Efficacy of flattening-filter-free beam in stereotactic body radiation therapy planning and treatment: A systematic review with meta-analysis
Summary
A linear accelerator with the flattening-filter removed generates a non-uniform dose profile beam. We aimed to analyse and compare plan quality and treatment time between flattened beam (FB) and flattening-filter-free (FFF) beam to assess the efficacy of FFF beam for stereotactic body radiation therapy (SBRT). The search strategy was based around 3 concepts; radiation therapy, flattening-filter-free and treatment delivery. The years searched were restricted from 2010 to date of review (October 2015). All plan quality comparisons were between FFF and FB plans from the same data sets. We identified 210 potential studies based on the three searched concepts. All articles were screened by two authors for title and abstract and by three authors for full text. Ten studies met the eligibility criteria. Plan quality was evaluated using conformity index (CI), heterogeneity index (HI) and gradient index (GI). Dose to organs-at-risk (OAR) and healthy tissues were compared. Differences between beam-on-time (BOT) and treatment time (T × T) were also analysed. Normalized percentage ratios of CI and HI demonstrated no clinical differences among the studied articles. GI displayed small variations between the articles favouring FFF beam. The BOT with FFF is substantially reduced, and appears to impact the frequency of intra-fraction imaging which, in turn, affects total treatment time. Based on planning tumour volume (PTV) coverage, dose to OAR and healthy tissue sparing, FFF beam is clinically effective for the treatment of cancer patients using SBRT. We recommend the use of FFF beam for SBRT based on these factors and the reported overall treatment time reduction.
Introduction
Although surgery is the mainstay of treatment for many cancers (primary or metastatic)1-3 radiation therapy with or without surgery is an effective treatment modality.2, 4-7 In early stage lung cancer, 3-year actuarial local control rates and survivals of 98% and 56%, respectively, can be achieved with radiation therapy.8 Patients who refuse surgery or whose medical co-morbidities precludes surgery are offered radiation therapy, with radiation doses of 55–70 Gy delivered over 4–7 weeks.1 Stereotactic body radiation therapy (SBRT) using intensity-modulated radiation therapy (IMRT) beams to deliver high radiation doses over a short period of time is an established treatment for small solitary lesions as well as small metastatic lesions.4, 9 This hypo- fractionated treatment regime (using daily image guidance) facilitates treatment margin reductions and results in excellent local control rates with minimal toxicity.1, 5, 9, 10
Stereotactic body radiation therapy can be used to deliver single doses of up to 30–34 Gy,11, 12 so relies heavily on precise target tracking, accurate immobilization and sophisticated treatment delivery techniques. This complex planning and treatment mean it can take as long as 90 minutes to immobilize, verify and treat the patient.6, 13 Such long treatment times can be uncomfortable and ultimately increase the risk of intra-fraction motion.14
Volumetric modulated arc therapy (VMAT) allows reduction of SBRT treatment times, but is still limited by the maximum dose rate of a conventional linear accelerator (linac) with a flattened beam (FB). Compensation of the forward-peak bremsstrahlung by a flattening-filter decreases the maximal dose output of a linac from ~2000 to 500 monitor units per minute (MU/min), increasing the treatment time.7 When the flattening-filter is removed, the dose rate can be increased by four times. This substantially shortens beam-on-time (BOT) and makes flattening-filter-free (FFF) beams an attractive alternative for SBRT.15 However, removal of the flattening-filter creates changes in beam dose profile and can affect plan dosimetry.
A FFF beam generates a non-uniform dose profile with lower mean energy in comparison to FB of the same nominal energy. FFF beams have reduced head scatter, leaf transmission, energy variation in the lateral direction and reduced peripheral dose.5, 7, 16 The dose at 20 cm distance from the field edge can on average be reduced by 23–31% for 6 and 10 MV SBRT plans.16 The non-uniformity of the FFF photon fluence can be compensated for by using intensity-modulation planning but it is uncertain whether the distribution is qualitatively equivalent to that of the FB plans.
We compared and analysed the plan quality of a FB and FFF beams across multiple treatment sites. We evaluated the plan quality using conformity index (CI), homogeneity index (HI) and gradient index (GI), and compared differences in healthy tissue sparing and total monitor units required for plan delivery. We analysed the effects of FFF beams on BOT and overall treatment time (T × T).
Methods
Search strategy
The PICO (population, intervention, comparison, outcome) approach was used to assess the eligibility of the included articles. Six databases (Medline, Embase, Pubmed, Cochrane Reviews, CINAHL and Google Scholar) were searched from 2010 until October 2015, as FFF beam is a new concept within radiation therapy. The search strategy was based around three concepts. These were: population- patients who received ‘radiation therapy’, intervention- ‘flattening-filter-free (FFF)’ and outcome- ‘treatment time’. Synonyms within each concept were mapped to medical subject headings (MeSH) or searched as a keyword, and combined with the ‘OR’ Boolean operator. Each concept was then combined with the ‘AND’ Boolean operator to generate a complete list of potentially eligible studies. We searched the published English medical literature.
Eligibility criteria and study selection
The population was restricted to humans receiving SBRT for cancer treatments. To maintain integrity of plan quality comparison, articles without direct comparison between IMRT or VMAT FFF plans and IMRT or VMAT FB plans from the same patient data set were excluded. For treatment time studies, data extracted from two subgroups of previously treated patients were accepted. The outcome of interest was plan quality comparison to assess the efficacy of FFF beam for SBRT planning and treatment. Beam-on-time and treatment time were also analysed. Studies reporting on cell survival and/or toxicity outcomes were excluded. Studies designed for physics experimental studies, case studies, supplementary articles and conference papers were excluded.
The final yield was imported into Endnote X7.4 and the titles and abstracts were screened independently for eligibility by two reviewers (TD and MP). Articles deemed suitable for full text review were imported into Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia. www.covidence.org) for further screening by two reviewers (TD: all papers, MP: A-K, BH: L-Z). Any disagreement was discussed and re-evaluated until a consensus was reached. A manual check of references from included articles together with citation tracking was performed.
Quality assessment
The quality of the included articles was assessed using the Downs and Black 199817 checklist. The checklist was adapted specifically for this review to address nine key areas: scope and purpose, selection criteria, test strategy, external and internal validity, analysis, interpretation and risk of bias. All articles were assessed with respect to these questions and a score given for each positive response. A score of 70% or more was deemed high quality. A summary of the quality assessment was recorded in Covidence and verified by second reviewer (BH).
Data extraction
One author (TD) completed the data extraction (verified by MP). A standardized spreadsheet was used to summarize the key items significant to the scope of the review. Information extracted from included studies included: sample size, study site, study focus and model and manufacturer of treatment planning system and linear accelerators. Results of CI, HI and GI were extracted for plan quality comparison as well as mean dose rate, total monitor units and dose to organs-at-risk (OAR). Studies that focused on T × T analysis had individual BOT, imaging time and overall treatment time extracted where possible. Means and standard deviations were extracted for calculations of effect sizes and meta-analysis. The beam energy of FFF and FB used in the study was noted to ensure comparison between similar energy was reported where possible.
Data synthesis
Plan quality
In addition to plan evaluation based on planning tumour volume (PTV) coverage and OAR dose, other plan quality metrics including the CI, HI and GI were also used to quantify plan quality.
Conformity index is generally defined as the ratio of the total volume enclosed by x% of the prescribed dose to the volume of the PTV enclosed by x% of the prescribed dose (Table S1).4-7
As seen in Table S1, variables adopted by each author utilize similar methods of calculation, with Dzierma et al.18 combining under-dose ratio (UR) with the Paddick overdose ratio (OR) to formulate CI. Zhang et al.19, combined both CI and target coverage (TC) to further assess plan quality. The conformity number (CN) is produced from these two equations, which removes uncertainty of plans that can result in a higher CI, while also having poor TC.
Conformity index value is always greater than unity, and a value closer to unity represents better target conformity.
The HI plan metric assesses the variation of radiation dose received by the PTV and commonly serves as a measure of dose homogeneity within the PTV volume (Table S2).4, 7, 18
Gradient index is universally proposed as the ratio of the volume covered by half the prescription dose to the total volume of the prescription dose, which measures the significance the dose gradient decreases outside the PTV (Table S3).18, 19
Because of the variation in formulas used to study the indices of included articles, direct comparison of CI, HI and GI number were not possible. Despite their differences, most studies agree that a number approaching unity, or closest to unity indicates a better plan based on PTV dose conformity, homogeneity and dose drop-off. The exception was Reggiori et al.4, which indicated that a number approaching 0 is better for HI.
We normalized the indices (to allow for plotting and assess patterns across included articles), and expressed them as percentage ratios. The normalized number is a relative between FB index and FFF index, with FB used as the controlled base line. We determined that a variation of ±2% from the controlled base line represented a clinically insignificant difference in plan quality between the two techniques. A ratio of 1.0 indicated no difference in plan quality based on the calculated indices between the two plans. A number smaller than 1.0 indicated FFF plan was better than FB plan. A percentage ratio greater than 1.0 suggested FB plan was better.
Treatment time
A standardized mean difference (SMD) with 95% confidence interval was used to calculate the effect size (ES) of BOT and T × T where results from two different subgroups were used. Effect size thresholds of 0.2, 0.5 and 0.8 were considered small, medium and large respectively.
Where BOT and T × T were obtained from the same sample, data were converted to a standardized paired difference (SPD), (or repeated measures Cohen's d) with 95% confidence interval using the Comprehensive Meta-analysis Version 2 statistical software package (Biostat Inc., Englewood, NJ, USA) (http://www.meta-analysis.com/).20 Calculation of the SPD with 95% confidence interval required information about the sample size, mean change in FB/FFF, and the standard deviation (SD) of the change. A further requirement was a measure of the pre–post test reliability (r).20-23 Where this was not reported, a conservative estimate of r = 0.5 was used.24 Individual study results were pooled in a meta-analysis (random effects model) where sufficient data were available from two or more studies.
Results
Yield
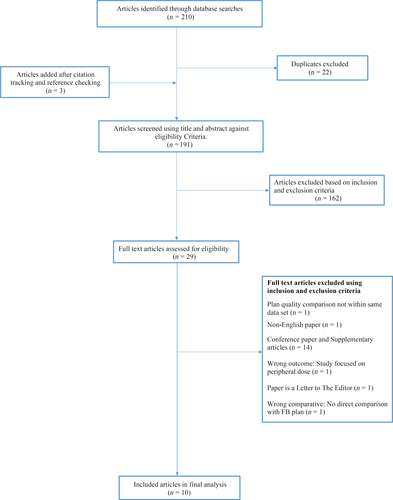
A search of the literature yielded 210 articles, with 22 articles duplicates. Of 191 articles (three articles added from citation tracking) screened by title and abstract, 29 articles were eligible for full text review. After the articles were screened against eligibility criteria, 10 articles remained for final analysis (Fig. 1).
Study quality assessment
Four of ten included articles5, 6, 15, 18 reported actual probability p- values or expressed p- values as <0.001 for the main outcomes investigated by this review. Two articles9, 10 reported using data from different subgroups of previously treated patients for their treatment time analysis. Where the authors did not supply information on how BOT and/or T × T was measured, we assumed BOT was obtained through test runs or during physics quality assurance procedures. For T × T, we assumed the time reported would exclude imaging time, set-up time and intra-fraction down time. Two of ten studies7, 18 cited total treatment time with no information on the method used to obtain this data. Boda-Heggemann et al.10, used previously published data obtained from a single-fraction dose of 12 Gy to compare data with single-fraction doses ranging from 5 to 12 Gy. Only this article scored less than our pre-specified high quality score (70%) using our assessment checklist, so was deemed to be low quality. No study was excluded based on quality assessments.
Study chjmirocteristics
Table S4 lists the statistical data of the indices and treatment time analysis for all the studied cases. Studies which reported total monitor units and plan quality comparison using CI, HI and GI were reported as normalized percentage ratios for easy comparison between studies.
Where mean dose rates were studied, only FFF plans mean dose rates were available to present in the table. We assumed 6 MV FB plan used the common dose rates of 600 MU/sec. Reggiori et al.4, conducted a two phase study using the same data sets in phase 2 to investigate the effects tumour size have on plan quality. The results of the calculated indices from each phase studied are listed in the table but results for phase 2 study (with fictitious volumes) was not included in the meta-analysis to avoid double counting data. One result of BOT is listed as ‘minutes’ rather than effect size, because standard deviation is missing from this study. Overall, the patient numbers were small (range 8–99, mean = 23.4) but a wide range of treatment sites were studied.
Plan quality
We found tumour coverage and dose to OAR compjmiroble between FB and FFF plans with FFF plans having marginally higher maximum (p > 0.05) and mean PTV doses (Lung: p = 0.004; Spine p = 0.348). See Table S5.
Dzierma et al.18 did not report dose to OAR but recorded percentage improvement in organs-at-risk sparing by 7 MV FFF plan as evaluated by the standardized values Smax and Smean with a 5% level of significance. There were four variations to the CI formulas used while HI and GI were each derived from three different formulas. Figure 2 show CI and HI's normalized percentage ratios evenly distributed favouring neither FB nor FFF plan. GI on the other hand demonstrated a shift in the normalized percentage ratios favouring FFF beams.
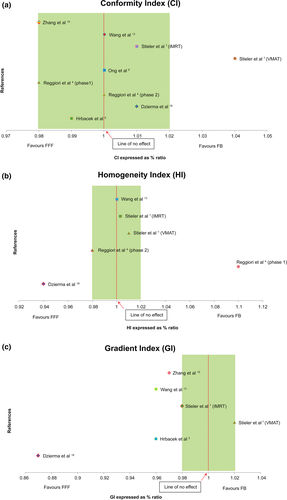
 ±2% band: Plans compjmiroble, no clinical differences.
±2% band: Plans compjmiroble, no clinical differences.Two articles found PTV size affected indices with larger PTV compromising CI and GI in FFF plans.4, 5 Lang et al., however, reported no effect between tumour size and plan quality15 with Ong et al., also found FFF to be suitable for PTV lengths of more than 10 cm and volume >200 cm.3, 6
Visually, there were minimal and insignificant differences in the dose distribution between the two plans with FFF plans exhibiting slightly larger areas of high dose regions. We found most (6/9) VMAT FFF plans recorded higher total MUs compared to FB plans, with FB mean total MU = 2919 and FFF mean total MU = 3065.
Treatment time analysis
Figures 3 and 4 show the results of standardized paired difference for BOT and T × T where data were collected from same sample group. Hrbacek et al.5 re-planned 6 MV FFF plans with 10 MV beams to compare effects beam energy have on BOT. The data from this substudy was also not included in the meta-analysis.
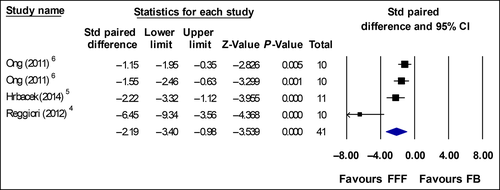
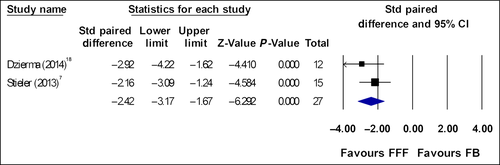
Prendergast et al. and Boda-Heggemann et al., analysed time using data from two different subgroups. Prendergast BOT effect size was: ES = −1.35 (p < 0.01) with 95% confidence interval −0.89 to −1.79. Treatment time ES = −1.73 (p < 0.01) with 95% confidence interval −1.25 to −2.19. Boda-Heggemann et al. T × T ES = −1.57 (p = 0.08) with 95% confidence interval −0.42 to −2.57, both studies showed significance time reduction favouring FFF beams.9, 10
Eight articles which studied time reported large and significantly faster BOT and T × T for FFF plans compared with FB plans. The planning doses across these eight studies were 5–25 Gy per fraction. Four studies with fraction doses of 10–25 Gy yielded ES or SPD of >2.0. Studies with fraction doses ranging from 5 to 18 Gy have overall ES and SPD between −1.1 and −1.5. When combined in a meta-analysis across 63 participants, there was a large and significant reduction in BOT and T × T with overall ES ~2.0. Mean BOT across the three studies for FB is 6.39 min and for FFF is 2.42 min. Mean T × T across the two studies are 11.21 min and 5.79 min for FB and FFF respectively.
Discussion
We aimed to investigate the efficacy of FFF beam for the planning and treatment of SBRT for primary and metastatic lesions. Because of SBRT's sophisticated treatment delivery techniques involving multiple co-planar and non-co-planar small fields/arcs, highly accurate tumour tracking and/or on-line image guidance is an important requirement. To achieve delivery of this highly accurate treatment technique, SBRT can take up to 90 minutes.6, 13 When breath-hold techniques are added (to treat non-small cell lung cancer (NSCLC)), SBRT treatment time could potentially increase because it is dependent on breath-hold duration. The faster dose rates of the FFF mode of a linear accelerator are attractive in comparison with FB because intra-fraction duration can be reduced by an average of 50%.4, 5, 7, 9, 10, 13, 18
When the time difference between 6 MV FFF and 10 MV FFF is compared, using 10 MV FFF for a single-fraction dose 7.5–10 Gy does not result in time advantage because of the limits of gantry rotational speed.6, 15 A mean time reduction of 50% (from raw data) was consistent throughout studies with single-fraction dose regimes between 6–25 Gy.4-7, 9, 13, 18 Bode-Heggeman et al.10, reported time reduction of approximately 40% with smaller fraction size. Lang et al., found 6 MV FFF only saves time if more than 1000 MU (~4 Gy) is needed to deliver the plan and 10 MV FFF only saves time if more than 2000 MU (10 Gy) is needed.15 To take advantage of this time reduction, sliding window IMRT and VMAT plans with faster MLC leaf speeds would be desirable. Lechner et al.25, found a reduction in T × T for IMRT when FFF is used. This was not seen with VMAT because the leaf speed of their conventional linac is 2 cm/s.
There were minimal differences in the CI and HI percentage ratios between the two techniques, the slight improvement in CI for the FFF plans was commonly associated with slightly higher maximum PTV doses (reported in all studies). For GI, there was improvement with FFF plans because the lower mean energy of a non-flattened beam produced less head scatter, leaf leakage and overall scattered radiation, resulting in faster dose drop-off outside the PTV.16
Although reduced peripheral dose in FFF beam would imply reduce dose to OAR (sparing healthy tissues) this is dependent on proximity of the OAR to tumour location so was not observed in three studies.4-6 Reggiori et al., Hrbacek et al. and Ong et al., performed plans on liver metastases, lung and lung/spine respectively. Maximum spinal cord dose and maximum heart dose for these three studies were slightly higher for FFF beams. This is consistent with the slightly higher maximum PTV doses for these three studies with FFF beams.4-6
Some have suggested that the lower mean energy beam (softer spectrum) of the FFF beam may mean a slightly higher integral dose to healthy tissue and might increase surface dose,26 surface dose in FB is increased with increased field sizes, and thus, it can be surmised that FFF beams, although produces a higher surface dose for small and medium field sizes compared to FB, is similar or even lower for larger field sizes.26
To minimize the effects of the softer spectrum, higher energies, 8 MV could be used in preference to 6 MV. Beam tuning of the FFF beam to produce the same percentage depth dose (PDD) as FB also minimizes this effect.
Target/organ motion is an inherent problem for all target dose variations. The interplay effect caused by the interplay between the motion of the target and the motion of the radiation beam defined by the multi-leaf collimator (MLC) aperture can complicate target dose accuracy. While the interplay effect in IMRT/VMAT technique is likely to average out over a large number of fractions,27 it is a concern when SBRT is used in conjunction with IMRT/VMAT techniques.
Stambaugh et al.28, suggested the interplay effect is negligible (<0.2%). It is also small (0.9% average, 2.2% maximum) when the target excursion is artificially increased to 2–3 cm. The interplay effect is significantly lower for the three and five fraction simulations28 when compared to single-fraction SBRT.
Under phantom conditions, Ong et al.29, found single-arc and single-fraction 2400 MU/min FFF RapidArc lung SBRT susceptible to interplay, while two arcs and ≥2 fractions, the effect is reduced to a level unlikely to be clinically significant.
Elekta linacs equipped with FFF beams are installed with Agility MLC which allows leaf speed of 6.5 cm/sec. Varian systems based both on design and physical mechanic variations result in decreased movement speed in comparison with Elekta linacs.7, 10 Although the nominal dose rate of FFF linac is 1200 MU/min and 2400 MU/min for 6 MV FFF and 10 MV FFF, respectively, this potential maximum value is not often achieved due to beam modulation and/or gantry rotational speed.26
Because of the variety in treatment techniques delivered with and without fluence modulation, the increased dose rate of a FFF beam does not necessarily directly translate into shorter treatment times if FFF is used for non-SABR techniques.26
One study reported time for the individual components that make up the overall treatment time. They found the overall treatment time was substantially less mainly because of the reduction in intra-fraction ‘down time’.9 Intra-fraction imaging was only ordered at the discretion of the treating radiation oncologist if there was concern regarding intra-fraction motion. CT was used three times more often in the conventional cohort than in the FFF cohort (25% vs. 84%), possibly because the clinicians assumed that shorter treatment times resulted in less intra-fraction motion.9 Although intra-fraction motion was not directly measured in this study, existing data support this assumption.
The dose comparison between FB and FFF plans for OAR (not in close proximity to PTV) and healthy tissues showed a slight reduction for FFF plans (not clinically insignificant; Table S5).
Only GI showed a marginal improvement in percentage ratios favouring FFF plans. Although the slight improvement in healthy tissue dose was minimal, it was significant as most studies (4 of 5) reported the same outcome. We found numerical improvement across the five articles but no clinically meaningful improvement in our stated endpoints.
Use of very high dose rates requires a stringent patient monitoring protocol during beam delivery because a maximum dose of 1 Gy can be delivered in 2.5 sec.6 The extent of dosimetric error caused by positional shifts during RapidArc FFF can be substantial and warrants further investigation. Another clinical concern that has also been raised is the potential increase in toxicity when using FFF beams with high dose rates. However, first clinical studies in patients treated with SBRT did not report unexpected toxicity.30 SBRT treatment volumes are commonly small and toxicity is usually mild. Increase in toxicity with SBRT treatments using FFF beams would therefore seem unlikely.15
Conclusion
We have shown the efficacy of intensity-modulated FFF beam on the planning for SBRT through improved dose to OAR and healthy tissue sparing. PTV coverage between the two plans was compjmiroble. Normalized percentage ratios of CI and HI showed negligible differences between FB and FFF beam, while GI showed some improvement favouring FFF beam. The plan quality of FFF beams was compjmiroble across multiple treatment sites. The BOT in FFF modality is substantially reduced, and appears to impact on the frequency of intra-fraction imaging. We recommend the use of FFF beam for the planning and treatment of SBRT because FFF plans are compjmiroble to FB plans, the faster BOT of FFF beam can minimize intra-fraction organ motion, reduce patient breath-hold time (lung SBRT) and overall treatment time.
Acknowledgements
The authors acknowledge Cathy Hargraves, Scott Jones and Anita Harriman for their insights and technical support.




