Cystic lung disease in adult Indigenous Australians in the Northern Territory of Australia
K Mishra: MBBS; R Fazal MBBS; T Howarth MPH; J Mutai FRANZCR; AX Doss FRANZCR; SS Heraganahally FRACP.
Abstract
Introduction
Indigenous Australians have a high prevalence of chronic lung diseases. However, no previous studies have reported on cystic lung disease in an Indigenous patient cohort.
Methods
This report describes 20 adult Indigenous patients noted to have incidental lung cysts on chest computed tomography (CT) while being referred to undergo lung function tests in the Northern Territory of Australia.
Results
Of the total 20 Indigenous patients demonstrating presence of pulmonary cysts on chest CT scan, 13/20 (65%) were males with a mean age of 49.9 years (range 24–74 years), with no significant difference in age between males and females. The majority reported a smoking history and spirometry demonstrated moderate reduction in lung function parameters. While there was no pattern in the size or location of cysts, most demonstrated multiple cysts (55% had ≥5 cysts) with bilateral involvement (65%), alongside a range of concurrent pulmonary radiological abnormalities. The aetiology for lung cysts was largely unknown.
Conclusion
This is the first report to illustrate cystic lung disease within an Indigenous population. Further radiology studies are required to investigate the causes and prognostications of cystic lung disease in Indigenous patients.
Introduction
In Australia, 3.3% of the population self-identify as First Nations Indigenous Australians (henceforth respectfully referred to as Indigenous patients/people/population). In the Northern Territory (NT) of Australia, approximately 30% of the population self-identify as of Indigenous descent, which is the highest across any Australian state or territory.1 Indigenous people residing in the Top End of the NT Australia are reported to have a higher burden of chronic respiratory conditions.2 Chest radiology is a basic fundamental modality in day-to-day clinical practice for the diagnosis, management and monitoring of several respiratory pathologies. Computed tomography (CT) of the chest is becoming increasingly utilised due to its ability to provide accurate and high-resolution images.3 While literature overwhelmingly supports the higher prevalence of pulmonary conditions in Indigenous people,2 ironically there are only anecdotal radiological studies that describe pulmonary diseases in Indigenous Australians that date back to the1970–1990s.4, 5 This creates an unacceptable deficiency in our knowledge, limiting the ability of health professionals to provide appropriate care in the diagnosis and in the management of several pulmonary disorders for Indigenous people.
Nevertheless, a recent study from our centre reported on chest CT findings among adult Indigenous patients undergoing lung function testing, and noted that chronic airway diseases such as chronic obstructive pulmonary disease and bronchiectasis were highly prevalent.6 However, we were intrigued to note that up to 5% of the study cohort (20/402) demonstrated the presence of pulmonary cysts.6 Due to the limited radiology data that exists in the literature on cystic lung disease in any Indigenous population globally, it is of interest to describe in this report the demographics, relevant clinical parameters and describe CT cyst characteristics specifically, in those 20 patients identified to have pulmonary cysts from our previous study.6
Methods
This retrospective study was conducted at the respiratory and radiology service based at the Royal Darwin Hospital (RDH), a university affiliated teaching hospital in the Top End Health Services (TEHS) region, NT of Australia. Study participants included were 20 Indigenous Australian adults from our previous report,6 who were identified to have lung cysts on chest CT scan while being referred to undergo a lung function test. This study is a part of a larger project assessing factors influencing and implications of chest radiological findings and lung function parameters among adult Indigenous Australians in the TEHS, NT of Australia. Further details regarding setting, chest CT scan data and study participants are available from our previous report.6 This study was approved by the Human Research Ethics Committee of the NT, TEHS and Menzies School of Health Research (reference no: HREC 2019-3445).
Results
Of the total 20 Indigenous patients demonstrating presence of pulmonary cysts on chest CT scan, 13/20 (65%) were males with a mean age of 49.9 years (range 24–74 years), with no significant difference in age between males and females (male 49 years vs. female 52 years). The mean body weight was 66 kg with a body mass index of 23 kg/m2, which was lower in males compared to females. The majority reported a smoking history and spirometry demonstrated a moderate reduction in lung function parameters (Table 1). One female patient had a diagnosis of Systemic Lupus Erythematosus (SLE).
| Clinical parameters | Results (n = 20) |
|---|---|
| Age (years) | 49.9 years (±14.4) |
| Male 49.3 (±14.7) | |
| Female 52.3 (±14.9) | |
| Sex (males) (n = 20) | 13 (65%) |
| Remoteness (n = 18) | Inner Regional (ASGS 3) (n = 3, 17%) |
| Very remote (ASGS 5) (n = 15, 83%) | |
| Weight kg (mean) (n = 18) | 65.8 kg (±15.8) |
| Males 63.6 (±15.4) | |
| Female 71.6 (±17.3) | |
| BMI kg/m2 (mean) (n = 18) | 23.3 kg/m2 (±5.9) |
| Males 21.8 (±4.9) | |
| Females 27.2 (±7) | |
| Smoking history (n = 18) | 18 (100%) |
| FVC % predicted (mean) (post BD) (n = 18)† | 60.2% (±14.4) |
| Male 58.4% (±16.4) | |
| Female 65.2% (±5) | |
| FEV1 % predicted (mean) (post BD) (n = 18) | 55% (±20.4) |
| Male 51% (±22.1) | |
| Female 67% (±6.4) | |
| FEV1/FVC absolute value (mean) (post BD) (n = 18) | 0.71 (±0.15) |
| Male 0.66 (±0.15) | |
| Female 0.83 (±0.03) | |
| DLCO (% predicted) (n = 16)‡ | 59 (±19.3) |
| Male 58 (±18.3) | |
| Female 60 (±18.4) | |
| TLC (% predicted) (n = 16)‡ | 58 (±18) |
| Male 55 (±18.3) | |
| Female 63 (±18.4) |
- † Out of the 20 patients 18 had acceptable and reproducible spirometry results. ‡Out of 20 patients 16 were assessed for DLCO and TLC parameters. ASGS, Australian Statistical Geography Standard; BMI, body mass index; DLCO, diffusing capacity of the lungs for carbon monoxide; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; TLC, total lung capacity.
Radiology findings
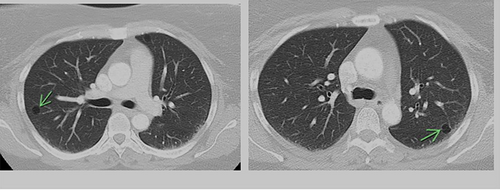
The majority of cysts were filled only by air (n = 19/20, 95%) and were thin walled (<2 mm, n = 18, 90%), with 11 patients (55%) showing five or more cysts. The distribution of cysts did not demonstrate any particular pattern, involving either upper, middle or lower lobes, although the right lobes did appear more commonly affected than the left (Table 2). However, the majority of patients demonstrated multiple cysts with bilateral involvement (n = 14/20 (70%)) and when patients demonstrated bilateral involvement, upper or lower zone involvement was slightly more common than mid zone involvement. The demographics and concurrent presence of other CT abnormalities alongside pulmonary cysts are shown in Table 3. Presence of Chronic obstructive pulmonary disease (COPD)/emphysema or bronchiectasis was observed frequently (COPD/Emphysema 10/20 (50%), bronchiectasis 11/20 (55%)). Twelve patients had a follow up CT available to assess change or progress in cyst size or numbers during the study window (2012–2020, mean 839 days between the CT scans). The vast majority remained stable with no change in size nor in numbers or occurrence of new cysts. Of these, 12 patients 7/12 (59%) had COPD/Emphysema, 5/12 (42%) had bronchiectasis. Among patients who demonstrated lung cysts with concurrent presence of COPD/emphysema or bronchiectasis, most cysts were distinct from that of radiological changes secondary to emphysema or bronchiectasis. Examples of cysts observed in the study patients are illustrated in Figures 1-5.
| Sex | Age | Cyst shape | Cyst filling | Number of cysts | Wall thickness (mm) | Clustering | Location |
|---|---|---|---|---|---|---|---|
| F | 63 | Rounded | Air | <5 | <2 | Non-clustered | RUL, RLL |
| F | 65 | Rounded | Air | <5 | <2 | Single cyst | LLL |
| F | 26 | Irregular | Air and fluid | ≥5 | 2–4 | Clustered | RLL, LLL |
| F | 53 | Rounded | Air | <5 | <2 | Non-clustered | RML, RLL |
| F | 63 | Rounded | Air | ≥5 | <2 | Clustered and non-clustered | RUL, RML, RLL, LLL |
| F | 45 | Rounded | Air | ≥5 | <2 | Non-clustered | RUL, RLL, LUL, LLL |
| F | 44 | Rounded | Air | <5 | <2 | Non-clustered | RLL, LLL |
| M | 74 | Irregular | Air | ≥5 | <2 | Clustered | RUL, LUL |
| M | 53 | Rounded | Air | <5 | <2 | Non-clustered | RLL, LLL |
| M | 38 | Rounded | Air | <5 | <2 | Single cyst | RLL |
| M | 64 | Irregular | Air | ≥5 | 2–4 | Clustered | RUL |
| M | 38 | Irregular | Air | ≥5 | <2 | Clustered | RUL, RLL, LUL |
| M | 52 | Rounded | Air | ≥5 | <2 | Clustered and non-clustered | RUL, RML, RLL, LUL, LLL |
| M | 74 | Rounded | Air | <5 | <2 | Non-clustered | RLL, L Lingular |
| M | 54 | Irregular | Air | ≥5 | <2 | Clustered | RUL, RLL, LUL, LLL |
| M | 33 | Rounded | Air | <5 | <2 | Single cyst | RML |
| M | 48 | Rounded | Air | ≥5 | <2 | Clustered and non-clustered | RUL, RML, RLL LUL, LLL |
| M | 49 | Irregular | Air | <5 | <2 | Single cyst | LLL |
| M | 33 | Rounded | Air | ≥5 | <2 | Clustered and non-clustered | RUL, RML, RLL, LUL, LLL, Lingular |
| M | 32 | Rounded | Air | ≥5 | <2 | Clustered | RUL, LUL |
- Clustering defined as each cyst being ≤10 mm apart from each other. LLL, left lower lobe; LUL, left upper lobe; RLL, right lower lobe; RML, right middle lobe; RUL, right upper lobe.
| Sex | Age | Lung nodules | Emphysema/COPD | Granuloma | Lung mass | Bronchiectasis | Small airways disease/inflammation | Atelectasis/collapse | Ground glass | Bullae |
|---|---|---|---|---|---|---|---|---|---|---|
| F | 63 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 |
| F | 65 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| F | 26 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| F | 53 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| F | 63 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| F | 45 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| F | 44 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| M | 74 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| M | 53 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| M | 38 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| M | 64 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| M | 38 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| M | 52 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| M | 74 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 |
| M | 54 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| M | 33 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 |
| M | 48 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 |
| M | 49 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| M | 33 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| M | 32 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
- COPD, chronic obstructive pulmonary diseases.
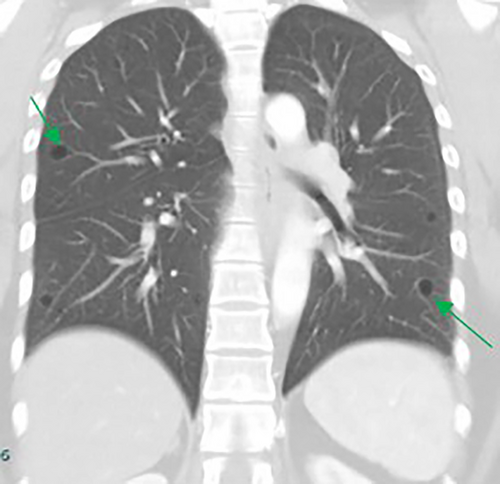
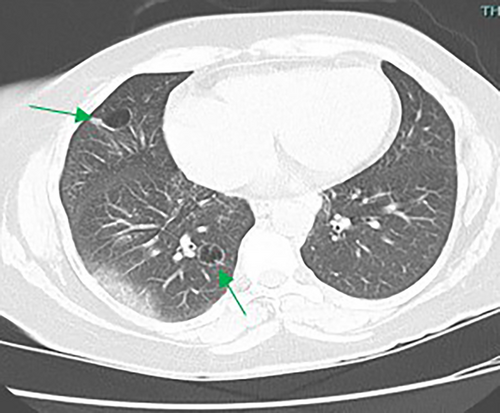
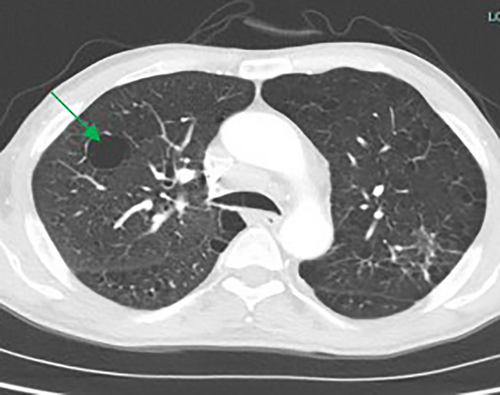
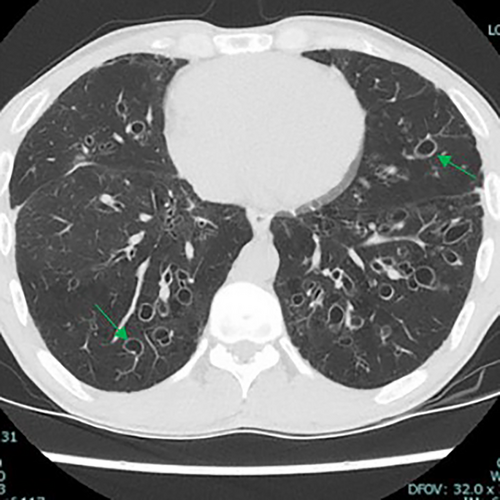
Discussion
To the best of the authors' knowledge, this is the first study reporting on pulmonary cysts in an Indigenous population and this report has demonstrated that lung cysts are far more common in Indigenous patients than that observed among non-Indigenous population reported in the previous literature.
A cyst is pathologically defined as an air space encapsulated in an epithelial or fibrous wall.7 Radiographically, a pulmonary cyst is typically rounded, well-defined parenchymal lucency or low attenuation area within the lung, exhibiting a well-defined interface with normal lung parenchyma.7, 8 Lung cysts are unique in comparison to other visceral cysts as they usually do not contain fluid.9 However, they may rarely demonstrate fluid attenuation or solid material and assume bizarre shapes.9 Typically a cyst exhibits a wall thickness ≤2 mm.7-9 Population screening studies have demonstrated that solitary or small numbers of cysts occur in 7.6% of healthy individuals over the age of 40 years, and can be attributed to normal ageing.8 Greater than five cysts are highly uncommon in asymptomatic individuals, and have only been documented in 0.9% of the population.8
While the pathogenesis of pulmonary cyst formation is poorly defined, the most widely acknowledged pathway leading to cystic lung disease is check-valve airway obstruction, followed by distal over-inflation, creating an air-filled cystic lesion.9 Cysts may originate from congenital, genetic, infectious, inflammatory, lymphoproliferative, neoplastic and smoking-related, or due to traumatic events and degenerative processes.7-11 Lung cysts have been categorised on the basis of their location, number, distribution and associated CT findings as follows: (i) subpleural cysts, either solitary or multifocal; (ii) parenchymal cysts without associated high-resolution computed tomography (HRCT) findings, either solitary or multiple; (iii) parenchymal cysts seen in association with nodules; and (iv) parenchymal cysts seen in association with ground-glass attenuation.7 Cystic adenomatoid malformations, pulmonary sequestrations and bronchogenic cysts are well described in infants,12 while the majority of adults with cystic lung disease have underlying lymphangioleiomyomatosis (LAM), pulmonary Langerhans cell histiocytosis (PLCH), Birt-Hogg-Dubé syndrome (BHD), or lymphoid interstitial pneumonia (LIP).7-11 The peak incidence of cystic lung disease is variable, but most commonly occurs in the third or fourth decade of life.7-11 Sex-based predilection is unclear. Depending on the number of cysts, underlying aetiology and age of the individual, patients may present asymptomatically or with nonspecific symptoms, including a chronic cough and shortness of breath.8, 10, 13, 14 Pneumothorax is the most common acute presentation of cystic lung disease, an important event leading to the diagnosis of diffuse cystic lung disease.8, 10
Patients' history, clinical examination, appropriate blood tests such as, full blood count, liver function tests including lactate dehydrogenase, auto-antibody screening, C-reactive protein, urinalysis looking for microscopic haematuria and lung function tests in conjunction with HRCT are helpful in narrowing the aetiology of lung cysts and in certain circumstances lung biopsy may be required.7, 15, 16 Due to the complex nature of cystic lung disease, algorithms have been proposed to determine aetiology and obtain accurate diagnosis and classification.7 Long term prognosis is variable and dependent on the course of associated illness, with a 33–50% mortality rate within 5 years of diagnosis.10 Overall, the prognosis of cystic lung disease is poor.
In this report we observed that Indigenous patients also demonstrate cysts, as described in previous literature for other populations, with most being rounded, air-filled and with a wall thickness of about two millimetres. However, more than 50% had greater than five cysts and several had irregular shaped cysts. Furthermore, a significant proportion demonstrated concurrent presence of other radiological abnormalities. Given the lack of any substantial published radiology data among Indigenous patients, the aetiology of lung cysts in our patients represented in this report are unclear and furthermore, the prognostication of cystic lung disease is largely unknown and poorly documented in the literature. However, it is plausible that patients demonstrating single or less than five cysts without concurrent pulmonary abnormalities may have a better long-term prognosis. A previous report from our centre reported on cystic lung disease in an Indigenous woman with Sjögren syndrome and amyloidosis,17 and one patient in this study had SLE. However, it may be reasonable to speculate there may be myriads of reasons for demonstrating cystic lung disease among Indigenous people, including if there is any genetic predisposition as observed in other ethnic populations in the previous literature.7-16 In the authors' experience and opinion, what is represented in this current study is only the tip of the iceberg, with an unprecedented number of Indigenous Australians presenting with cystic lung disease in clinical practice on a day-to-day basis in this region, including patients as young as 20 years old with bilateral extensive lung cysts and few presenting with respiratory failure with cystic lung disease.
Indigenous patients are reported to have a heightened burden of chronic respiratory diseases, alongside lower lung function parameters, including quite advanced and complex radiology findings.6, 18-27 In this case study, a significant proportion of patients were observed to have emphysema or bronchiectasis and to demonstrate cysts that in the majority were considered to be distinct either from emphysema or bronchiectasis. The historical definition of cystic lung disease by default has only been derived from the non-indigenous populations and suggests that lungs cysts are a distinct entity as opposed to the more common cyst-like lung diseases of emphysema and bronchiectasis.28-30 It is unknown if the significantly increased respiratory disease burden from emphysema and bronchiectasis in the Indigenous population and perhaps lung cysts may or may not represent a spectrum of distinct pathological entities, and if there is any underlying genetic predisposition that is expressed more in the Indigenous population due to environmental or other extrinsic factors, for example: smoking. In this study almost 100% of the patients had a smoking history, when data was available. In other ethnic populations smoking has been established to have a clear linkage to PLCH, however, it is unknown if this is the case for Indigenous Australians. In view of this, an Indigenous specific lung cyst classification could be considered as proposed in Box 1.
Box 1. Proposed pulmonary cysts classification for Indigenous patients
- Type 1C (well circumscribed, thin walled, rounded, and C for clustered)
- Type 1NC (well circumscribed, thin walled, rounded, and NC for non-clustered)
- Type 2C (irregular shaped, non-rounded, thin walled, and C for clustered)
- Type 2NC (irregular shaped, non-rounded, thin walled, and NC for non-clustered)
- Type 3 C and 3 NC (Both Type 1 and 2 Cysts)
Nonetheless, the presence of cystic lung disease can further perpetuate to adverse outcomes, especially in a population already with pre-existing higher burden of advanced chronic respiratory conditions. Hence, further dedicated efforts/studies are required to investigate the aetiopathogenesis and radio-histopathological correlation/data to advocate diagnostic and management pathways in order to reduce the respiratory related morbidity and mortality among Indigenous people. We hope our study may be of interest to other colleagues caring for Indigenous patients with pulmonary disorders not only in Australia, but also in other Indigenous peoples globally, and encourage further chest radiology related research in this population.
Limitations
The results of this study are restricted to participants retrieved from the database maintained from our previous study. Hence, they may not represent all Indigenous patients undergoing chest CT from the TEHS region of the NT of Australia, and this may have introduced bias in the observed outcomes in this study. Indeed, much lower numbers represented in this study due to limited dataset utilised of what could be the true unprecedented higher prevalence of cystic lung disease exists in this Indigenous population. Moreover, this study does not compare the findings of Indigenous patients with lung cysts to non-Indigenous populations. Furthermore, this report consists of limited number of patients (n = 20) based in the Northern Territory, hence the conclusions may not be generalisable to other Indigenous populations. Nevertheless, this is the first study investigating cystic lung disease in an Indigenous population and there is scope for further prospective studies.
In conclusion, there is currently limited published evidence on the aetiology, classification and prognostication of cystic lung disease in Indigenous population. Our study demonstrates that in the Indigenous population, lung cysts are more common in males in the fourth decades of life and the majority of patients have a smoking history. The size and distribution of cysts does not demonstrate any particular pattern. However, the majority are multiple and bilateral, moreover, the aetiology is largely unknown. The results of this report are an important avenue to further investigate cystic lung disease in the Indigenous population, with the ultimate goal identifying and addressing barriers which can close the gap in life-expectancy between Indigenous and non-Indigenous populations.
Acknowledgements
We acknowledge the wonderful contribution of the RDH medical staff, including rural and remote community Aboriginal health workers and RDH patients travel division for co-ordinating care for Aboriginal people living in the remote and rural communities in the TEHS, NT region. We also would like to thank our research assistant Ms Ara Perez in the data collection. Open access publishing facilitated by Flinders University, as part of the Wiley - Flinders University agreement via the Council of Australian University Librarians.
Funding statement
None.
Conflict of interest
All authors declare no conflicts of interest for this study.
Patient consent
Not applicable/retrospective study.
Open Research
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




