Are there systematic barriers to participation in cancer treatment trials by Aboriginal and Torres Strait Islander cancer patients in Australia?
The authors have stated the following conflict of interest: Gail Garvey is a member of the Cancer Australia Research and Data Advisory Group.
Abstract
Objective: To identify factors that may systematically reduce opportunities for Aboriginal and Torres Strait Islander Australians to participate in cancer clinical trials.
Methods: Analysis of online documents from the Australia and New Zealand Clinical Trials Registry for cancer treatment trials (Phase 3, 4 or Not Applicable) with at least one Australian site, registered in 2014–2018.
Results: Among 365 eligible trials, most (89%) had sites only in major cities/inner regional areas, but 39% of Aboriginal and Torres Strait Islander Australians live outside these areas. Seven cancer types accounted for 58% of cancers among Aboriginal and Torres Strait Islander people, but only 46% of trials addressed these cancers. Most trials specified exclusions relating to comorbidities/health status. A substantial minority of trials (38%) explicitly referred to investigator opinion/judgment as a relevant determinant of patient eligibility.
Conclusion: Aboriginal and Torres Strait Islander patients appear to have a reduced opportunity to participate in trials because of where they live, their type of cancer and their general health status, as well as for less transparent reasons relating to investigator judgment.
Implications for public health: Greater transparency and greater scrutiny of barriers to trial participation for Aboriginal and Torres Strait Islander Australians are needed to ensure equitable access.
Clinical trials are increasingly recognised as having an integral role in the Australian healthcare system.1 There have been several recent initiatives aimed at improving Australia’s competitiveness in attracting trials by reducing start-up times, maximising patient recruitment and retention and improving governance.2-7 Funding for clinical trials has also increased; for example, the Commonwealth Government’s Medical Research Future Fund has allocated $614.2m in funding to increase clinical trials activity over ten years.8
Cancer accounted for the largest number of registered clinical trials in Australia between 2006 and 2015, with more than 1,870 trials (nearly one in five trials overall).9 Access to clinical trials has been recognised as important for cancer patients.8 For individual patients, trial participation provides an opportunity to receive the latest treatments and the best possible care.10 At the cancer population level, clinical trial participation is positively associated with increased survival and decreased mortality, and greater participation can accelerate advances in treatment.10
Cancer is the second leading cause of death for Aboriginal and Torres Strait Islander Australians,11 and outcomes are poorer for Aboriginal and Torres Strait Islander people who get cancer.12 The Optimal Care Pathway for Aboriginal and Torres Strait Islander People with Cancer13 report and the National Clinical Trials Governance Framework5 explicitly recognise the need to ensure equitable access to trials for Aboriginal and Torres Strait Islander Australians.
Little is known about the current levels of trial participation by Aboriginal and Torres Strait Islander people and whether there are systematic barriers reducing their opportunity to participate. Previous research, largely in the US, has highlighted the importance of study design factors, including stringent inclusion and exclusion criteria, in reducing opportunities for trial participation by members of particular population groups.14-16
The aim of this study was to describe, based on freely available information, the current clinical trial landscape as it relates to Aboriginal and Torres Strait Islander participation, with a specific focus on identifying areas requiring more substantial investigation. Publicly available, on-line documentation from the Australia and New Zealand Clinical Trials Registry (ANZCTR; www.anzctr.org.au) was examined for clinical trials relating to cancer treatment to assess the evidence for systematic barriers to participation by Aboriginal and Torres Strait Islander patients. We aimed to identify any factors that could systematically exclude Aboriginal and Torres Strait Islander patients, such as comorbidities, smoking, lack of English proficiency, geography and cancer type. As the ANZCTR does not include information about the Aboriginal and Torres Strait Islander status of participants in registered trials, it was not possible to examine participation directly.
Methods
Data source
- Registry = ANZCTR or ClinicalTrials.gov;
- Country of recruitment = Australia (+/- other countries);
- Condition category = Cancer;
- Study type = Interventional;
- Intervention code = Treatment: Drugs or Treatment: Surgery or Treatment: Devices or Treatment: Other;
- Ethics application status = Approved;
- Healthy volunteers = No;
- Phase = 3 or 4 or 3/4 or Not Applicable (N/A); and
- Registration date range = 1/1/2014 to 31/12/2018.
The search was conducted and records accessed and downloaded on 6 and 7 March 2019. No ethics approval was required for this project as no human participants were involved.
Trials specified as being Phase 0, 1 or 2 were excluded because the potential benefit to the patient is much less clear in these earlier phases. Phase 3 trials involve a comparison of the risks and benefits of a new versus standard treatment, while Phase 4 trials investigate longer-term risks and benefits. The label Phase N/A is intended to be used for non-drug trials.
Data abstraction
Relevant information was abstracted from the trial documentation and recorded on a paper form, then entered onto an Excel spreadsheet. Data abstracted from the on-line protocol included: trial registration number; title; phase; main condition studied (type of cancer); intervention code; age group; sex; main inclusion and exclusion criteria (e.g. comorbidities, smoking status, language); location of sites (hospital locations; states/territories); target sample size; and primary funding source. The extent of detail in the documentation varied from trial to trial. Analysis is based on the information as it was recorded in the online record.
Variables of interest
Key variables in the analysis included phase, location of trial site(s), cancer type, treatment type(s), target sample size, and selected inclusion and exclusion criteria.
Remoteness category for all listed sites was determined by visually inspecting Australian Bureau of Statistics (ABS) Maps using overlays for 2016 Remoteness Area and 2016 Local Government Area.17 Sites were categorised as major cities, inner regional, outer regional, remote or very remote. Trials without a specific site listed (some of which are internet-based) were excluded from geographic analysis and proportions shown are based on the number of non-missing values.
Treatment type was coded as Drugs, Surgery, Device and/or Other. The ‘Other’ treatment category includes a variety of interventions, from radiotherapy to exercise and diet, psychotherapy, models of care, stem cell transplants and more. Trials could include more than one treatment type.
Target sample size referred to all sites, not just Australian sites. For studies for which target sample size was not specified (e.g. because recruitment had been completed), final sample size was used if available.
Coding of selected inclusion and exclusion criteria
Selected inclusion and exclusion criteria were coded using a combination of pre-specified categories for language proficiency, smoking status and selected comorbidities, along with free-text response for other criteria of potential interest. Specified comorbidity categories included cardiovascular, respiratory, liver and renal disease/dysfunction and diabetes. Inadequate organ function was coded to the relevant organ if it was named (e.g. inadequate liver function) or if specific laboratory values were given (e.g. serum creatinine not within a specified range); if not, it was recorded as ‘inadequate organ function (not specified)’. Performance status, a measure of physical functioning in daily living activities, was coded as positive if any range was specified, regardless of the specific measurement tool used.
Following initial coding, free-text entries were reviewed to identify any other exclusions of interest. Three relatively common categories were identified, including: 1) a statement indicating that ‘other inclusion/exclusion criteria may apply’; 2) exclusions based on a combination of psychological and/or psychiatric conditions and/or substance abuse history, generally in the context of a patient’s ability to comply with the study protocol; and 3) a statement indicating that the judgment or opinion of the investigator should be used in determining eligibility, generally expressed in terms of potential risk relating to one or more of patient compliance, study aims and/or patient safety. All trial documents were subsequently reviewed and coded as positive or negative on each of these three criteria.
Comparison data on population distribution and cancer incidence
The location of trial sites and the types of cancers studied in included trials were compared with Australian data on population distribution and cancer incidence. Population distribution by remoteness category was based on ABS estimates for June 2016.18 Data on the most common cancer types diagnosed in Aboriginal and Torres Strait Islander Australians in 2009–2013 were from the Australian Institute of Health and Welfare.12
Data analysis
Phase 3 and Phase 4 trials were combined in all analyses due to small numbers of Phase 4 trials. Simple descriptive statistics were calculated using Stata V13 (Stata Corporation, College Station, TX). The comparison of Phase 3/4 versus Phase N/A trials was conducted using χ2 tests for categorical variables and Wilcoxon Rank-sum tests for continuous variables.
Results
A total of 424 potentially eligible records was identified. Of these, 59 trials (14%) were excluded for the following reasons: no Australian sites listed (n=3); trial withdrawn (n=3); ‘rollover’ trial (n=2); cancer not the main focus (n=23); stated ‘purpose’ of the intervention related to diagnosis (n=5), prevention (n=4), or education/counselling/training (n=18) rather than treatment; or the trial was aimed at offspring rather than cancer patients (n=1). There remained 365 trials for analysis, including 266 Phase 3 (73%), 11 Phase 4 (3%) and 88 Phase N/A (24%) trials.
Phase 3/4 versus Phase N/A trials
Characteristics of included trials are shown in Table 1. Phase 3/4 and Phase N/A trials were significantly different from one another with respect to the treatments and patients studied, sponsorship, and trial location and size. Most Phase 3/4 trials were relatively large, multi-national, industry-sponsored drug trials, while the majority of Phase N/A trials were smaller, Australian-only, not industry-sponsored, and studied ‘other’ treatments.
All included trialsa (n=365) |
Phase 3/4 (n=277) |
Phase N/A (n=88) |
P-valueb |
||||
|---|---|---|---|---|---|---|---|
No. |
% |
No. |
% |
No. |
% |
||
Location |
<0.001 |
||||||
Australia only |
98 |
27 |
21 |
8 |
77 |
88 |
|
Australia and other countries |
267 |
73 |
256 |
92 |
11 |
12 |
|
Primary sponsor |
<0.001 |
||||||
Industry |
230 |
63 |
222 |
80 |
8 |
9 |
|
Other |
135 |
37 |
55 |
20 |
80 |
91 |
|
Sex |
<0.001 |
||||||
Males only |
41 |
11 |
23 |
8 |
18 |
20 |
|
Females only |
37 |
10 |
20 |
7 |
17 |
19 |
|
Both males and females |
287 |
79 |
234 |
84 |
53 |
60 |
|
Treatment typec |
|||||||
Drug(s) |
272 |
74 |
269 |
97 |
3 |
3 |
<0.001 |
Surgery |
26 |
7 |
13 |
5 |
13 |
15 |
0.001 |
Device(s) |
31 |
8 |
1 |
<1 |
30 |
34 |
<0.001 |
Otherd |
70 |
19 |
18 |
6.5 |
52 |
59 |
<0.001 |
State/Territory of sitesc |
|||||||
New South Wales |
287 |
79 |
240 |
87 |
47 |
53 |
<0.001 |
Victoria |
264 |
72 |
233 |
84 |
31 |
32 |
<0.001 |
Queensland |
200 |
55 |
169 |
61 |
31 |
35 |
<0.001 |
South Australia |
155 |
42 |
145 |
52 |
10 |
11 |
<0.001 |
Western Australia |
145 |
40 |
124 |
45 |
21 |
24 |
<0.001 |
Tasmania |
44 |
12 |
40 |
14 |
4 |
5 |
0.01 |
Australian Capital Territory |
33 |
9 |
29 |
10 |
4 |
5 |
0.09 |
Northern Territory |
9 |
2 |
7 |
2 |
2 |
2 |
0.89 |
Site locations in Australiae |
|||||||
Major cities only |
245 |
71 |
180 |
66 |
65 |
87 |
0.001 |
Inner regional only |
3 |
1 |
3 |
1 |
0 |
0 |
0.36 |
Outer regional only |
3 |
1 |
1 |
<1 |
2 |
3 |
0.06 |
Major cities and inner regional only |
60 |
17 |
53 |
20 |
7 |
9 |
0.04 |
Major cities and outer regional only |
15 |
4 |
15 |
6 |
0 |
0 |
0.04 |
Inner regional and outer regional only |
0 |
0 |
0 |
0 |
0 |
0 |
--- |
|
Major cities, inner regional and outer regional |
21 |
6 |
20 |
7 |
1 |
1 |
0.05 |
Range |
Median (IQRf) |
Range |
Median (IQRf) |
Range |
Median (IQRf) |
P-valueb |
|
Target sample sizeg |
6-5,956 |
440 (149-706) |
13-5,956 |
544 (303-780) |
6-1,900 |
50 (27.5-143) |
<0.001 |
Number of Australian sitesh |
1-43 |
5 (3-8) |
1-43 |
6 (4-9) |
1-25 |
2 (1-3) |
<0.001 |
- Notes:
- a: Includes Phase 3, 4 and Phase N/A trials, registered on ANZCTR (or ClinicalTrials.gov and linked to ANZCTR) between 1/1/2014 and 31/12/2018.
- b: For comparison of Phase 3/4 versus Phase N/A, using χ2 test for categorical variables and Wilcoxon Rank-sum test for continuous variables.
- c: Categories are not mutually exclusive. Trials could have more than one treatment type and/or be in more than one State/Territory and percentages may therefore add up to more than 100%. Percentages for State/Territory are based on the number of trials for which sites were specified (n=347).
- d: Includes a wide range of other interventions, including (but not limited to) radiotherapy, exercise and diet, psychotherapy, models of care and stem cell transplants.
- e: Categories are mutually exclusive. Percentages are based on the number of trials for which sites were specified (n=347). There were no trial sites in Remote or Very Remote areas.
- f: IQR, Interquartile range.
- g: Final sample size was used if target sample size was not specified.
- h: Excludes trials for which specific sites were not specified (n=18).
Location and size of trial sites
Eighteen trials did not include information about specific sites. Of the 347 trials for which site information was available, New South Wales had the largest number of trials, followed by Victoria and Queensland (Table 1). The majority of trials had sites in major cities only (71%), and there were no trial sites in remote or very remote areas.
The geographic distribution of trial sites differed considerably from the distribution of the Aboriginal and Torres Strait Islander population by remoteness category (Figure 1). While the majority of Aboriginal and Torres Strait Islander Australians live in major cities and inner regional areas (61%), a substantial minority (19%) live in remote and very remote areas.
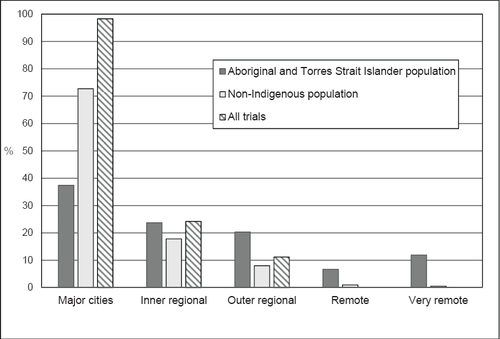
Population distribution and trial sites by remoteness area.
Types of cancer studied
Figure 2 shows the types of cancers studied in Phase 3/4 and Phase N/A trials. Lung cancer and leukaemia were the most common in Phase 3/4 trials, while trials relating to breast cancer, prostate cancer or any cancer were the most common in Phase N/A trials.
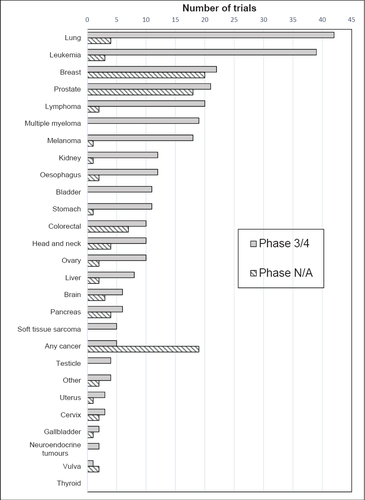
Number of cancer treatment trials by cancer type: Phase 3/4 vs Phase N/A, trials registered in 2014-2018.
There was a gap between the cancers studied in Phase 3/4 trials and the most common cancers in the Aboriginal and Torres Strait Islander population (Table 2). The seven most common cancers (lung, breast, colorectal, prostate, head & neck, uterine and liver) accounted for 58% of new cases among Aboriginal and Torres Strait Islander people in 2009–2013,12 compared with 46% of Phase 3/4 trials in 2014–18. By contrast, 60% of Phase N/A trials addressed the top seven Aboriginal and Torres Strait Islander cancers, although this was largely due to trials relating to breast and prostate cancer.
Cancer type |
Aboriginal and Torres Strait Islander population |
All trials |
Phase 3/4 |
Phase N/A |
|---|---|---|---|---|
% of new casesa |
% of trials |
% of trials |
% of trials |
|
1. Lung |
14.8 |
12.6 |
15.2 |
4.6 |
2. Breast (female) |
12.1 |
11.5 |
7.9 |
22.7 |
3. Colorectal |
9.6 |
4.7 |
3.6 |
8.0 |
4. Prostate |
8.2 |
10.7 |
7.6 |
20.4 |
5. Head and neck |
6.5 |
3.8 |
3.6 |
4.6 |
6. Uterine |
3.5 |
1.1 |
1.1 |
1.1 |
7. Liver |
3.5 |
2.7 |
2.9 |
2.3 |
Top 5 cancers combined |
51.1 |
42.5 |
37.6 |
58.0 |
Top 7 cancers combined |
57.9 |
45.8 |
41.2 |
60.2 |
- Note:
- a Source: AIHW 2018.12
Trial exclusion criteria
Exclusions based on comorbidities and/or health status were commonly specified in trial documentation, especially for Phase 3/4 trials (Table 3). For example, exclusion for one or more of the following comorbidities was specified in 74% of Phase 3/4 trials and 32% of Phase N/A trials: cardiovascular, renal, liver or respiratory disease/dysfunction or diabetes. Language proficiency was more likely to be stated as a requirement in Phase N/A trials, especially those involving treatment with psychotherapy. Very few trials specified exclusion on the basis of smoking status. About one in eight Phase 3/4 trials (12%) included a statement to indicate that ‘other criteria may apply’; that is, the documentation provided online did not include all relevant information.
Type of exclusion |
Phase 3/4 trials (n=277) |
Phase N/A trials (n=88) |
P-valuea |
||
|---|---|---|---|---|---|
No. |
% |
No. |
% |
||
Comorbidities/health status |
|||||
Diabetes |
22 |
7.9 |
6 |
6.8 |
0.73 |
Cardiovascular diseaseb |
147 |
53.1 |
19 |
21.6 |
<0.001 |
Renal diseaseb |
119 |
43.0 |
9 |
10.2 |
<0.001 |
Respiratory diseaseb |
68 |
24.6 |
11 |
12.5 |
0.02 |
Liver diseaseb |
175 |
63.2 |
7 |
8.0 |
<0.001 |
Any of the above |
205 |
74.0 |
28 |
31.8 |
<0.001 |
Above plus inadequate organ functionc |
214 |
77.3 |
29 |
33.0 |
<0.001 |
Above plus inadequate performance statusd |
245 |
88.4 |
49 |
55.7 |
<0.001 |
Other factors |
|||||
Language |
6 |
2.2 |
26 |
29.6 |
<0.001 |
Smoking status/history |
3 |
1.1 |
2 |
2.3 |
0.40 |
‘Other criteria may apply’ |
33 |
11.9 |
0 |
0.0 |
0.001 |
Psychological/psychiatric conditions and/or substance abusee |
66 |
23.8 |
20 |
22.7 |
0.83 |
Investigator judgmentf |
106 |
38.3 |
31 |
35.2 |
0.61 |
- Notes:
- a: P-value for comparison based on χ2 test.
- b: Includes inadequate organ function based on specified values
- c: Organ(s) and/or cut-off values not specified
- d: Any exclusion based on a specified performance status score.
- e: Includes any statement with explicit mention of psychological and/or psychiatric conditions and/or substance abuse history. This was generally in the context of a patient’s ability to comply with the study protocol.
- f: Includes any statement explicitly indicating that the judgment or opinion of the investigator should be used in determining eligibility. This was generally expressed in terms of potential risk relating to one or more of: patient compliance; study aims; and/or patient safety.
Nearly one in five trials included an exclusion relating to psychological and/or psychiatric conditions and/or a history of substance abuse, with similar proportions for Phase 3/4 (24%) and Phase N/A trials (23%). A substantial minority of trials (38% of Phase 3/4 and 35% of Phase N/A) included a statement to indicate that investigator judgment or opinion should be used in deciding on patient eligibility.
Discussion
This analysis of publicly available, online documents relating to Phase 3/4 and Phase N/A trials registered in 2014–2018 has identified factors that could systematically exclude Aboriginal and Torres Strait Islander patients from cancer treatment trials. In addition to a mismatch with respect to both trial location and cancer types studied, exclusion based on comorbidities was common. There were also substantial risks of exclusion based on implicit criteria, such as investigator judgment/opinion.
There was a mismatch between where trials are run and where Aboriginal and Torres Strait Islander people live, and this was the case for both Phase 3/4 and Phase N/A trials. Many Aboriginal and Torres Strait Islander people with cancer need to travel to major cities and regional areas to access treatment, so they may be treated in places where there are trials running. However, many trials involve follow-up visits that would make it difficult if not impossible for them to participate. Several trials were internet-based studies with no specific sites listed. While in theory, such trials could increase participation by people who live away from trial centres, in practice the benefits are likely to be limited by lower levels of access to and affordability of digital technology for Aboriginal and Torres Strait Islander Australians, particularly, but not exclusively, in remote areas.19 The lack of trial sites in remote areas is not surprising, given the level of infrastructure, resources, expertise and critical mass needed to run trials. The development of tele-trials may eventually enable more patients from remote areas to access trials run by established clinical trial units in urban areas,20 but distance remains an important challenge.
There was also a mismatch between the types of cancers studied and the most common cancers among Aboriginal and Torres Strait Islander people, especially in Phase 3/4 trials.
Although there was a somewhat better match in terms of cancer types for Phase N/A trials overall, the emphasis on breast and prostate cancer and the nature of Phase N/A interventions mean that other common cancer types are likely to be under-studied, especially those with low survival and/or late presentation, such as lung cancer. Importantly, because most trials included restrictions relating to the specific tumour type, prior treatment/response to previous treatment, time since diagnosis, etc., a patient could have the type of cancer being investigated (e.g. breast cancer), but still not be eligible to participate in a given trial.
Exclusions based on comorbidities and/or inadequate organ function were very common, especially in Phase 3/4 trials. This is more likely to have an impact on eligibility for Aboriginal and Torres Strait Islander patients than for other patients, given the higher levels of comorbidity in the Aboriginal and Torres Strait Islander population, both in general11,21 and among cancer patients.22 While such exclusions may be appropriate and necessary to protect people from being exposed to unacceptable risk, it has been argued that in many cases exclusions are overly – and unnecessarily – strict, and that the advantages derived from using narrow eligibility criteria may be outweighed by reduced generalisability of trial results.10 In any case, improvements in treatment are needed for people with comorbidities as well as those without, and the inclusion of a broad range of patients not only improves generalisability but is also necessary to determine the level of diversity in response to treatment.10
Exclusions based on psychological or psychiatric illness or a history of substance abuse were relatively common. While these were usually justified on the basis of increased risk of noncompliance with the study requirements, they were often worded in a way that appeared to leave substantial room for interpretation. Explicit statements regarding investigator judgment were also relatively common, usually in relation to the participant’s ability to comply with the protocol (including follow-up), or having a condition or characteristic that could compromise the aims of the study or increase the risk to the participant. While on one level it makes sense to be sure that the trial fits the person and the person fits the trial, on the other hand, this raises the possibility that some patients (including some Aboriginal and Torres Strait Islander patients) will be seen as being ‘risky’ or ‘too hard’, and therefore excluded, possibly unfairly and/or unnecessarily. Importantly, the growing evidence in the literature of the extent of implicit bias among healthcare providers and its relationship to lower quality of care23-26 suggest that such exclusions could occur even in the absence of conscious decision-making.
Ford and colleagues conceptualised trial participation as a function of barriers and promoters in the areas of: 1) awareness of and about trials; 2) the opportunity to participate; and 3) a person’s acceptance or refusal of that opportunity.14 Many potential barriers to trial participation by ethnic minority groups (mostly in the US) have been identified, but arguably the two most important are barriers relating to opportunity: 1) study design (including rigid inclusion and exclusion criteria and other design aspects such as location, focus, timing and duration);14-16 and 2) ‘gatekeeping’ (whether or not a care provider actually discusses potential trial participation with a given patient).16,27-28 The results reported here suggest that both types of barriers are also relevant for Aboriginal and Torres Strait Islander Australians.
Limitations
The main limitation of this study relates to the completeness and quality of documentation, which is unknown. Analysis is based on the information as it was recorded. Given that one-third of Phase 3/4 studies explicitly indicated that ‘other criteria may apply’, it is likely that the study under-estimates the proportion of studies with specific exclusion criteria. There is also potential for error due to the coding of free-text, particularly with respect to investigator judgment. A positive response was not recorded if there was any ambiguity or doubt, which means that the proportion of studies with exclusion based on investigator judgment may be under-estimated. This study was limited to cancer treatment trials; the extent to which the results apply to other clinical trials is uncertain.
Implications for public health
The changing landscape of clinical trials in Australia6-7 has increased the need for scrutiny of potential – and actual – barriers to trial participation by Aboriginal and Torres Strait Islander Australians. The National Clinical Trials Governance Framework highlights equity as a core principle, and explicitly states the importance of respect and cultural safety to ensure equity for Aboriginal and Torres Strait Islander Australians.5 However, the move to reduce trial start-up times through streamlined ethics and governance approvals1-2 runs counter to the need for greater scrutiny and protection to ensure cultural safety for Aboriginal and Torres Strait Islander trial participants.29 This tension will need to be resolved if equitable access is to be a reality.
Given the paucity of information about the participation of Aboriginal and Torres Strait Islander people in cancer clinical trials, this study was intended to describe the current landscape based on freely available information and to identify areas requiring more substantial investigation. Further research, underpinned by the systematic collection and reporting of Indigenous status of potential and actual trial participants as part of a national minimum data set,30 is needed to understand what is actually occurring in practice and to determine how best to ensure fair access to culturally safe cancer clinical trials for Aboriginal and Torres Strait Islander Australians.
Acknowledgements
This study was supported by the NHMRC-funded Centre of Research Excellence in Targeted Approaches To Improve Cancer Services for Aboriginal and Torres Strait Islander Australians (TACTICS; #1153027). JC was funded by an NHMRC Research Fellowship (#1058244). GG was funded by an NHMRC Investigator Grant (#1176651). The views expressed in this publication are those of the authors and do not necessarily reflect the views of the funder. All authors had full access to all of the data (including statistical reports and tables) in the study.




