The Acoustic Ecology of Coastal Dolphins by Assessing the Structural Variability of Sounds and the Influence of Contextual Factors
ABSTRACT
The acoustic ecology of a previously unexamined dolphin population in the Mediterranean was assessed by investigating how sound emissions and acoustic features are influenced by concurrent conditions. Whistles and click-trains emission rates were compared among different environmental, social and behavioural conditions. Structural variability of 3928 good/high-quality vocalizations was analysed in relation to contexts through a two-stage approach. First, two multivariate MANOVA-models were fitted considering the entire set of acoustic parameters extracted from whistles and click trains, to investigate the effect of concomitant factors on the overall acoustic structure of each vocalization. Subsequently, GLMM models were applied to each acoustic feature individually to explore its response to different contextual factors. Emission rates increased significantly with calves and in larger groups, with also a positive effect of socialization on whistles and of muddy/sandy seabed and depth on impulsive sounds. The multivariate approach showed that all contextual factors influenced sounds’ structure, with whistles being strongly affected by behaviour and calves’ presence. The GLMM models highlighted that each acoustic parameter varied differently in response to specific factors, with (1) increasing trends in whistles’ duration and inflection points during interaction with fishery and decreasing ones during socializing, and (2) decreasing inter-click-intervals and increasing click-repetition-rates in larger groups and during interactions with fishery. These results provide new findings on the acoustic plasticity of bottlenose dolphins and a more comprehensive view of the magnitude of the social, environmental and behavioural influence, highlighting how the complexity of the species’ acoustic repertoire has yet to be unravelled at the local level.
1 Introduction
Sound plays an essential role in the ecology and behaviour of several marine organisms, enabling prey identification and predator avoidance, communication and reproduction, navigation and orientation in the environment (Montgomery and Radford 2017). The characterization of sounds in terms of the acoustic capability of the species and the investigation of the interaction between the species and the habitat as mediated by sound are part of the rapidly growing field of marine bioacoustics (Clark et al. 2011; Montgomery and Radford 2017) and of the emerging field of ecoacoustics (Gage and Farina 2017; Schoeman et al. 2022). Numerous biotic, abiotic and anthropogenic factors can influence, in space and time, the acoustic plasticity of a species (i.e., the variation in the use of the acoustic repertoire and changes/shifts in the spectral/temporal characteristics of specific calls) and the individual acoustic phenotype; together these factors form the acoustic ecology of a given species (Nowacek 2005; Van Opzeeland et al. 2010). Biotic factors, such as the presence of conspecifics, predators or prey, can influence vocalizations (‘vocalization’ is used here to refer to any acoustic call emitted by an individual, without regard to the anatomical feature that produced it) and lead to variations in acoustic activity (Branstetter and Mercado 2006; Sayigh 2014); the abiotic component can influence the acoustic production, for example, when changes in temperature, pH or depth affect the transmission and reception of sounds (Erbe, Duncan, and Vigness-Raposa 2022), ultimately resulting in variations in the source level and acoustic structure of the calls (Miller et al. 2014); the anthropogenic component can affect the expression of acoustic patterns, for instance when underwater noise impacts the soundscapes of the ecosystems (Duarte et al. 2021), elicits acoustic adjustments in some components of the species’ repertoire (Perez-Ortega et al. 2021; Rako-Gospić et al. 2021), produces masking of the species signals and reduces their ability to sense the surrounding environment (Clark et al. 2009; Erbe et al. 2016; Erbe et al. 2022).
Cetaceans are widespread marine predators that feed on organisms at multiple trophic levels, playing a pivotal role in maintaining the structure of an ecological community (Heithaus et al. 2008; Kiszka, Woodstock, and Heithaus 2022) and in the functioning of marine ecosystems (Hocking et al. 2017). They evolved to rely heavily on underwater sounds for all life functions and processes, using them as their primary sensory and communication modality, like so defining their ‘active space’, and the extent and nature of interactions with their surrounding environment to fulfil their ecological role and needs (Burnham and Duffus 2023). Nevertheless, the basis of vocalizations of most cetaceans and the size and composition of their repertoires, as well as the influence of environmental and context factors modulating their expression, remain insufficiently described (Azzolin et al. 2021; Pace, Lanfredi, et al. 2021; Papale, Azzolin, et al. 2021). The common bottlenose dolphin (Tursiops truncatus), probably the most studied cetacean species so far, is a wide-ranging apex predator with a complex acoustic repertoire. Because of its cosmopolitan distribution, ecology (it is a generalist predator feeding on fish, shrimps and squids, usually using foraging techniques that are shaped on habitat type and prey availability; Sargeant and Mann 2009; Berens McCabe et al. 2010; Ridgway et al. 2014) and the relatively easy access in coastal environments, it is often used as model species in numerous acoustic studies (Gannon et al. 2005). Like other odontocetes, its vocalization suite mainly includes whistles and impulsive sounds (Herzing 2000; Luís, Couchinho, and dos Santos 2016, Luís et al. 2021; Jones et al. 2020), but the repertoire also comprises sequences of multi-unit rhythmic signals (Pace, Tumino, et al. 2022) and several other less studied sounds (Jones et al. 2020). Whistles are frequency-modulated (∼2–20 kHz; Madsen et al. 2012; Kriesell et al. 2014) narrowband omnidirectional sounds lasting between 0.1 and 5.4 s (Kaplan and Reiss 2017) used primarily for communication, individual recognition and maintenance of social relationships and cohesion in groups (Janik and Sayigh 2013; MacFarlane et al. 2017). They can be extremely variable in the acoustic characteristics (Oswald et al. 2022) and emission rates (ERs), possibly reflecting differentiation patterns at between-individual, population and species levels (La Manna et al. 2022). Impulsive sounds mainly include echolocation clicks and burst pulses. Typically providing information about the surrounding environment and the detection of potential prey, echolocation clicks are high-frequency (with an accepted reference maximum of 120–150 kHz), directional signals generally emitted in series (click trains) lasting about 0.1 to 3.0 s, characterised by variable inter-click intervals (ICI) depending on the echolocation task, distance to the target and other influencing aspects (Norris et al. 1961; Wood and Evans 1980; Au 1993; Surlykke and Nachtigall 2014; Jones et al. 2020). Burst pulses, often categorised onomatopoeically with relatively little consistency between different studies (Killebrew et al. 2001; Jones et al. 2020), are sequences of clicks with very high repetition rates (RRs; ICI about 0.003–0.006 s) and a clear beginning and end point (Herzing 2000). Their function is still much debated: They seem to play an important role in both feeding events and intraspecific communication, and during ‘arousal’ behaviours such as aggression and alarm (Killebrew et al. 2001; Blomqvist and Amundin 2004; Luís et al. 2021), suggesting the possible functions of encoding group identity and mediating behaviours within the group (Herzing 2014). The different expressions and use of this variegate set of sounds depend on several influencing factors, mainly related to environmental gradients, context and local foraging ecology (Nowacek 2005; Tellechea 2020; Marian et al. 2021). All odontocetes, including common bottlenose dolphins, have high acoustic plasticity, being able to adjust the acoustic parameters of the emitted signals according to different habitat characteristics, social and behavioural settings, geographical sites and anthropogenic pressures (Papale et al. 2015; La Manna et al. 2020, 2022; Gregorietti et al. 2021; Jones et al. 2020; Luís et al. 2021; Perez-Ortega et al. 2021). For example, whistles’ acoustic structure may vary with group behaviour, size and composition (Quick and Janik 2008; Heiler et al. 2016; La Manna et al. 2020), and impulsive sounds arrangements may be adjusted as a function of the surrounding scenario (Finneran 2013; Luís, Couchinho, and dos Santos 2016). However, several knowledge gaps in the extent of the variations and the weight of each influencing factor exist, and possible local peculiarities related to distinct geographical units are still under investigation. Here, we address this issue in a previously unexamined population of common bottlenose dolphins in the central Mediterranean Sea by (1) assessing the call rate and variability of echolocation click trains, burst pulses and whistles recorded during visual sightings of a group of dolphins near the Tiber river estuary (Italy), and (2) considering the overall emission contexts. Although this type of investigation is not new, we examine these different vocalisation types together during any dolphin encounter, applying for the first time a two-stage analytical approach to test whether environmental, social and behavioural factors significantly influence their acoustic structure and production patterns. Multivariate models were first fitted on the entire set of acoustic parameters extracted from each vocalization and subsequently, linear models were fitted on each acoustic feature individually, to investigate the effect of concomitant factors on both the overall acoustic structure of each vocalisation and the response of each acoustic parameter. Our aim is to understand whether the complex interplay of local environmental, social and behavioural components, rather than each alone, acts as a driving force in influencing the emission rates and shaping the vocalisations’ structure of the population.
2 Materials and Methods
2.1 Study Area
The study area is located in the Tyrrhenian Sea and covers about 1300 km2; it includes the estuary of the Tiber River, which flows into the sea through the two mouths of Fiumara Grande and Fiumicino (Figure 1). The seabed of the region is mainly constituted by soft sediments (muddy and/or sandy), due to the large volumes of sedimentary inputs from the Tiber River, but it also embraces coralligenous outcrops and seagrass meadows (Posidonia oceanica) (Casoli et al. 2020; Ventura et al. 2022). Spilling significant amounts of sediments, waste and pollutants, the waters of the Tiber River cause high-level of turbidity, contributing to enriching the surrounding area with organic material and favouring the development of a diverse marine community (Ardizzone, Belluscio, and Criscoli 2018). Since the 1960s, at about 5.5 km off the Tiber River mouths, there are two crude oil and petroleum products unloading structures (single point mooring [SPM] called R1 and R2), and south of the mouths, at about 12 km off the coastline, is located the fully submerged MPA ‘Secche di Tor Paterno’. Within a radius of 750 m around SPMs, navigation, anchoring, diving and fishing are prohibited, thus providing a protected habitat for the aggregation of demersal and pelagic species, which are exploited by common bottlenose dolphin groups (Pace, Di Marco, et al. 2021; Triossi, Willis, and Pace 2013). Some specific activities are permitted in the MPA (diving through authorised centres for a limited number of visitors per day; authorised recreational fishing from small boats beyond 45 m depth; authorised small-scale artisanal fishing under strict restrictions); transit, anchoring and freediving are not allowed.
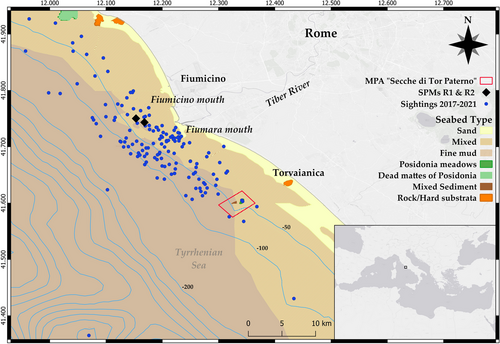
Over the past 10 years, the species have been regularly recorded in the study area (Caruso et al. 2024; Martino et al. 2021; Pace et al. 2019, Pace, Di Marco, et al. 2021, Pace, Ferri, et al. 2022, Pace, Panunzi, et al. 2022), noticing the constant presence of females with their offspring (Pedrazzi, Giacomini, and Pace 2022). The area is affected by multiple anthropogenic activities such as tourism, ship traffic (small-to-large pleasure boats) and recreational, artisanal and commercial fishing (Ardizzone, Belluscio, and Criscoli 2018). Common bottlenose dolphins have often been observed interacting with these fisheries, particularly trawling (Pace et al. 2019, Pace, Ferri, et al. 2022).
2.2 Data Collection
Acoustic and visual data on focal groups of bottlenose dolphins were collected in the Tiber River estuary area during boat-based daily surveys. On-board a sailing vessel Benetau Oceanis 41.1, the sampling effort consisted of a 5-month period (between June and October each year), to maximize suitable conditions for navigation and searching) over 5 years (2017–2021). An adaptive sampling method (Martino et al. 2021) was employed to establish survey routes, considering sea state and bathymetry. Sampling days were chosen according to wind speed and wave height, by consulting the weather and sea forecasts in advance. This way, surveys were conducted only in favourable conditions (i.e., sea state ≤3 Douglas, wind force ≤3 Beaufort, no rain, no fog).
A detailed description of boat-based daily surveys and data collection procedures is reported in Pace, Di Marco, et al. (2021), Pace, Ferri, et al. (2022), Pace, Panunzi, et al. (2022). Observations were performed by at least three operators using 7 × 50 binoculars and the naked eye, at a steady speed of 4–6 knots. On many occasions, fishing trawlers operating in the area were detected, identified through AIS vessel tracking apps, and then followed to eventually spot interacting individuals/groups. In the case of sighting of a group of dolphins, animals were slowly approached by reducing the speed to 1–2 knots and then followed in parallel to collect pictures for photo-identification and behavioural purposes, minimise disturbance and avoid causing alterations in their behaviour.
When a group of bottlenose dolphins was sighted, several variables (including potential factors influencing vocalizations’ acoustic structure) were recorded in real-time or estimated later via photographs analysis. These included group size, age class composition (based on the classification followed in Pace, Di Marco, et al. (2021)) and consequently presence/absence of calves in the group, predominant behaviour (i.e., behavioural state in which more than 50% of the animals are involved; Mann 1999), interaction with fishing gears/vessels (trawl nets or gillnets/pots), seafloor depth and seabed type. Simultaneously, passive acoustic monitoring was conducted. Acoustic recordings were collected by using (1) omnidirectional hydrophones, that is, two Colmar GP0280 provided by CIBRA-Pavia University in the field seasons 2017–2018 (sensitivity –168.8 dB re 1 V/µPa, flat frequency response from 1 to 30 kHz ±5 dB), with a bandwidth 5 Hz–90 kHz, and (2) a towed array of two Aquarian Audio H1c-2018 provided by Nauta in the field seasons 2019–2021 (sensitivity −199 dB re 1 V/µPa, flat frequency response from 20 to 4 kHz ±4 dB), with a bandwidth <0.1 Hz to >100 kHz; and (3) a digital sound interface Roland Quad Capture UA55 (16–24-bit, 192 kHz sampling format, USB connection). Data were deemed comparable between the two sampling periods, as the hydrophones shared basic technical properties.
Real-time visualization of the spectrograms was achieved through a Windows-based laptop with the software SeaPro (CIBRA, University of Pavia). During sightings, SeaPro was switched to recording mode to collect acoustic emissions and store them as 10-min uncompressed wav files for the entire duration of the encounter. No other cetacean species other than the common bottlenose dolphin was visually detected during recordings.
2.3 Acoustic Analysis
The acoustic analysis was conducted on 1541 wav files, corresponding to 13 773 min of recording. Whistles and impulsive sounds were selected through the visual inspection of the spectrograms using the software Raven Pro 1.6 (Cornell Laboratory of Ornithology, Ithaca, NY, USA). Impulsive sounds were distinguished into two main types: (i) echolocation click trains (CT; e.g., Norris et al. 1961; Wood and Evans 1980; Au 1993; Surlykke and Nachtigall 2014; Buscaino et al. 2015, Buscaino et al. 2021; Jones et al. 2020) and (ii) short burst pulses (SBP; e.g., Herzing 2000; Killebrew et al. 2001; Luís, Couchinho, and dos Santos 2016). The following spectrogram settings were chosen to optimize signals’ visualization: (a) whistles: Hamming window, size 1024, DFT 1024, overlap 50%, hop size 512, frequency resolution 187.5 Hz; (b) impulsive sounds (CT and SBP): Hamming window, size 512, DFT 512, overlap 50%, hop size 256, frequency resolution 375 Hz. A quality score (Q) was assigned to each analysed sound depending on the signal-to-noise ratio (SNR) (Luís, Couchinho, and dos Santos 2016; La Manna et al. 2022): (i) whistles: WQ1 = whistle fairly audible and with a contour not clearly visible on the spectrogram (start/end points not measurable); WQ2 = whistle audible and with a contour clearly visible on the spectrogram from the beginning to the end; WQ3 = whistle clearly audible and predominant on the spectrogram; (ii) impulsive sounds: PQ1 = sound fairly audible with clicks not clearly visible on the spectrogram (start/end points not measurable); PQ2 = sound audible with clicks clearly visible on the spectrogram from the beginning to the end (start/end points measurable); PQ3 = sound clearly audible with clicks predominant on the spectrogram (start/end points measurable). Only good to high-quality (Q2 and Q3) whistles and impulsive sounds (Figures S1 and S2) were further analysed to extract the acoustic parameters (Papale, Azzolin, et al. 2021, Papale, Alonge, et al. 2021; Pace and Pedrazzi 2024) reported in Table 1 (see also Figures S3 and S4). The selection tool available in Raven Pro 1.6 was used to automatically extract duration (DT), minimum (LF) and maximum Frequency (HF) from WQ2-WQ3 vocalizations. Start frequency (SF), end frequency (EF) and inflection points (IP) of whistles were instead manually measured from the spectrograms. The number of pulses (NP) composing PQ2-PQ3 sounds was counted on the spectrogram and used afterward to calculate click repetition rates (RR) and ICI. Considering that only frequencies up to 96 kHz have been recorded, impulsive sounds’ frequency measurements included the frequency at the lower limit of the entire click series only.
| Whistles (W) | Code | Definition |
|---|---|---|
| Minimum frequency (Hz) | LF | Frequency at the lower limit of the whistle |
| Maximum frequency (Hz) | HF | Frequency at the upper limit of the whistle |
| Start frequency (Hz) | SF | Frequency at the beginning of the whistle |
| End frequency (Hz) | EF | Frequency at the end of the whistle |
| Frequency range (Hz) | FR | Difference between whistle maximum frequency and minimum frequency |
| Inflection points (number) | IP | Mathematic definition in sine function of a change from positive to negative or negative to positive slope in the whistle contour |
| Duration (s) | DT | Total duration calculated as the difference between whistle ending time and beginning time |
| Impulsive sounds (CT, SBP) | Code | Definition |
|---|---|---|
| Minimum frequency (Hz) | LF | Frequency at the lower limit of the entire click series |
| Number of pulses | NP | Number of clicks composing the sound |
| Duration (s) | DT | Time interval from the first click to the last click of the series |
| Repetition rate (pulses/second) | RR | Number of pulses per second |
| Inter-click-interval (ms) | ICI | The time interval between clicks in a series (1/RR) |
2.4 Context Analysis
Three types of potential factors influencing whistles and pulsed sounds’ acoustic structure were considered: (i) environmental: three categories of seabed type (muddy, sandy and mixed) remotely obtained from the EMODnet website (https://emodnet.ec.europa.eu/en) and two categories of depth (< 50 m and > 50 m) extracted from the GEBCO chart (https://www.gebco.net/) for each sighting location (Figure 1; the map was generated using the software QGIS, Version 2.18.16); (ii) social: three categories of group size based on the number of photo-identified individuals (<15, between 15 and 30, and >30 individuals) and two categories of calve occurrence (presence/absence); (iii) behavioural: three categories of observed activity (feeding, socializing, and interaction with fishery).
2.5 Statistical Analysis
Kruskal–Wallis and Wilcoxon–Mann–Whitney tests were carried out to assess differences in ERs among environmental, social and behavioural factors (Zar 2010). Post hoc Dunn Test with Bonferroni correction was applied in case of significant differences among classes. Non-parametric tests were adopted as ERs do not assume a normal distribution of data.
The acoustic parameters extracted for each type of vocalization (W, CT and SBP) were used to investigate the variability of their acoustic structure among the three contexts (environmental, social and behavioural). Descriptive statistics (Mean, SD, CV, Median, and 95% CI) were calculated from good-to-high-quality sounds of each type both on the overall dataset and for each context. SBPs were not further analysed because of the small sample size (n = 81).
First, as a preliminary investigation of the possible significant effect of the concomitant environmental, social and behavioural factors on the overall acoustic structure of each vocalization, two MANOVA models (multivariate analysis of variance) were fitted. Multivariate analysis allows for the simultaneous consideration of all the parameters that describe the acoustic structure, taking into account also the interaction among them, and providing a first summarised view of the vocalization's variability in relation to different context factors. The first model (M1) was developed on whistles, using six out of seven acoustic parameters as response variables (frequency range was excluded since it was highly correlated with both maximum and minimum frequency); the second model (M2) was fitted on CT, using minimum frequency, inter-click-interval, repetition rate and duration as response variables. In both models, all context factors (n = 5; i.e., sea bottom type, depth, group size, presence/absence of calves, behavioural activity) were used as independent variables.
As a second step, generalised linear mixed models (GLMMs) were fitted separately on each acoustic parameter of whistles and click trains to investigate in detail their individual responses to different contextual factors. A Gaussian distribution was used for all acoustic parameters except for inflection points to which a negative binomial was applied. To consider the temporal variability, the date of each recording was entered as a random factor in all models. This allows us to take into account that vocalizations recorded during the same sighting are likely to be more related to each other. All five context factors considered in the MANOVA were used as explanatory variables and the best-fitting combination of predictors for each acoustic parameter was identified following a forward selection procedure based on the AIC (Akaike information criteria) and likelihood ratio test (Zuur et al. 2009). Non-normal variables were log-transformed. Multicollinearity among covariates was checked using the variance inflation factors (VIFs). The best model was validated through the graphical inspection of residuals (Q-Q plot of the residuals to check for normality; residual vs. fitted values plots to verify homogeneity).
All analyses were performed in R 4.0.3 (http://www.r-project.org). GLMMs were fitted using the glmer function of the lme4 package in R (Bates et al. 2015).
3 Results
3.1 Whistles
A total of 19 441 whistles were collected in 107 out of 146 common bottlenose dolphin sighting occasions, 74 of which contained good to high-quality whistles (WQ2 and WQ3) that accounted for 2294 vocalizations (Table 2). Descriptive statistics per context are included in Tables S1–S5).
| Acoustic features | Mean ± SD | CV | Median | 95% CI |
|---|---|---|---|---|
| Minimum frequency (Hz) | 5918 ± 1763 | 0.30 | 5812 | 5846–5991 |
| Maximum frequency (Hz) | 15 282 ± 3785 | 0.25 | 15385 | 15 127–15 437 |
| Start frequency (Hz) | 7703 ± 2835 | 0.37 | 7384 | 7587–7820 |
| End frequency (Hz) | 11 531 ± 707 | 0.62 | 11009 | 11 242–11 821 |
| Frequency range (Hz) | 9364 ± 3200 | 0.34 | 9026 | 9233–9495 |
| Number of inflection points | 1.31 ± 1.75 | 1.33 | 1 | 1.23–1.38 |
| Duration (s) | 0.96 ± 0.58 | 0.60 | 0.86 | 0.94–0.99 |
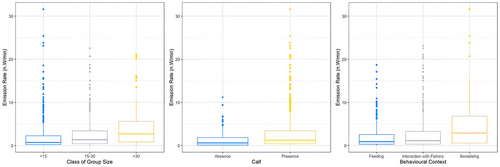
Whistles were mostly recorded in any of the following conditions: muddy bottom and depth > 50 m (57%), presence of calves (91%) and during interactions with fishing activities (45%; Table 3). However, their emission rates did not vary significantly among seabed types and at different depths, while significant differences emerged (1) between presence and absence of calves, with significantly higher ER values in the presence of calves (Mann–Whitney: W = 30713, p < 0.05), (2) among different classes of group size (Kruskal–Wallis: χ2 = 39.498, df = 2, p < 0.05), with ER significantly increasing in larger groups (Dunn Test: < 15 vs. 15–30: Z = −4.978, p-value adj. < 0.05; < 15 vs. > 30: Z = −5.098, p-value adj. < 0.05; 15–30 vs. > 30: Z = −2.500, p-value adj. < 0.05) and (3) between three behavioural contexts (Kruskal–Wallis: χ2 = 30.501, df = 2, p < 0.05) with social behaviour showing significantly higher ER values (Dunn Test: Feeding–Socializing: Z = −5.489, p-value adj. <0.05; Int. Fish.–Socializing: Z = −4.483, p-value adj. <0.05; Feeding-Int.Fish.: Z = −1.845, p-value adj. > 0.05; Figure 2).
| Whistles | Click trains | |||||
|---|---|---|---|---|---|---|
| ER (mean ± SD) | N | ER (mean ± SD) | N | |||
| Environmental Variables | Seabed type | Muddy | 2.71 ± 4.41 | 11 138 | 4.24 ± 3.22 | 26 132 |
| Sandy | 3.71 ± 3.29 | 520 | 3.93 ± 2.28 | 704 | ||
| Mixed | 2.59 ± 3.61 | 7783 | 3.55 ± 3.29 | 22 674 | ||
| Depth | <50 m | 2.69 ± 3.64 | 8339 | 3.53 ± 3.26 | 22 598 | |
| >50 m | 2.65 ± 4.37 | 11 102 | 4.25 ± 3.22 | 26 912 | ||
| Social variables | Group size | <15 | 2.28 ± 4.19 | 6199 | 3.38 ± 2.88 | 20 521 |
| 15–30 | 2.65 ± 3.41 | 9977 | 4.29 ± 3.26 | 24 388 | ||
| > | 4.99 ± 5.92 | 3265 | 5.07 ± 4.98 | 4601 | ||
| Calves | Presence | 2.92 ± 4.3 | 17 698 | 4 ± 3.36 | 42 516 | |
| Absence | 1.43 ± 2.03 | 1743 | 3.26 ± 2.64 | 6994 | ||
| Behavioural variables | Behavior | Feeding | 2.23 ± 3.37 | 4087 | 3.9 ± 3.11 | 17 358 |
| Socialising | 4.95 ± 5.81 | 4485 | 3.82 ± 2.54 | 2839 | ||
| Interaction with fishery | 2.73 ± 4.04 | 9414 | 4.51 ± 3.55 | 22 053 | ||
The MANOVA model (M1) highlighted that all context factors have a significant effect on whistles’ structure (seabed type: Pillai's trace = 0.053, F12,4114 = 9.434, p < 0.01, ηp2 = 0.03; depth: Pillai's trace = 0.020, F6,2056 = 6.935, p < 0.01, ηp2 = 0.02; group size: Pillai's trace = 0.045, F12,4114 = 7.83, p < 0.01, ηp2 = 0.02; calves: Pillai's trace = 0.020, F6,2056 = 7.079, p < 0.01, ηp2 = 0.02), with the greatest values showed by behavioural activity (behaviour: Pillai's trace = 0.198, F12,4114 = 37.59, p < 0.01, ηp2 = 0.10).
The outputs of the best GLMMs fitted separately on the six whistles’ acoustic parameters (Table 4; details in Table S6) showed that each parameter varied differently in response to specific context factors. Minimum and start frequency appeared to be negatively influenced by the interaction with fishery, while duration and inflection points appeared to significantly increase during interaction with fishing gears and to decrease in social contexts. Calves’ presence had a positive and significant effect on minimum frequency, end frequency and duration, which conversely was negatively influenced by muddy seabed, together with inflection points.
| Minimum frequency | Start frequency | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| cR2 = 0.43 | mR2 = 0.07 | cR2 = 0.41 | mR2 = 0.03 | ||||||
| Fixed effects | Estimate | SE | t value | p | Fixed effects | Estimate | SE | t value | p |
| Intercept | 6312.429 | 453.102 | 13.932 | ** | Intercept | 8641.665 | 717.415 | 12.046 | ** |
| Socialising | −386.691 | 172.53 | −2.241 | * | Int with fishery | −1188.955 | 305.819 | −3.888 | ** |
| Int with fishery | −533.514 | 193.195 | −2.762 | * | Random effect | SD (Intercept) | Residual | ||
| Calf Presence | 1017.137 | 317.297 | 3.206 | ** | Date | 1893 | 2357 | ||
| Random effect | SD (Intercept) | Residual | End frequency | ||||||
| Date | 1195 | 1489 | cR2 = 0.39 | mR2 = 0.05 | |||||
| Maximum frequency (log) | Fixed effects | Estimate | SE | t value | p | ||||
| cR2 = 0.30 | mR2 = 0.0003 | Intercept | 8729.67 | 1381.47 | 6.319 | ** | |||
| Fixed effects | Estimate | SE | t value | p | Calf presence | 3129.26 | 991.2 | 3.157 | ** |
| Intercept | 9.617 | 0.029 | 335.577 | ** | Random effect | SD (Intercept) | Residual | ||
| Random effect | SD (Intercept) | Residual | Date | 3516 | 4758 | ||||
| Date | 0.152 | 0.235 | Duration (log) | ||||||
| Inflection points (negative binomial) | cR2 = 0.37 | mR2 = 0.11 | |||||||
| cR2 = 0.46 | mR2 = 0.11 | Fixed effects | Estimate | SE | t value | p | |||
| Fixed effects | Estimate | SE | z value | p | Intercept | 0.58716 | 0.05463 | 10.748 | ** |
| Intercept | 0.2328 | 0.1873 | 1.243 | * | Socializing | −0.07228 | 0.02617 | −2.762 | * |
| Socializing | −0.3604 | 0.1387 | −2.599 | * | Int with fishery | 0.09942 | 0.02847 | 3.492 | ** |
| Int with fishery | 0.2971 | 0.1467 | 2.025 | * | Calf presence | 0.12162 | 0.04501 | 2.702 | * |
| Seabed Mud | −0.5303 | 0.2202 | −2.408 | * | Seabed mud | −0.12546 | 0.04096 | −3.063 | ** |
| Random effect | SD (Intercept) | Random effect | SD (Intercept) | Residual | |||||
| Date | 0.8057 | Date | 0.1478 | 0.2307 | |||||
- * p < 0.05; **p < 0.01.
3.2 Impulsive Sounds
A total of 50 238 impulsive sounds were collected in 123 out of 146 common bottlenose dolphin encounters. Good- to high-quality sounds (PQ2 and PQ3) were recorded in 96 of these 123 encounters, accounting for a total of 1715 vocalizations, of which 1634 were click trains (CT) and 81 were SBP (Table 5). Descriptive statistics per context are reported in Tables S1–S5.
| Acoustic features | CT (n = 1634) | SBP (n = 81) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | CV | Median | 95% CI | Mean ± SD | CV | Median | 95% CI | |
| Minimum frequency (Hz) | 7109 ± 3641 | 0.51 | 6112 | 6932–7285 | 7145 ± 3183 | 0.44 | 6882 | 6441–7849 |
| Inter-click-interval (s) | 0.13 ± 0.06 | 0.47 | 0.11 | 0.12–0.13 | 0.005 ± 0.004 | 0.8 | 0.004 | 0.005–0.006 |
| Repetition rate (pulses/second) | 10.9 ± 7.8 | 0.71 | 8.7 | 10.5–11.3 | 240 ± 101 | 0.42 | 253 | 218–263 |
| Duration (s) | 2.0 ± 2.2 | 1.1 | 1.3 | 1.9–2.1 | 0.08 ± 0.04 | 0.5 | 0.07 | 0.07–0.09 |
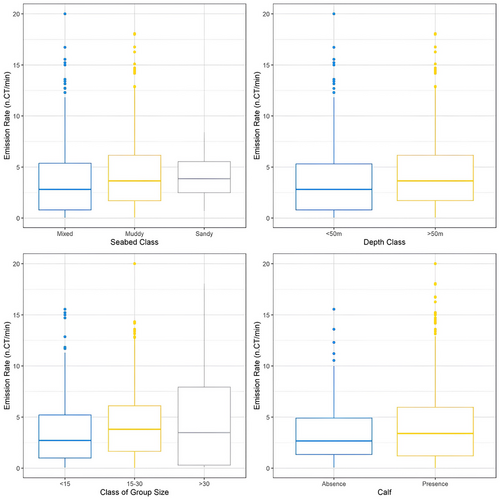
CTs (N = 49 510) were mostly recorded in any of the following conditions: on muddy bottom (53%), at a bottom depth > 50 m (54%), in presence of calves (86%) and during interactions with fishing activities (45%; Table 3). Their emission rates varied significantly among different seabed types (Kruskal–Wallis: χ2 = 24.453, df = 2, p < 0.05), particularly between mixed and muddy substrates (Dunn Test: Mixed-Mud: Z = −4.902, p-value adj. <0.05; Mixed-Sand: Z = −1.180, p-value adj. > 0.05; Mud-Sand: Z = −0.050, p-value adj. > 0.05) and significantly increased at higher depth (Mann–Whitney: W = 189381, p < 0.05). Click trains’ ERs also varied significantly with social context, showing higher values in presence of calves (Mann–Whitney: W = 113371, p < 0.05) and significant differences among classes of group size (Kruskal–Wallis: χ2 = 24.1, df = 2, p < 0.05; Dunn Test: <15 vs. 15–30: Z = −4.792, p-value adj. <0.05; <15 vs. > 30: Z = −2.128, p-value adj. > 0.05; 15–30 vs. > 30: Z = −0.266, p-value adj. > 0.05; Figure 3). No differences emerged among behavioural classes.
The MANOVA model (M2) highlighted that almost all environmental, social and behavioural factors have a significant effect on CTs’ acoustic structure, except for seabed type (depth: Pillai's trace = 0.020, F3,1366 = 9.495, p < 0.01, ηp2 = 0.02; group size: Pillai's trace = 0.076, F6,2734 = 18.085, p < 0.01, ηp2 = 0.04; calves: Pillai's trace = 0.025, F3,1366 = 11.783, p < 0.01, ηp2 = 0.03; behaviour: Pillai's trace = 0.046, F6,2734 = 10.735, p < 0.01, ηp2 = 0.02). As for whistles, the output of the best GLMMs fitted on CTs showed that each acoustic parameter varied individually in response to different context's variables (Table 6; details in Table S7). In particular, minimum frequency and click repetition rate resulted significantly higher during interaction with fishery and ICI lower in interaction with fishing gears. Group size had a negative effect on ICI as well, with bigger groups significantly affecting ICI. Conversely, it appeared to have a positive and significant influence on click repetition rate (RR).
| Minimum frequency (log) | Duration (log) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| cR2 = 0.43 | mR2 = 0.07 | cR2 = 0.13 | |||||||
| Fixed effects | Estimate | SE | t value | p | Fixed effects | Estimate | SE | t value | p |
| (Intercept) | 8.434 | 0.159 | 52.905 | ** | Intercept | 0.9279 | 0.02854 | 32.51 | ** |
| Int with fishery | 0.134 | 0.046 | 2.889 | ** | Random effect | SD (Intercept) | Residual | ||
| Random effect | SD (Intercept) | Residual | Date | 0.199 | 0.512 | ||||
| Date | 0.293 | 0.436 | |||||||
| Inter-click interval (log) | Click repetition rate (log) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| cR2 = 0.13 | mR2 = 0.03 | cR2 = 0.22 | mR2 = 0.06 | ||||||
| Fixed effects | Estimate | SE | t value | p | Fixed effects | Estimate | SE | t value | p |
| Intercept | 0.133 | 0.01 | 24.306 | ** | Intercept | 2.265 | 0.053 | 42.678 | ** |
| Int with fishery | −0.011 | 0.01 | −2.13 | * | Int with fishery | 0.088 | 0.041 | 2.121 | * |
| Group size >30 | −0.029 | 0.01 | −2.661 | * | Group size >30 | 0.345 | 0.099 | 3.503 | ** |
| Random effect | SD (Intercept) | Residual | Random effect | SD (Intercept) | Residual | ||||
| Date | 0.019 | 0.06 | Date | 0.193 | 0.433 | ||||
- *p < 0.05; **p < 0.01.
4 Discussion
The production, use, and reliance on a wide variety of sounds by several marine species in different contexts indicate that acoustic interaction with the surrounding environment is a complex biological function, crucial for daily life activities and survival (Tyack 1998). Vocalizations are a great information pathway used to study many aspects of species ecology, behaviour and conservation (Obrist et al. 2010; Clay, Smith, and Blumstein 2012; Penar, Magier, and Klocek 2020; Lewis, Williams, and Gilman 2021; Stein and Rachlow 2023). In particular, behavioural bioacoustics is increasingly emerging as a powerful tool to understand the context and function of species repertoires over different sites and across ecological scales (e.g., Oestreich et al. 2024). Here, we analysed the vocalizations of the bottlenose dolphin geographical unit living nearby the Tiber River estuary, in the central Tyrrhenian Sea. We reported original data on both whistles and impulsive sounds registered under different contextual conditions. By using recordings collected during visual sightings, we applied for the first time a finer analytical approach fitting two sequential models to investigate whether different context-dependent factors may affect sounds’ structure and modulate their usage as already observed in other populations worldwide. Results showed that we were able to capture more subtle variations than expected, obtaining a set of information useful for characterizing peculiarities at the local level and refining our knowledge on the nuances of the complex bottlenose dolphin acoustic repertoire in different conditions.
Although both vocalization types were recorded in all analysed conditions, the emission rates and the acoustic parameters of whistles and impulsive sounds varied significantly in different contexts, suggesting a well-defined link between the type of situation, its putative valence and the expression of the two calls in that situation. More specifically, both ERs were significantly higher in the presence of calves and larger groups, but differences between the two vocalizations emerged in relation to behavioural and environmental contexts, with whistles having significantly higher ERs during socialization, and ERs of CTs varying significantly with seabed types and depth. The presence and the differential expression of whistles in different behavioural contexts are not surprising considering their specific communicative function in intraspecific social interactions, individual identification, coordination of group activities and group movements (e.g., Janik and Sayigh 2013; Lammers and Oswald 2015; MacFarlane et al. 2017); on the other hand, impulsive echolocation sounds are primarily used to catch, orientate and navigate (e.g., Au 1993, 2004), possibly explaining the stronger influence of environmental factors on their ERs, with higher values during feeding activities and interactions with fishery for possible depredation purposes.
Acoustic parameters were influenced mainly by the behavioural and social context. In particular, the structural characteristics of both vocalization types were affected by the interaction with fishery (mainly trawling). These results highlight a common pattern emerging when the complexity of environmental, social and behavioural contexts requires a finer transmission of the signals’ information content. In the case of aroused settings, like opportunistic feeding behind trawlers, an increase in signal length and modulation (a higher number of inflection points for whistles, and a higher repetition rate for impulsive sounds) occurred. For whistles, this is the opposite of other findings in the Mediterranean Sea (Rako-Gospić et al. 2021), which reported that during interaction with trawling vessels, whistles were shorter and with a lower number of inflection points. In our study, common bottlenose dolphins following trawling vessels emit longer and frequency-modulated whistles to possibly overcome the noise generated by the vessels (e.g., La Manna et al. 2019) and better coordinate feeding during a potentially risky situation. Since food-related whistles may increase motivation of a caller and aid the sharing of information (King and Janik 2015), and a stronger modulation has been linked to high level of arousal and alertness, in particular in relation to boats (Perez-Ortega et al. 2021), it is reasonable to assume that catching prey items behind trawlers can be considered a demanding situation, and modulation can be a potential indicator of the individual state and its communication needs in a danger context. In addition, the augmented emission rates of click trains in this context may indicate multiple influencing factors, ranging from a more complex echolocation task in the proximity of the trawling net to the necessity of prey item selection (Ridgway et al. 2014; La Manna et al. 2023). Considering the scarcity of literature that focuses on bottlenose dolphin acoustics during interaction with trawling fishery (Di Nardo et al. 2023), the results here reported can further contribute to the understanding of the acoustic repertoire variability in such a context.
The pattern of increasing and fine-tuning whistle parameters was observed in the presence of calves as well, a context that requires a well-targeted and efficient communication exchange between mother–calf pairs (King et al. 2016). The use of so-called ‘Motherese’ has been documented in bottlenose dolphins, with females observed increasing the frequency of their whistles in the presence of calves, likely to enhance bonding and attention (Sayigh et al. 2023). Indeed, social context and calves’ presence influenced the structure of whistles (i.e., duration, minimum frequency and inflection points), with mostly decreasing values during socializing (i.e., minimum frequency, inflection points and duration) and increasing with calves (i.e., minimum frequency, end frequency and duration). This means that context-dependent plasticity in this bottlenose dolphin geographical unit is driven by local social properties and behavioural activity experienced, as observed in other Mediterranean sites (La Manna et al. 2020). However, vocalization functions are achieved and maintained as long as sounds are efficiently transmitted through the aquatic medium. Therefore, the habitat features, the social environment and the environmental noise play a key role in regulating the sounds’ characteristics. The estuarine area here investigated is indeed a remarkable site in terms of a variety of environmental attributes, population peculiarities and anthropogenic activities (Ardizzone, Belluscio, and Criscoli 2018; Pace, Tumino, et al. 2022, Pace, Ferri, et al. 2022), all well-known elements affecting the acoustic features of dolphins’ vocalizations (Miller et al. 2014). Forrest (1994) pointed out that absorption due to the type of seafloor can influence the structure of sounds, but in our study, we were unable to assess with certainty whether the properties of the seabed led to differential frequency use by the emitting individuals, or whether the acoustic signal picked up by the hydrophone was distorted by environmental characteristics and background noise. In addition, despite higher ERs of both whistles and clicks in larger groups (where several individuals may emit together and make signal detection challenging), the acoustic structure was marginally influenced by this social factor, possibly to preserve vocalizations’ function, and allow the passage of information in communication processes and the recognition of the emitter by the receiver (Dunlop et al. 2022; Madsen, Siebert, and Elemans 2023). Finally, the observation of specific changes in the structure of the sounds (i.e., increasing the duration of the signals and reducing the frequencies to levels that allow efficient propagation) may be a strategy to overcome environmental noise, as observed in other populations (Erbe, Duncan, and Vigness-Raposa 2022; Larsen et al. 2022).
In conclusion, results from this study have provided new findings on the bottlenose dolphin acoustic plasticity, and a more comprehensive view of the magnitude of the social, environmental and behavioural influence, highlighting how the complexity of the species’ acoustic repertoire has yet to be unravelled at the local level. Vocalizations can be more than just cues demonstrating presence and occupancy: since they are signals with ecological and social significance encoded in their acoustic properties (Wood et al. 2021), integrating behavioural data in acoustic surveys would be crucial for species management and a promising opportunity to detect changes in the ecosystems these soniferous species belong to. Although several studies have described the relationship of sounds with ecological, social anthropogenic and genetic factors (La Manna et al. 2020; May-Collado and Wartzok 2008; Papale, Azzolin, et al. 2021; Tellechea 2020), the interaction of these variables on the expression of certain bottlenose dolphin sounds has been studied only recently. This opens several investigation perspectives on structural and contextual variability in sound production in species that can handle their acoustic repertoire to balance the effects that concomitant and combined features may have on the functional role of each vocalization.
Acknowledgments
We thank Roma Natura-Tor Paterno AMP for the logistic support; ‘I Barcaroli del Dollaro’ and the local professional/recreational fishing community for their help; Margherita Silvestri, Chiara Di Marco, Sara Ferri, Alessandro Frachea, Sara Marini, Sara Verni and Carla Tumino for their field assistance in 2019–2020. We also thank Sapienza University of Rome for the support under Program RM1221816AE34004. We wish to dedicate this work to Gianni Pavan (September 21, 1960–May 26, 2023). All the authors profoundly feel the great loss to bioacoustics and marine science that Gianni's passing represents. He was constantly and deeply close to the Sapienza University of Rome research team until the end and was a sincere friend to D.S.P. Thank you, Gianni, for the generosity you always shared your knowledge, for the warmth you welcomed us, and for being so significantly part of our lives.
Open access publishing facilitated by Consiglio Nazionale delle Ricerche, as part of the Wiley - CRUI-CARE agreement.




