tender and swollen joint count; and ≥ 20%, ≥ 50% and ≥ 70%, respectively, improvement in at least 3/5 remaining ACR core measures: patient assessment of pain; patient and physician global assessment of disease activity; self-assessed disability (Health Assessment Questionnaire); and C-reactive protein. ACR, American College of Rheumatology; ITT, intention-to-treat; PP, per protocol.
In addition to swollen joints and tender joints, significant improvement was noted in all ACR core components. The difference in both treatment groups was not significant.
A significant decline in mean tender joint count was observed in both the groups after adalimumab treatment. On Day 28, mean reductions of 6.4 and 7.8 were reported for the test and reference groups, respectively. At week 12 the mean reductions were 10.5 and 11.7 for test and reference groups, respectively.
A similar degree of improvement was observed in swollen joint count in both the groups. At week 4, mean reductions of 5.5 and 6.5 were reported for test and reference groups, respectively. At week 12 the mean reductions were 8.2 and 9.2 for test and reference groups, respectively.
Significant improvements were observed in patient global assessment of disease activity, physician global assessment of disease activity and Disability Index of the HAQ. The summary of ACR core components is presented in Table 4.
| ACR core component | PP analysis | ITT analysis | ||
|---|---|---|---|---|
| Adalimumab (test) (N = 50) | Adalimumab (reference) (N = 53) | Adalimumab (test) (N = 60) | Adalimumab (reference) (N = 59) | |
| Tender joint count score (0–28) | ||||
| Baseline (Day 1) | 16.6 ± 6.09 | 17.4 ± 6.32 | 16.7 ± 5.98 | 17.2 ± 6.22 |
| Change from baseline at | ||||
| Week 4 (Day 28) | −6.4 ± 5.67† | −7.8 ± 6.21† | −6.5 ± 5.52† | −7.5 ± 6.12† |
| Week 12 (Day 84) | −10.5 ± 5.95† | −11.7 ± 7.19† | −10.7 ± 5.80† | −11.5 ± 6.98† |
| Swollen joint count score | ||||
| Baseline (Day 1) | 11.7 ± 5.57 | 12.4 ± 5.24 | 11.5 ± 5.42 | 12.1 ± 5.29 |
| Change from baseline at | ||||
| Week 4 (Day 28) | −5.5 ± 5.00† | −6.5 ± 5.81† | −5.5 ± 4.91† | −6.2 ± 5.75† |
| Week 12 (Day 84) | −8.2 ± 5.77† | −9.2 ± 6.02† | −8.1 ± 5.55† | −8.9 ± 5.94† |
| Patient assessment of pain | ||||
| Baseline (Day 1) | 66.5 ± 12.38 | 66.4 ± 11.11 | 67.0 ± 11.89 | 66.8 ± 11.53 |
| Change from baseline at | ||||
| Week 4 (Day 28) | −15.5 ± 11.18† | −15.8 ± 14.87† | −15.0 ± 11.84† | −16.2 ± 14.84† |
| Week 12 (Day 84) | −30.0 ± 17.66† | −28.4 ± 16.75† | −30.1 ± 17.52† | −29.1 ± 17.10† |
| Patient global assessment of disease activity | ||||
| Baseline (Day 1) | 66.2 ± 11.91 | 64.8 ± 10.57 | 65.3 ± 12.72 | 65.1 ± 10.96 |
| Change from baseline at | ||||
| Week 4 (Day 28) | −14.8 ± 11.47† | −16.5 ± 14.56† | −13.5 ± 13.89† | −16.7 ± 14.89† |
| Week 12 (Day 84) | −30.5 ± 16.75† | −28.3 ± 18.11† | −29.4 ± 18.18† | −28.8 ± 18.31† |
| Physician global assessment of disease activity | ||||
| Baseline (Day 1) | 63.4 ± 12.02 | 63.9 ± 10.39 | 63.1 ± 12.77 | 64.0 ± 10.63 |
| Change from baseline at | ||||
| Week 4 (Day 28) | −15.2 ± 13.86† | −16.8 ± 16.05† | −14.2 ± 14.80† | −16.5 ± 16.33† |
| Week 12 (Day 84) | −29.2 ± 18.35† | −28.6 ± 18.02† | −28.5 ± 19.71† | −29.1 ± 18.09† |
| Disability index of the HAQ | ||||
| Baseline (Day 1) | 1.7 ± 0.62 | 1.6 ± 0.61 | 1.7 ± 0.61 | 1.6 ± 0.58 |
| Change from baseline at | ||||
| Week 4 (Day 28) | −0.5 ± 0.48† | −0.5 ± 0.48† | −0.5 ± 0.45† | −0.5 ± 0.46† |
| Week 12 (Day 84) | −0.8 ± 0.63† | −0.7 ± 0.60† | −0.8 ± 0.61† | −0.8 ± 0.59† |
| CRP | ||||
| Baseline (Day 1) | 11.0 ± 12.72 | 10.5 ± 12.90 | 11.2 ± 12.39 | 10.5 ± 12.74 |
| Change from baseline at | ||||
| Week 4 (Day 28) | −2.5 ± 19.36 | −6.8 ± 12.77† | −3.0 ± 18.04 | −6.6 ± 12.69† |
| Week 12 (Day 84) | −5.5 ± 12.66† | 0.7 ± 26.98 | −5.8 ± 12.45† | 0.4 ± 26.38 |
| ESR | ||||
| Baseline (Day 1) | 53.9 ± 21.45 | 53.2 ± 20.33 | 11.2 ± 12.39 | 10.5 ± 12.74 |
| Change from baseline at | ||||
| Week 4 (Day 28) | −5.4 ± 16.82† | −2.5 ± 15.62 | −5.3 ± 15.55† | −1.9 ± 15.76 |
| Week 12 (Day 84) | −8.6 ± 19.76† | −5.4 ± 17.35† | −9.0 ± 19.88† | 6.1 ± 16.98† |
| DAS28-CRP | ||||
| Baseline (Day 1) | 5.8 ± 0.88 | 5.8 ± 0.83 | 5.8 ± 0.87 | 5.8 ± 0.82 |
| Change from baseline at | ||||
| Week 4 (Day 28) | −1.3 ± 0.92† | −1.5 ± 1.04† | −1.2 ± 0.93† | −1.5 ± 1.04† |
| Week 12 (Day 84) | −2.1 ± 1.09† | −2.1 ± 1.21† | −2.1 ± 1.05† | −2.1 ± 1.17† |
| DAS28-ESR | ||||
| Baseline (Day 1) | 6.8 ± 0.78 | 6.9 ± 0.81 | 6.8 ± 0.76 | 6.9 ± 0.80 |
| Change from baseline at | ||||
| Week 4 (Day 28) | −1.1 ± 0.83† | −1.2 ± 1.00† | −1.1 ± 0.82† | −1.2 ± 0.99† |
| Week 12 (Day 84) | −2.0 ± 1.10† | −2.1 ± 1.15† | −2.0 ± 1.04† | −2.1 ± 1.11† |
- †Significant compared to baseline using paired t-test. Values presented as mean ± SD. ACR, American College of Rheumatology; CRP, C-reative protein; DAS28, Disease Activity Score of 28 joints; ESR, erythrocyte sedimentation rate; HAQ, Health Assessment Questionnaire; ITT, intention-to-treat; PP, per protocol.
At baseline the DAS28-CRP score in the test group was 5.8 ± 0.88 and in the reference group it was 5.8 ± 0.83. On Day 28, the mean reductions of 1.3 and 1.5 were reported for test and reference groups, respectively. At week 12 the mean reduction of 2.1 was observed for each group. There was no significant difference in any of the parameters between the two treatment groups at baseline and the end of the study.
An efficacy subset analysis showed no significant difference between the treatment groups with respect to age, gender and weight on ACR criteria response and DAS28 (CRP/ESR).
At week 12 of the study, anti-drug antibodies were observed in two samples of patients treated with test adalimumab (titer values of 25 and 800), and one sample from a patient treated with reference adalimumab (titer value of 200). Low-level antidrug antibodies were observed in two baseline samples (before the drug treatment) at Visit 1 with titer values of 25 and 50.
All the analytical runs in the study were compared in terms of negative control, positive control parameters and screening cut points and were found to be within ±20% of the assay acceptance criteria.
Overall, test adalimumab (Exemptia) and reference adalimumab (Humira) were safe and well tolerated in this study. A total of 28 adverse events were reported in 17 subjects by MedDRA preferred term among treatment groups, and in addition three serious adverse events were reported during the study. The distribution of adverse events was comparable between the treatment groups. There were 13 adverse events reported in seven subjects in the test group, whereas in the reference group 15 adverse events were reported by 10 subjects. Pyrexia, headache and cough were commonly reported in both the treatment groups. Three serious adverse events were reported in this study, namely, pyrexia, dizziness and cough. Two serious adverse events were reported in the test group and one serious adverse event in reference group. All serious adverse events were completely resolved.
Three system organ classes with higher numbers of adverse events were gastrointestinal disorder, general disorder and administration site condition and infection and infestation. The majority of adverse events were mild in intensity and not related to the study drug. All adverse events were completely resolved.
There were no persistent changes from baseline in laboratory parameters in any group and are presented in Table 5. General examination shows no significant abnormal sign in any treatment group except two cases of pallor at Visit 3 in the reference group. No other systemic abnormality was observed throughout the study, except for musculoskeletal disorders; however, the proportion of abnormality was comparable in both treatment groups.
| Laboratory parameters | Visit | Adalimumab (test) (N = 60) | Adalimumab (reference) (N = 60) |
|---|---|---|---|
| Hemoglobin (gm/dL) | Screening visit | 11.6 ± 1.48 | 11.7 ± 1.48 |
| Visit 5 (Day 84) | 11.8 ± 1.43 | 11.7 ± 1.50 | |
| Total red blood cells count (106/μL) | Screening visit | 4.4 ± 0.64 | 4.4 ± 0.47 |
| Visit 5 (Day 84) | 4.4 ± 0.42 | 4.4 ± 0.55 | |
| Total leucocyte count (103/μL) | Screening visit | 9.2 ± 2.66 | 8.6 ± 2.44 |
| Visit 5 (Day 84) | 8.4 ± 2.65 | 8.1 ± 2.68 | |
| Total platelet count (103/μL) | Screening visit | 329.7 ± 105.78 | 311.2 ± 92.85 |
| Visit 5 (Day 84) | 276.9 ± 72.69 | 265.1 ± 81.32 | |
| Alkaline phosphatase (U/L) | Screening visit | 89.9 ± 28.60 | 84.1 ± 22.71 |
| Visit 5 (Day 84) | 80.4 ± 26.81 | 78.9 ± 28.14 | |
| Aspartate aminotransferase (U/L) | Screening visit | 22.5 ± 11.39 | 21.0 ± 10.60 |
| Visit 5 (Day 84) | 22.0 ± 11.94 | 21.8 ± 9.88 | |
| Alanine transaminase (U/L) | Screening visit | 21.6 ± 12.01 | 20.6 ± 14.22 |
| Visit 5 (Day 84) | 20.3 ± 10.89 | 21.7 ± 12.85 | |
| Bilirubin (Total) (mg/dL) | Screening visit | 0.3 ± 0.18 | 0.3 ± 0.11 |
| Visit 5 (Day 84) | 0.3 ± 0.15 | 0.3 ± 0.20 | |
| C-reactive protein (mg/L) | Screening visit | 20.8 ± 29.07 | 19.8 ± 33.12 |
| Visit 5 (Day 84) | 5.4 ± 6.32 | 10.9 ± 26.86 | |
| Erythrocyte sedimentation rate (mm) | Screening visit | 54.7 ± 19.59 | 50.8 ± 18.23 |
| Visit 5 (Day 84) | 45.0 ± 13.91 | 47.1 ± 18.64 | |
| Blood urea nitrogen (mg/dL) | Screening visit | 9.2 ± 2.76 | 9.3 ± 3.20 |
| Visit 5 (Day 84) | 10.6 ± 3.50 | 10.6 ± 3.41 | |
| Creatinine (mg/dL) | Screening visit | 0.5 ± 0.19 | 0.6 ± 0.21 |
| Visit 5 (Day 84) | 0.6 ± 0.17 | 0.6 ± 0.19 |
- Data are presented as mean ± SD.
Discussion
Cadila Healthcare Limited of the Zydus Group has developed an adalimumab biosimilar (Exemptia; ZRC-3197). Extensive physicochemical and biological comparability data showed the test adalimumab (Exemptia) to be highly similar to the reference adalimumab.15
The present study was designed to evalutate the efficacy, tolerability and safety of test adalimumab in comparison with reference adalimumab treatment in patients with RA. Subjects participated in this study were on stable MTX monotherapy for at least 4 weeks and had moderate to severe inadequately controlled RA.
Sample size was estimated before the regulatory approvals and the study initiation. As there was no guideline available on how much equivalence margin to keep for the adalimumab biosimilar study, a further evolution of guidance is required. However, at the end of the study, the actual biosimilarity had 90% CIs and was found to fall within a rigorous equivalence margin recommended for pharmacokinetics and pharmacodynamics studies (±20%).
Also the immunogencity assessment was performed up to 12 weeks and the study was not powered to assess immunogencity.
Adalimumab is associated with rapid clinical response rate following intravenous16 and subcutaneous injection.17 In this study patients were administered adalimumab 40 mg subcutaneously every other week for 12 weeks, which is in accordance with the statement of international consensus on biological agents.18, 19
In this study the efficacy was assessed using the ACR improvement criteria8, 9 and the DAS.13
Such composite criteria required improvement in multiple variables, and were more rigorous than those in which improvement is measured in only one or some selected clinical variables.
Results of the study indicate that test adalimumab (Exemptia) was effective and well tolerated in RA patients and was highly comparable with reference adalimumab (Humira). After treatment with test adalimumab (Exemptia) 82% of subjects had an ACR20 response, 46% of subjects had ACR50 response and 14% of subjects had ACR70 response at week 12 in per protocol populations. This was quite comparable with the treatment with reference adalimumab, where 79.2% of subjects had ACR20 response, 43.4% of subjects had ACR50 response and 15.1% ACR70 response on Day 84 in per protocol populations. No statistically significant difference between treatments groups were observed in ACR20, ACR50 and ACR70 responses. During this study, the imrovement in ACR response at the end of the study as compared to baseline reflects the combined effects of MTX and adalimumab treatments.
The efficacy and safety of adalimumab originator product were assessed in four randomized, double-blind studies in patients with active RA. The drug was evaluated in combination with MTX (12.5–25 mg) or as monotherapy or with other DMARDs, of which two studies were carried out to assess the efficacy of originator adalimumab in combination with MTX, where patients receiving adalimumab 40 mg every other week had achieved ACR20 response rate of 63% and 65%; ACR50 response of 52% and 39%; and ACR70 response of 24% and 21% at 6 months.20
In another study by Bombardieri et al.,21 the effectiveness of reference adalimumab was assessed in RA patients, where after 12 week of treatment 60% of patients reported ACR20 response and 33% reported ACR50 response. It was also reported that 60–70% of patient who do not responsd to MTX treatment achieved an ACR20 response with TNF antagonist treatment.22
In another study by Huang et al., the efficacy and safety of adalimumab plus MTX was assessed in a multicenter, randomized, bouble-blind parallel group and placebo-controlled clinical study. After 12 weeks of adalimumab 40 mg every other week, 57% of patients achieved ACR20 response and 32.2% patients achieved ACR50 response.23 The study therefore showed comparable responses with those reported in Bombardieri et al. The small difference in results among the various studies may be explained by differences in study inclusion criteria.
In the current study, over a period of 12 weeks the improvement in DAS28-CRP and DAS28-ESR scores were clinically relevent and statistically similar in test and reference groups. No significant similar was observed between treatment groups both at baseline and at the end of the study. In this study, 64% and 66% of subjects showed reduction in DAS28-CRP score of at least 1.2 units after 12 weeks of test and reference treatment, respectively. In this manner, whether by the ACR or the DAS criteria, both the drugs in the current study gave highly comparable reduction in scores and with no statistically significant difference.
Furthermore, an efficacy subset analysis of subject population showed no significant difference between the treatment groups with respect to age, gender and weight on ACR criteria and DAS28 (CRP/ESR).
Adalimumab carries out its function by binding to TNF-α and thereby preventing its binding to its receptor.24 Besides its ability to bind to the target antigen, the efficacy of any antibody drug also depends upon its circulating half-life.25 Varying levels of circulating half-life of a drug are known to impact the drug efficacy.26, 27 Comparable efficacy between the two drugs, test adalimumab (Exemptia) and adalimumab (AbbVie), is therefore also an indirect measure of the overall comparability or biosimilarity of the two drugs in their antigen-binding property and other physicochemical characteristics. This conclusion on biosimilarity of the two drugs has also been confirmed in extensive physicochemical and biological comparability studies.15 Additionally, circulating half-life of a drug can also be impacted by anti-drug antibodies (ADA) elicited against it as a result of its immunogenicity.28
Immunogenicity refers to the ability of a protein antigen to elicit an immune response, resulting in the production of ADA against itself. The antibodies generated can be either neutralizing or non-neutralizing in nature.28 The non-neutralizing antibodies can impact the circulating half-life of the drug, affecting the bioavailability. Neutralizing antibodies bind to the binding site of the therapeutic protein and neutralize it, thus possibly preventing or reducing the ability of biologic therapies from working, and potentially leading to disease progression.29-35 In some rare cases ADA have also been associated with safety.28, 36 It was reported that patients treated with adalimumab monotherapy had higher rates of antibody development than those on concomitant MTX.20 In this study, the results indicate that the drug products test adalimumab (Exemptia) and reference adalimumab (Humira) are similar with respect to immunogenicity response in patients with RA. This suggests that the two drugs were quite comparable in their aggregates and had similar folding properties, as has been separately reported.15
Overall, adalimumab test and reference were well tolerated and their safety profiles were comparable in this study. The inceidence of adverse events and serious adverse events were comparable between the treatment groups. The majority of adverse events were of mild intensity and not related to the study drug and all were completely resolved.
In this study, injection site reactions were not evident in any group, although it is a commonly reported adverse event with adalimumab.20
Like other TNF-blocking agents, the association of adalimumab with tuberculosis has been reported in many clinical studies. It was also reported that the incidence of tuberculosis reactivation increased at higher doses than the recommended dose.20 In the current study the incidence of tuberculosis was very low at 1.7% (n = 1) compared to a previously reported study.20 Results of laboratory investigations and proportion of abnormality were comparable in both treatment groups. Though longer duration of assessment with large sample sizes will provide better results.
Overall, the results of this study demonstrated high degree of biosimilarity with respect to efficacy, tolerability and safety of test adalimumab (Exemptia) and the reference adalimumab (Humira) treatment in patients with RA.
Disclaimer
AbbVie and Humira are registered trademarks of AbbVie Inc., North Chicago, Illinois, USA. Exemptia is a trademark owned by Cadila Healthcare Ltd.
Acknowledgments
This study was sponsored and funded by Cadila Healthcare Limited, the Zydus Group Company, India. In collaboration with the investigators, the sponsor of the trial contributed to the design of the study, collection of data, statistical analysis and interpretation and reporting of the results.
All authors accept full responsibility for the study, had full access to all the data and take responsibility for the integrity of the data and the accuracy of the analysis. The corresponding author had the final responsibility to submit for publication.
The authors would like to thank Dr. Gunjan Jariwala and Dr. Kevinkumar Kansagra, Senior Scientist, ZRC, Ahmedabad for project management, Mr. Harilal, Principal Scientist for bio-analytical support, Mr. Hitesh Chauhan, Director, Biometry, Cliantha Research Limited, Ahmedabad an independent clinical research organization for statistical assistance and data management and Dr. Jayesh Bhatt, Senior Scientist, ZRC, Ahmedabad for preparation of the manuscript.




