Palliative radiotherapy is effective for both well- and poorly differentiated neuroendocrine neoplasms
E O'Reilly: MBChB, FRANZCR; L Lao MBChB, FRANZCR; B Woodhouse BSc (Hons); K Sharples PhD; C Print MBChB, PhD; B Lawrence MSc, FRACP.
Abstract
Introduction
The outcomes of palliative radiation therapy (RT) for neuroendocrine neoplasms (NEN) are seldom reported. We investigated outcomes following palliative radiotherapy in a cohort of patients with NENs. We hypothesised that well-differentiated NEN will be less likely to have a clinical response than poorly differentiated NEN.
Methods
Patients who received at least one course of palliative RT were identified using the New Zealand NETwork! Registry. Patients with Merkel cell carcinoma, pulmonary small cell carcinoma or asymptomatic patients were excluded. Clinical response to RT within 90 days and overall survival were analysed alongside clinical variables (fractionation, RT site, tumour differentiation and tumour primary site).
Results
The cohort comprised 79 patients, with 147 courses of palliative RT delivered. Clinical response was measurable for 100 courses, with clinical response rate of 76%. A course delivered to a well-differentiated NEN was associated with 2.02-fold (95% CI 0.67, 6.12; P = 0.21) increase in odds of a clinical response compared to a poorly differentiated NEN. Median overall survival from the first fraction of RT was 94 days (95% CI 80, 138 days). Overall survival was higher in well-differentiated NEN than in poorly differentiated NEN (HR 0.2, 95% CI 0.10–0.40, P-value < 0.001); 30-day mortality was 7%. There were significantly reduced odds of clinical response for non-bone sites, and for courses >10 fractions compared to a single fraction.
Conclusion
Palliative RT is an appropriate option for management of symptoms in patients with both well- and poorly differentiated metastatic NEN.
Introduction
Neuroendocrine neoplasms (NEN) are an uncommon form of malignancy that can begin in any organ containing neuroendocrine cells. The incidence of these tumours is increasing. There were 1736 patients diagnosed with NEN in New Zealand in the 5 years between 2008 and 2012, with an age adjusted 9% increase in incidence over this 5-year period.1 This is similar to data from the United States where the annual incidence of NEN increased 6.4-fold between 1973 and 2012.2, 3 Population-based data have shown that 20–45% of these patients have metastatic disease at diagnosis.1, 3, 4
Data from population-based studies suggest that palliative RT may be underutilised in patients with NEN. In a cohort of patients with metastatic bone disease due to gastroenteropancreatic NEN and lung carcinoid tumours, 42% of patients reported pain related to their bone metastases at diagnosis or during follow-up – and yet only 26% of this cohort received palliative RT.5 This contrasts with a population-based study of 51,610 patients with metastatic disease from breast, prostate, lung or colorectal cancer where 41% received palliative RT.6 Patients with NEN often suffer from prolonged disease-related symptoms, compared to other types of malignancy where the symptom trajectory is reported to decrease over time, creating additional opportunity for utilisation of RT in NEN.7
Well-differentiated low-grade NENs have traditionally been thought of as less likely to respond to RT.8 This anecdotal maxim may reduce the use of RT for this group of patients. This retrospective report aimed to describe outcomes following palliative RT in patients with neuroendocrine neoplasms, and describe factors that may be associated with clinical response and survival. The primary outcome was clinical response to RT within 90 days, and secondary outcomes included overall survival, 30-day mortality and clinical response to re-irradiation. We hypothesised a lower rate of clinical response with well-differentiated NENs compared to poorly differentiated NENs.
Methods
Patients in the Auckland region with neuroendocrine neoplasms who received at least one course of palliative RT between 1995 and 2012 were identified in the New Zealand NETwork! Registry (NETR). NETR is a retrospective database that includes all NEN in New Zealand diagnosed 2008–2012, and all NEN in the Auckland region diagnosed from 1995 to 2012. The NETR identified cases by searching the New Zealand Cancer Registry using ICD-O morphology codes and searching public and private histology records in all 20 District Health Boards. Classification of NEN was defined as per WHO 2010 nomenclature. Pulmonary small cell carcinoma was excluded from the NETR. Merkel cell carcinoma were included in the NETR but excluded from our analysis (sensitivity to RT is already well documented). Included both in the NETR and in our analysis were gastroenteropancreatic (GEP) NENs, bronchopulmonary carcinoids, extrapulmonary small cell carcinoma, large cell carcinoma, medullary thyroid carcinoma, paraganglioma, pheochromocytoma and NENs of other primary sites such as gynaecological and genitourinary.
Patients who received palliative RT but were asymptomatic were excluded from the analysis. Patients were also excluded if they had adenocarcinoma with neuroendocrine differentiation, or if clinical response within 90 days could not be assessed due to lack of clinical records within that time period. Patients were still included if data are absent due to death within 90 days after RT, or if there are clinical records available but they do not give an indication of response.
Data recorded for each patient included gender, date of birth, date of death, primary site of their NEN and tumour differentiation. This information was sourced from NETR. Categories of differentiation include ‘Well’, ‘Poorly’ and ‘Other’. ‘Other’ differentiation includes those with mixed histology, or the ambiguous historic term neuroendocrine carcinoma (which in the past meant a NEN displaying invasion or spread, rather than the current meaning of a poorly differentiated NEN).
Clinical information was gathered from individual patient records, through the clinical record department at Auckland City Hospital. This included the indication for palliative RT, the site of palliative RT, dose and fractionation of the palliative RT, response to the specified course of palliative RT within 90 days, and whether it was re-treatment of that site.
For analysis purposes, the site of RT was divided into bone and other. Others included any non-bone site. The indication for RT was divided into: Dysphagia, haemostasis, neurology, respiratory symptoms, pain, ‘neurology and pain’, ‘respiratory symptoms and pain’ and others. The dose and fractionation of RT was divided into either single fraction, 2–9 or >10 fractions.
The proportion of courses where the patient was classified as having a clinical (symptomatic) response within the 90 days following first day of RT treatment was assessed. Response was defined in a binary fashion as ‘response’ (included complete response, partial response) or ‘no response’ (included no change, or progressive symptoms). The response assessment was based on the description of current symptoms documented in the patient's clinical record, within the 90 days following the start date of RT. If a symptom was not directly referred to in the clinician's follow-up documentation, response was recorded as ‘not available’. Time to response was measured from the first day of each course of RT to the date of the clinic letter where the response was documented, but was not included in the analysis.
Tumour stream was divided into the three largest groups for analysis – thoracic, GEP and ‘other’ (other included medullary thyroid, genitourinary, gynaecological, head and neck, paraganglioma and unknown).
The 30-day mortality was calculated from the first day of each course of RT. Overall survival was calculated from the start of each patient's first course of RT. Patients who were still alive at the point of data collection were censored at the date they were last known to be alive, as determined from their clinical notes.
Data were collected under ethical approval 12/NET/60: A national multi-centre retrospective registry to facilitate epidemiological description, diagnosis and management of Neuroendocrine Tumours amongst New Zealanders. This was approved by the Northern A Health and Disability Ethics Committee via the HDEC-Expedited Review pathway. No informed consent was sought, as this was a retrospective audit of data only.
Statistical analysis
The proportion of RT courses which were classified as having a clinical response within 90 days following the start date of RT was calculated using a logistic regression model with robust standard errors to account for the multiple courses of RT per person. Proportions and 95% confidence intervals were calculated from the fitted model using the inverse logit formula. This was calculated for all courses, and then we limited the data to each patient's first course only.
Proportions of RT courses with clinical response were compared according to fractionation, radiation site, differentiation and tumour stream using logistic regression models with robust standard errors.
The 30-day survival following each course of RT was calculated using Kaplan–Meier methods to estimate the overall proportion who died within 30 days of treatment; 95% confidence bands were estimated using the jackknife estimator.
Overall survival was calculated and plotted using Kaplan–Meier methods, and median survival was reported with a 95% confidence interval.
The proportion of patients who died within 30 days of receiving their first fraction of RT was calculated to give 30-day mortality, for both ‘all courses’ and each patients ‘first course only’.
The absolute and relative risk of a clinical response was calculated by comparing fractionation (single vs. multi-fractionation) for bone site and non-bone site, and P-value calculated.
Results
Population characteristics
Overall, 262 patients were identified in the NETR database who received RT. Of those, 178 were excluded from analysis, for reasons outlined in the consort diagram (see Fig. 1). The most common reasons for exclusion were Merkel cell histology (n = 79) followed by inability to access adequate clinical notes (n = 35).
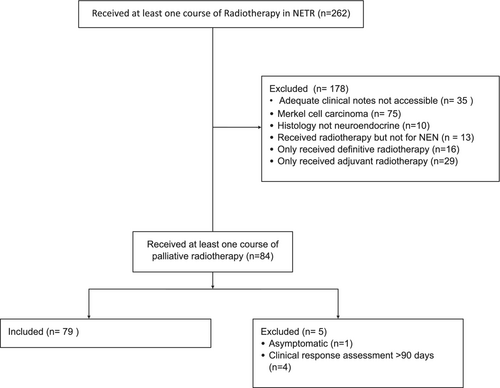
The characteristics of the study population are shown in Table 1. The median age was 63 years, and 41.8% were female. The most common primary tumour stream was thoracic (34.2%). The majority of patients had poorly differentiated NEN (67.1%).
| Overall, N = 79 (%) | |
|---|---|
| Age | |
| Mean (SD) | 63.2 (14.0) |
| Median (IQR) | 63.0 (55.5, 74.0) |
| Gender | |
| F | 33 (41.8) |
| M | 45 (57.0) |
| Unknown | 1 (1.3) |
| Tumour stream | |
| Thoracic | 27 (34.2) |
| GEP | 20 (25.3) |
| Other/unknown | 32 (40.5) |
| Tumour differentiation | |
| Poorly | 53 (67.1) |
| Well | 18 (22.8) |
| Other | 8 (10.1) |
| Outcome | |
| Alive | 3 (3.8) |
| Died | 76 (96.2) |
RT treatment details
A total of 79 patients received at least one course of palliative RT and were eligible for analysis. A total of 147 courses of palliative RT were delivered to the 79 patients. A total of 26 patients had multiple courses of RT. The courses of RT included in the analysis were delivered between 2003 and 2012.
The most common indication for RT was pain (58.5%). Symptoms included in the ‘other’ category included – one patient each with urinary symptoms and rectal symptoms, four patients with oedema, one patient with gastric obstruction and one patient with dysphonia. The most common site for a course of RT was axial bone (40.1%). Nine percent of the courses of RT were re-treatments (13 courses in total). It was most common to receive 2–9 fractions of RT (50.3%), or a single fraction (29.9%). The median dose for all courses of radiotherapy was 8 Gy (range 6–8 Gy) for a single fraction, 20 Gy (range 12–30 Gy) for 2–9 fractions and 30 Gy (range 20–50 Gy) for 10 or more fractions.
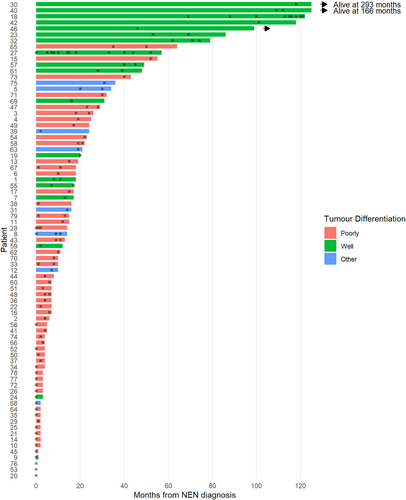
The Swimmer plot (Fig. 2) illustrates when each patient received their course or courses of palliative RT with regards to diagnosis of NEN and death, and shows that the majority appear to receive their treatments near the end of life. Note patients 27 and 18 received treatment most frequently out of all patients, with 16 and 12 courses of RT respectively. All other patients received <5 courses.
Follow-up
In this study, 100 of 147 (68.0%) courses of RT had information on clinical response documented in clinical records within 90 days from the first day of RT. Of the 47 without information on clinical response documented, 24 courses had documentation available within these 90 days without specific mention of the symptom that they were treated for. 23 courses did not have clinical follow-up documented and were in patients who had died within 90 days of their first day of that course of RT.
Clinical response
Limiting the data to the first course for each patient (n = 52), the probability of the patient having a clinical response within 90 days is 0.67 (95% CI 0.54, 0.79). Of all 100 courses of RT in which clinical response was measured (which includes both first course for each patient and all subsequent courses), the probability of the patient having a clinical response within 90 days is 0.76 (95% CI 0.65, 0.84). Clinical responses for all courses of RT are shown in Table 2.
| Clinical response within 90 days | Overall, N = 147 (%) | ||||
|---|---|---|---|---|---|
| Yes, N = 76 (%) | No, N = 24 (%) | Unknown deceased at 90 days, N = 23 (%) | Unknown alive at 90 days, N = 24 (%) | ||
| 30-day mortality | |||||
| No | 73 (96.1) | 24 (100.0) | 15 (65.2) | 24 (100.0) | 136 (92.5) |
| Yes | 3 (3.9) | 0 (0.0) | 8 (34.8) | 0 (0.0) | 11 (7.5) |
| Dose | |||||
| Single fraction | 24 (31.6) | 3 (12.5) | 9 (39.1) | 8 (33.3) | 44 (29.9) |
| 2–9 fractions | 41 (53.9) | 14 (58.3) | 8 (34.8) | 11 (45.8) | 74 (50.3) |
| 10 or more | 11 (14.5) | 7 (29.2) | 6 (26.1) | 5 (20.8) | 29 (19.7) |
| Radiation site | |||||
| Abdomen | 3 (3.9) | 2 (8.3) | 1 (4.3) | 0 (0.0) | 6 (4.1) |
| Bone – appendicular | 15 (19.7) | 0 (0.0) | 8 (34.8) | 5 (20.8) | 28 (19.0) |
| Bone – axial | 34 (44.7) | 9 (37.5) | 5 (21.7) | 11 (45.8) | 59 (40.1) |
| Brain | 2 (2.6) | 5 (20.8) | 2 (8.7) | 1 (4.2) | 10 (6.8) |
| Head and neck | 2 (2.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (1.4) |
| Lung | 3 (3.9) | 2 (8.3) | 1 (4.3) | 0 (0.0) | 6 (4.1) |
| Mediastinum | 6 (7.9) | 4 (16.7) | 1 (4.3) | 0 (0.0) | 11 (7.5) |
| Oesophagus | 3 (3.9) | 1 (4.2) | 1 (4.3) | 1 (4.2) | 6 (4.1) |
| Pelvis | 6 (7.9) | 1 (4.2) | 3 (13.0) | 4 (16.7) | 14 (9.5) |
| Subcutaneous | 2 (2.6) | 0 (0.0) | 1 (4.3) | 2 (8.3) | 5 (3.4) |
| Indication (symptoms) | |||||
| Dysphagia | 3 (3.9) | 2 (8.3) | 1 (4.3) | 0 (0.0) | 6 (4.1) |
| Haemostasis | 5 (6.6) | 0 (0.0) | 2 (8.7) | 1 (4.2) | 8 (5.4) |
| Neurology | 4 (5.3) | 4 (16.7) | 2 (8.7) | 3 (12.5) | 13 (8.8) |
| Pain | 50 (65.8) | 9 (37.5) | 12 (52.2) | 15 (62.5) | 86 (58.5) |
| Pain and neurology | 7 (9.2) | 2 (8.3) | 3 (13.0) | 2 (8.3) | 14 (9.5) |
| Respiratory | 4 (5.3) | 5 (20.8) | 2 (8.7) | 0 (0.0) | 11 (7.5) |
| Other | 3 (3.9) | 2 (8.3) | 1 (4.3) | 3 (12.5) | 9 (6.1) |
| Retreatment | |||||
| No | 66 (86.8) | 23 (95.8) | 22 (95.7) | 23 (95.8) | 134 (91.2) |
| Yes | 10 (13.2) | 1 (4.2) | 1 (4.3) | 1 (4.2) | 13 (8.8) |
| Tumour differentiation | |||||
| Poorly | 32 (42.1) | 14 (58.3) | 16 (69.6) | 11 (45.8) | 73 (49.7) |
| Well | 37 (48.7) | 8 (33.3) | 4 (17.4) | 10 (41.7) | 59 (40.1) |
| Other | 7 (9.2) | 2 (8.3) | 3 (13.0) | 3 (12.5) | 15 (10.2) |
- Some courses are on the same patient (repeated treatments).
When analysed by tumour differentiation, 32 courses of 46 courses of RT to a patient with a poorly differentiated tumour experienced clinical response (69.6%), and 37 of 45 courses of RT to a patient with a well-differentiated tumour had a clinical response (82.2%). Overall, courses of RT to a patient with a well-differentiated tumour are associated with 2.02-fold the odds of having a clinical response compared to a poorly differentiated tumour (see Table 3). The odds of a clinical response to RT were less favourable for multiple fractions compared to a single fraction (2–9 fractions, Odds 0.37, P = 0.06; >10 fractions, Odds 0.20, P = 0.02) and to a non-bone site compared to a bone site (Odds 0.33, P = 0.04).
| Odds ratio | Lower CI | Upper CI | P-value | |
|---|---|---|---|---|
| Dose | ||||
| Single fraction | 1 | – | – | – |
| 2–9 fractions | 0.37 | 0.13 | 1.06 | 0.06 |
| 10+ fractions | 0.2 | 0.05 | 0.74 | 0.02 |
| Radiation site | ||||
| Bone | 1 | – | – | – |
| Other radiation site | 0.33 | 0.12 | 0.94 | 0.04 |
| Primary site | ||||
| Thoracic | 1 | – | – | – |
| GEP | 2.15 | 0.42 | 10.98 | 0.36 |
| Other/unknown | 1.85 | 0.57 | 5.93 | 0.3 |
| Tumour differentiation | ||||
| Poorly | 1 | – | – | – |
| Well | 2.02 | 0.67 | 6.12 | 0.21 |
| Other | 1.53 | 0.48 | 4.86 | 0.47 |
Survival and 30-day mortality
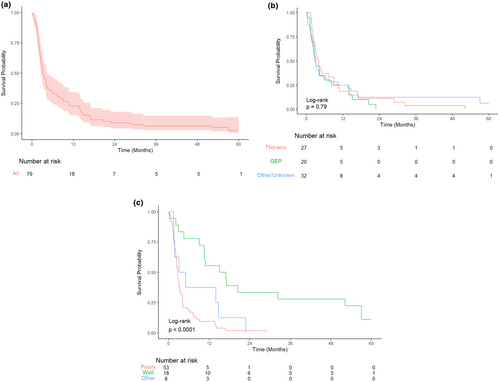
Median overall survival for all patients was 94 days from the start of their RT course (95% CI 80, 138 days). In this study, 5-year overall survival following the first course of palliative RT was 2.5% (95% CI 0.5%, 13.6%).
There were 11 deaths within 30-days following a RT course, of 147 analysed courses which gave a 30-day mortality of 7%. The 30-day mortality counting each patients first course only (n = 79) is 8%. There was no significant difference in overall survival analysed by tumour stream. There was significantly improved survival for well-differentiated tumours compared to poorly differentiated, (HR 0.20, 95% CI 0.10–0.39, P-value <0.001) but not for ‘other’ differentiation compared to poorly differentiated. See Figure 3 for Kaplan–Meier survival curves.
Retreatment
A total of 13 courses of RT were assessed to be ‘re-treatments’ at previously treated sites (11% of all courses of palliative RT). Five of these courses were for the same patient, to three different bony sites. In this study, 11 of these re-treatments were to the axial bone, one to the pelvis and one to the head and neck region. In addition, seven courses were for a single fraction of radiation, five were 2–9 fractions and one was for 10 or more fractions. Noting a small number of cases, 11 of these courses had data for clinical response, and 10 of these 11 achieved a response.
Discussion
This retrospective report utilises data from the New Zealand NETwork! Registry to assess outcomes following palliative RT in patients with neuroendocrine neoplasms, and describe patterns of use of palliative RT in these patients.
The number of patients and courses of RT analysed in this study is relatively large compared to similar reports. A single systematic review8 has assessed the use of external beam RT in the treatment of GEP neuroendocrine tumours. The authors identified 11 studies with a total of 176 patients. Four studies in their review reported on RT for patients with metastatic disease, containing 85 patients. Three studies did not report response rates. Due to the heterogeneity of the data, they could not draw a conclusion on the impact of external beam RT for a specific tumour grade or primary. One retrospective study has looked specifically at outcomes in 28 patients with NEN who received palliative RT for bone metastases.9 Therefore, the current study of 79 patients adds significant new data to this understudied area.
The rate of clinical response for all courses of RT in our study was 76%. There was no evidence of lower response rate in well-differentiated NEN when compared to poorly differentiated NEN. Instead, the odds of response were higher for well-differentiated NEN. Although we acknowledge that caution must be taken in overstating differences in small subsets, this does challenge our hypothesis, and suggests that palliative RT can be effective for symptom management in both well- and poorly differentiated NEN.
There was significantly reduced odds of response for courses of RT to a non-bone site, compared to bone. This may reflect a wider spectrum of complexity and variability within RT plans for non-bone sites. There were significantly reduced odds of clinical response for those receiving 10+ fractions of RT compared to a single fraction. It is uncertain if this is clinically relevant given small numbers in this subgroup. There may also be unknown confounding factors, including the possibility of higher pre-treatment pain levels, presence of complex neuropathic pain or bulkier lesions in this group which may have contributed to a decision for utilising the fractionated RT. This may also reflect on the era of RT, with increasing use of single fraction RT for palliation of bone metastases over time10 and other palliative treatments available at that point for the patients. There is now well-established evidence11 to support a similar response rate between a single fraction of RT and multiple fractions for palliative RT to uncomplicated bone metastases, for bone pain.
The Royal College of Radiologists have recommended 30-day mortality rates of higher than 20% following palliative RT as an indicator of patient harm. The 30-day mortality reported in a systematic review of 18 studies of palliative RT was lower than this, at 9–15.3%.12 The 30-day mortality in our study was also low, at 7%.
There are several factors that should be considered when interpreting this data. When analysing each patient's first course only (rather than including multiple courses in the same patients), we noted a slightly lower response rate of 67%, compared to 76% when analysing all courses. This suggests that patients were more likely to be offered multiple courses of RT if they had a good response to the first course, leading to the higher overall response rate when the analysis included multiple courses per patient. However, response rates for palliative RT to the bone in other malignancies have been reported as 61–62% (single fraction and multiple fraction), which is not dissimilar to the response rate in our cohort.11 There are variable reported response rates for non-bony sites of RT depending on the site and indication.13 There was no use of stereotactic RT in this cohort.
Additionally, we excluded 23 patients from the response analysis due to death within the 90 days, with no response documentation available. Excluding these patients from the response rate calculation requires us to assume that these people who died had the same probability of response as those who did not, which is unknown. However, palliative RT is not expected to impact on overall survival in general, so it could be assumed that their chance of response is similar. The lack of follow-up documentation could be due to the deteriorating patient not being well enough to attend follow-up, or clinicians hoping to spare these patients extra appointments.
The timeframe of 90 days post RT for clinical response assessment was chosen due to the broad period at which patients were reviewed for a post-treatment assessment. Prior studies reporting on outcomes from palliative RT have varied time points at which response is measured, from 1- to 3-month post-treatment.13
This study is limited by its retrospective design. As mentioned earlier, the study population was of reasonable size compared to previous reports on this topic in the literature; however, it is limited by small numbers in subgroups, which made showing clinically meaningful differences less likely. The response assessment was based on clinician reported symptoms in patient records, which relies on both accurate interpretation of the clinicians intended message, and on the accuracy of the clinician assessment. When assessing the overall impact of palliative RT, it is important to consider toxicity of the treatment and quality of life data. Due to this report's retrospective nature, it was impossible to accurately collect information on these factors.
A significant limitation was the lack of data regarding other therapy which the patient had received in the time immediate to or following the RT. It is possible that some or all of the effect attributed to the palliative RT was related to other treatments given around that time, such as analgesia, chemotherapy, somatostatin analogues, targeted biologic therapies or radionuclides.
In conclusion, and noting the limitations of this retrospective study, the rate of clinical response in this cohort indicates that palliative RT is a suitable option for symptom management in patients with both well- and poorly differentiated NEN. This challenges a prevailing orthodoxy that low-grade NENs do not benefit from palliative RT.
Ethical approval
Ethical approval 12/NET/60: A national multi-centre retrospective registry to facilitate epidemiological description, diagnosis and management of Neuroendocrine Tumours among New Zealanders. This was approved by the Northern A Health and Disability Ethics Committee via the HDEC-Expedited Review pathway. No informed consent was sought, as this was a retrospective audit of data only.
Acknowledgement
Open access publishing facilitated by The University of Auckland, as part of the Wiley - The University of Auckland agreement via the Council of Australian University Librarians.
Conflict of interest
No relevant conflicts of interest
Open Research
Data availability statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




