Utilizing 18F-fluoroethyl-l-tyrosine positron emission tomography in high grade glioma for radiation treatment planning in patients with contraindications to MRI
Abstract
Introduction
Patients with high grade glioma (HGG) and contraindications to magnetic resonance imaging (MRI) are dependent on contrast-enhanced computerized tomography (CT) scan imaging for radiation therapy (RT) target volume delineation. This study reviews the experience with the utilization of 18F-fluoroethyl-l-tyrosine positron emission tomography (FET-PET) to define residual disease post craniotomy and optimize RT planning.
Methods
Patients with HGG and a contraindication to MRI managed with radiation therapy between 2007 and 2015 were identified. RT target volumes including gross tumour volume (GTV) defined by CT-alone and the biological target volume (BTV) defined by PET-CT were recorded. Clinical target volumes (CTV) were created from the GTV and BTV respectively using standard protocol volume expansion. The expanded BTV was termed clinical target volume biological (CTV-B). Union and intersection between CTV and CTV-B, conformity index, volumetric parameters and individual patient outcomes were analysed.
Results
Six patients fit study criteria. There was a mean increase in CTV-B from CTV by 31.6% with a conformity index of 0.78. Two out of six patients had FET-PET avid disease outside the constructed PTV when delineated by CT-alone. One patient with CT-only planning had a new contrast-enhancing mass within 1 month of completing RT, suggesting potential geographical miss.
Conclusion
Patients with contraindication to MRI the addition of FET-PET can improve target volume delineation for RT Planning.
Introduction
Treatment paradigms for the management of high grade gliomas (HGG) involve maximum safe surgery followed by concurrent chemoradiotherapy. Magnetic resonance imaging (MRI) forms the basis of target volume delineation for radiation planning of HGG and has become a crucial element for the delivery of accurate and sophisticated adjuvant RT (radiotherapy). Pre and post-operative MRI are fused with the patient simulation computerized tomography (CT) data into the RT Planning systems. The Gross Tumour Volume (GTV) is delineated using these images and an expansion of this volume is used to create a Planning Target Volume (PTV). MRI provides detail on both enhancing as well as non-enhancing tumour, the latter which is poorly defined on CT. MRI for RT planning is a prerequisite for all recent HGG clinical trial protocols.
As the magnetic field of MRI can be disruptive to the properties of ferrous based objects, medical devices such as pacemakers are determined to be contraindications for MRI. As a diagnosis of HGG is managed as a relative medical emergency there is often inadequate time for pacemaker replacement with a modern MRI compatible device. Additionally, the pacing wires may be adherent to cardiac structures limiting the removal of all ferrous material. As a result, these patients often undergo adjuvant treatment planning and follow-up using simple diagnostic approaches such as CT imaging.
Fluorodeoxyglucose Positron Emission Tomography (FDG PET) imaging is a recognized modality in the staging of glioma. Uptake of FDG is determined by glucose metabolism and expression of GLUT-1 receptors and, to a lesser degree, cellular proliferation.1 Overexpression of GLUT-1 receptors occurs with greater frequency in higher grade tumours. However, there is high avidity of FDG within normal cerebral cortex. As such, the demarcation of tumour and normal brain is limited particularly in lower grade gliomas where relative uptake into the tumour is frequently reduced compared to uptake in normal cortex. Amino acid PET tracers such as (18) F-Fluoro-Ethyl-Tyrosine (FET) and 3′-Deoxy-3′[(18)F]-Fluorothymidine (FLT) offer an alternate imaging pathway with FET uptake reflecting uptake via the system L transporter LAT12 while FLT uptake correlates with cell proliferation and thymidase kinase-1 activity.3 As opposed to FDG, there is little uptake in normal brain. These tracers can be used to demarcate regions of both high and low grade tumour with greater accuracy than FDG. This has been demonstrated to assist in the presurgical evaluation for tumour biopsy and provide a means of response evaluation in management of glioblastoma.4
This study aims to review the experience with the target volume delineation for patients who have contraindications to MRI, and assess the additional information provided by utilization of FET-PET to define residual disease post craniotomy and optimize RT planning.
Methods
All adult patients diagnosed with HGG between May 2007 and July 2016 referred to the Cancer Centre were entered into a prospective database. This project was approved by the local Institutional Ethics Review Board. Patients with a contraindication to MRI, a pathological diagnosis of HGG, appropriate diagnostic and follow-up imaging available and at least 6 months of follow-up were included in the analysis.
Patients who had contraindications to MRI were identified by the absence of a MRI defined structure in the RT planning data. These patients were audited in regards to technique of RT target volume identification using CT or combined CT and PET scan. Acquisition of GTVs were standardized with one treating Radiation Oncologist with adherence to standard voluming protocols. Treated Clinical Target Volumes (CTVs) as defined by CT alone and Biological Target Volumes (BTVs) defined by combined CT and PET as well as subsequent treated Planning Target Volume (PTV) were recorded and compared.
Surgical resection
All patients underwent maximal safe resection prior to referral for adjuvant treatment. Individual patient surgical reports were analysed to determine the extent of resection and was noted as biopsy alone, sub-total resection or gross total resection.
FET-PET scan technique
All studies were acquired on a Siemens Biograph mCT PET/CT scanner with extended axial field of view (FoV) and time of flight (ToF) imaging capability, with 20 minute dynamic and 10 minute static acquisitions following a 3 minute FET infusion period. The standard injected dose of FET was 150 MBq.
Reconstruction parameters included three dimensional ordered subset expectation maximization (3D OSEM) with two iterations and 21 subsets with point spread function (PSF) recovery and ToF. Scatter correction was model-based and attenuation correction was CT-based. Post-reconstruction Gaussian filtering was performed with filter size of 5 mm.
RT target volume delineation
All patients had standard non-contrast CT simulation with immobilization by an individual Kevlar thermoplastic mask system. Instead of pre and postoperative MRI, contrast enhanced CT scans, and where available FET and FDG PET scans were fused with the non-contrast CT scan and entered into the Varian Eclipse Planning system. The quality of fusion was confirmed with comparison of manually generated CT scan evident structures (surgical cavity, ventricular system and pons) using the non-contrast planning CT as the reference. A single clinician was used for the planning process.
A GTV was created using the available imaging modalities including contrast CT with or without FET-PET where available. The rules for GTV creation were based on the EORTC-NCI 22981/26981 for GBM or EORTC CATNON for anaplastic glioma. This was designed for MRI based planning and incorporated the surgical cavity and postoperative enhancing tissue; and for the patients without MRI similar principles applied to the CT created volume. Where FET-PET was available, additional FET-avid regions were incorporated into the GTV, creating a BTV. The CTV and clinical target volume-biological (CTV-B) was a uniform expansion of the GTV and BTV respectively by 15 mm respecting anatomical and structural boundaries within the brain. The CTV or CTV-B were expanded by 3 mm to form a PTV. The union, intersection and conformity were analysed between the CTV and CTV-B as well as the volumetric difference between CTV and CTV-B if any.
The preoperative contrast-enhancing tumour on CT was volumed to confirm coverage of region of brain, but not used for volume delineation.
RT plan generation
Static intensity modulate radiotherapy (IMRT) was used in all patients with the dose prescription for patients less than or equal to 75 years with GBM either 60 Gy in 30 fractions over 6 weeks, or 40 Gy in 15 fractions over 3 weeks for the elderly subgroup aged greater than 75 years. For anaplastic glioma the plan was 59.4 Gy in 33 fractions over six and a half weeks. Coverage of 95% of the PTV by 100% of the dose was the dosimetry target.
Radiation therapy delivery
Treatment was delivered with IMRT using 6MV photons on a Varian Trilogy or TrueBeam STx Linear Accelerator. Daily IGRT was performed with the on-board imager (OBI) verifying position based on middle cranial fossa and orbital bone landmarks, and weekly Cone Beam CT to exclude any significant shift of intracranial contents arising from oedema, intercurrent haemorrhage or tumour reduction.
Systemic therapy management
All patients under the age of 75 with GBM were managed with concurrent, followed by adjuvant temozolomide (TMZ) for six months following the completion of RT. Patients with anaplastic glioma, underwent sequential treatment with radiotherapy followed by adjuvant TMZ. The elderly subgroup, over the age of 75, were treated with hypofractionated RT without TMZ.
Patient follow-up
All patients were followed up with clinical review and CT scans 2 monthly for the first 6 months if on TMZ. Patients not having TMZ or 6 months after TMZ were reviewed 3 monthly with CT scans. FET-PET was used at time of progression, where available, at clinicians discretion.
Statistical considerations
All patients had clinical and dosimetric data entered on an Excel database at our institution and updated for outcome events.
Study endpoints were analysed related to three outcomes: (i) the differences between CTV and CTV-B and subsequent PTVs generated, including conformity indices (ii) clinical outcome of site of relapse; and (iii) presence of a potential ‘geographical miss’.
The variation between CTV and CTV-B were analysed using Wilcoxon analysis. Significance was determined at P = 0.05 level. Respective CTV and CTV-B were analysed in regards to the volume of union, intersection, conformity index and the percentage increase in volume from CTV to CTV-B.
Clinical outcome of the treated patients were recorded as progression or no progression. At time of relapse, the relapse scan was fused into the radiation planning system and relapse was analysed in relation to previously generated PTV contour line. Failure was classed as central (within PTV contour), marginal (intersecting or within 20 mm of PTV contour), regional (≥20 mm from PTV contour but within same lobe of brain) or distant (not within same lobe of brain or any other distant site). These assessments were conducted by the same radiation oncologist for all cases.
A potential geographical miss was recorded for each patient if either the GTV defined by FET-CT was not covered by PTV generated by CT alone (for patients with FET-PET Planning) or presence of a regional relapse within 12 months of RT (for patients planned without FET-PET).
Results
329 patients with HGG were managed from May 2007 to July 2015 of which six patients were identified as having contraindication to MRI. Five patients had a cardiac pacemaker and one patient had metal fragments from prior gunshot wound. The individual patient details are described in Table 1.
| Characteristics | Patients (n = 6) |
|---|---|
| Gender | |
| Male | 5 |
| Female | 1 |
| Median age | 66 (range 44–81) |
| Histology | |
| GBM | 5 |
| Anaplastic astrocytoma | 1 |
| Location | |
| Frontal | 1 |
| Parietal | 1 |
| Temporal | 4 |
| Surgery | |
| GTR | 5 |
| STR | 1 |
| Biopsy | 0 |
| Chemotherapy | |
| Concurrent and adjuvant TMZ | 4 |
| Adjuvant TMZ | 1 |
| Sequential TMZ | 1 |
| Radiotherapy dose | |
| 60 Gy/30 fractions | 4 |
| 59.4 Gy/33 fractions | 1 |
| 40 Gy | 1 |
- AA, anaplastic astrocytoma; GBM, glioblastoma multiforme; GTR, gross tumour resection; STR, sub-total resection; TMZ, temozolomide.
Variations in target volume delineation
Four patients had BTVs generated using CT and FET-PET, whilst two patients had GTVs generated by CT scan alone.
The Individual CTV, CTV-B, union and intersection, conformity index (CI) and percentage increase in CTV-B from original CTV were noted (Table 2). The median CTV and CTV-B was 137.9 cm3 and 205.2 cm3. There was a median volume increase in 38.3 cm3 from CTV to CTV-B. The CI was defined as the intersection between CTV and CTV-B divided by the union between CTV and CTV-B. A CI of one indicated perfect concordance between CTV and CTV-B, whereas, a CI of zero indicates complete discordance between the two volumes. There was a mean increase in volume from CTV to CTV-B by 31.6% for patients with available FET-PET, with a mean conformity index of 0.78.
| Patient | Imaging | Pre-op tumour volume (cm3) | CTV CT (cm3) | PTV (cm3) | CTV-B (cm3) | CTV-B-CTV (cm3) | Percentage increase in BTV | Conformity Index |
|---|---|---|---|---|---|---|---|---|
| 1 | CT | 55.6 | 110.6 | 168.1 | N/A | N/A | N/A | N/A |
| 2 | CT | 27.2 | 149.2 | 210.2 | N/A | N/A | N/A | N/A |
| 3 | PET | 57.3 | 256.2 | 233.6 | 263.5 | 7.3 | 2.85 | 0.97 |
| 4 | PET | 29.8 | 70.6 | 105.5 | 127 | 56.4 | 79.89 | 0.56 |
| 5 | PET | 100.8 | 241 | 26.8 | 307.8 | 66.8 | 27.72 | 0.78 |
| 6 | PET | 49.9 | 126.7 | 136.6 | 146.9 | 20.2 | 15.94 | 0.83 |
- BTV, biological target volume; CT, computerized tomography; CTV, clinical target volume; PET, positron emission tomography; PTV, planning treatment volume.
Two of these four patients had regions of FET uptake that were more than 20 mm from the GTV based on CT alone, and these areas of FET uptake were not covered by the PTV generated by CT alone (Fig. 1). These two patients would be defined as a potential geographical miss based on CT Planning.
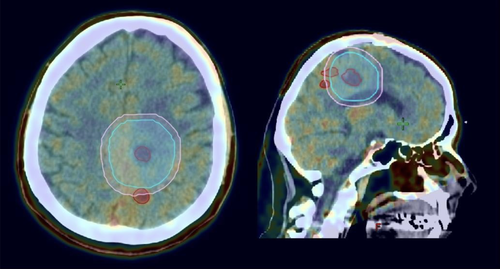
One of these patients developed a post RT contrast-enhancing mass at the site of prior FET uptake (which was encompassed within the subsequent PTV) and proceeded to repeat craniotomy with pathology demonstrating necrotic tumour only. This patient had pacemaker and leads changed to a MRI compatible device due to difficulties in surveillance post RT.
Site of tumour progression
Four patients had tumour relapse, two confirmed by repeat craniotomy, one by a persistent enlarging contrast enhanced mass and one with extensive CT and FET changes. Two of the relapses were central, one was marginal and the other regional. There were no distant relapses seen in our patient cohort. The marginal relapse occurred in a patient planned without FET Planning; with the relapse occurring 20 mm inferior from PTV edge at one month post RT. This may be considered a geographical miss based on CT Planning given the site of relapse and the early timeframe post RT (Fig. 2).
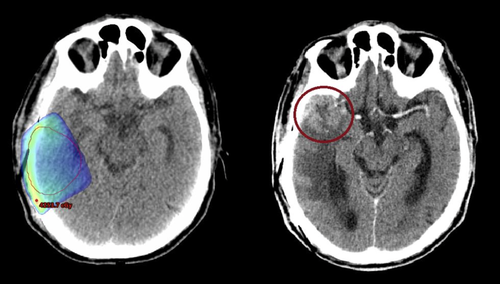
Potential geographical miss
Of the six patients, based on the study definitions, three would have a potential geographical miss if managed by CT Planning without FET. One due to a regional recurrence within months of treatment completion and the other two due to FET-avid lesions outside the PTV planned by CT-alone when FET was available.
Discussion
MRI plays an integral part in the diagnosis, treatment planning, progress and recurrence of malignant brain tumours, predominantly due to high soft tissue image quality.5-7 Radiotherapy treatment protocols from EORTC/RTOG have incorporated MRI sequences in aiding Radiotherapy treatment voluming and target acquisition, exploiting the improved spatial recognition over CT alone. Additional information from T2 weighted imaging and FLAIR have also been used in RTOG protocols to sequentially boost areas of potential high risk.8 MRI has also played an increasing role in as a means to detect early progression in HGG. Based on the EORTC 26981/22981 study of adjuvant radiation therapy with temozolomide,9 a patient will receive seven MRI scans in the initial 12 months following diagnosis of a glioblastoma multiforme. Concurrent improvements in interventional cardiology procedures have also resulted in a greater utilization of implantable cardiac devices such as permanent pacemaker (PPM) and implantable cardioverter-defibrillator (ICD)10 thus a greater chance for a patient with a device to be referred for MRI. Despite this, no clear modern guidelines exist for radiotherapy voluming and follow-up in instances where MRI is contraindicated.
Given the obvious advantages of MRI based imaging, one option, as successfully undertaken by a patient in the current series, is to have the pacemaker replaced with a compatible device. However, the ability to remove the old sensors may be limited due to longstanding wires being incorporated into cardiac tissue. This was an issue for a second patient in the current series who remains unable to have a compatible PPM inserted because of old attached sensing wires. Current guidelines remain that MRI is not a safe procedure in patients with PPM/ICD given the potential for various interactions which can be life threatening.10
Functional imaging with PET is becoming more common in the evaluation, treatment planning and follow-up of HGGs.11-14 Improvements in radiopharmaceutical tracers, particularly FET-PET have increased the specificity and sensitivity of glioma detection.15-17 FET-PET has also been utilized in radiotherapy planning of HGG. Niyazi et al., reported a small series of 17 patients with GBM with radiotherapy volumes generated with either MRI alone or MRI and FET-PET, showing that FET-PET yielded larger volumes which where geographically in different regions to MRI-based voluming alone for the majority of patients.18 Other small studies have shown similar results with the addition of FET-PET over MRI based target volume acquisition.19, 20 There was no literature available in comparing CT-based radiotherapy voluming and PET.
Our small study shows that FET-PET can be utilized in aiding radiotherapy planning in instances where there is contraindication to MRI use. The four patients who had FET-PET as part of their radiotherapy planning had a mean increase in their CTV by 31.6% (range 2.9–79.9%) and a mean conformity index of 0.78. Although this increase in CTV to BTV did not prove to be statistically significant (P = 0.068), likely due to the small sample size, all four patients did have a quantitative increase in their BTV. This shows that FET-PET not only yields a potentially greater target volume but also highlights areas not covered by the original CT-based plan. Two of the four patients had FET-PET avid disease outside the PTV volumed by CT alone and one patient not having FET-PET based planning had a marginal recurrence shortly after completion of radiotherapy. This suggests that half of our patient population had a potential geographical miss with CT alone based radiotherapy planning. Although other studies have showed similar results with addition of FET-PET to voluming over MRI, the limitation of our study is the small numbers which makes our results somewhat difficult to interpret.
In conclusion, FET-PET is a useful tool in aiding CT-based voluming in patients who have clear contraindications to MRI and could be used in aiding radiotherapy planning and follow-up.




