Early positive tactile stimulation reverses the increase of anxiety and decrease of sociality induced by early chronic mechanical pain in mandarin voles
Abstract
Animals may experience early negative (mechanical pain: being retrieved using an incisor by parents or attacked) or positive stimulation (being licked and groomed) that may affect emotional and social behaviors in adulthood. Whether positive tactile stimulation can reverse adverse consequences on emotional and social behaviors in adulthood resulting from chronic mechanical pain and underlying mechanisms remain unclear. This study used a tail-pinching model during development to simulate mechanical pain experienced by pups in high-social mandarin voles (Microtus mandarinus). Subsequently, brush-like positive tactile stimuli were applied to the backs of the mandarin voles. Various behavioral tests were used to measure levels of anxiety, depression, and sociability. The results showed that early tail-pinching delayed the eye opening of pups, increased levels of anxiety, reduced levels of sociality in male mandarin voles, and impaired social cognition in females during adulthood. Brushing on the back reversed some of these effects. While mandarin voles that were exposed to tail-pinching during development were exposed to sub-threshold variable stress as adults, they were more likely to show a stress-induced increase of anxiety-like behavior, reduction of sociability, and impairment of social cognition, displaying heightened susceptibility to stress, particularly in males. However, back-brushing reversed some of these effects, implying that these adults display enhanced stress resilience. In addition, tail-pinching reduced levels of serum oxytocin and increased corticosterone levels in serum, but back-brushing reversed these effects. Overall, it was found that positive tactile stimulation reversed increases in anxiety and impairments of social behavior induced by negative stimulation in male mandarin voles via alteration of oxytocin and corticosterone levels.
INTRODUCTION
Early life experiences can affect the development of the brain and behavior producing long-term consequences for animals and humans. These consequences are dependent on the types of experiences. Neonatal stress, such as maternal separation, produces negative consequences (Mooney-Leber & Brummelte 2020; Salberg et al. 2020), while positive experiences, such as enriching environments, improve brain and behavioral development (Richards et al. 2012; Balikci et al. 2020). However, less is known about the long-term effects of different types of experiences on emotional and social behaviors and their underlying neuroendocrine mechanisms. Whether the interaction between negative and positive early-life experiences can affect emotion and sociability in adulthood remains unknown.
Neonatal mammals are often retrieved by their parents using incisors, attacked and even killed by other adults, and human infants often experience abuse that may induce mechanical pain, constituting a negative experience (Vella et al. 2004). Simultaneously, they also receive parental care such as licking and grooming in many mammals and patting in humans, which are considered positive experiences. What consequences can be caused by the interaction between these two types of stimulation and the underlying mechanisms remain unclear. Additionally, tactile stimulation (TS) that simulates licking and grooming from parents, an enriching experience, can positively affect brain and behavioral development. Neonatal TS could reduce levels of anxiety and depression (Balikci et al. 2020; Roversi et al. 2020). It can also reverse increases in anxiety-like behavior and pain sensitivity induced by neonatal isolation in male and female adult Sprague-Dawley rats (Imanaka et al. 2008). However, whether TS can reverse the adverse effects of early mechanical pain on emotion and sociability and its neuroendocrine mechanisms remains unclear.
In addition, early social experiences may not affect behavioral development in adulthood directly but can influence susceptibility to stress. Early life stress causes long-term changes in the hypothalamic-pituitary-adrenal (HPA) axis response to stress in adulthood and increases susceptibility to depression (Juruena 2014; Juruena et al. 2015). Individuals living in a stressful environment early in life could cope with a challenging adult environment better displaying enhanced stress resilience (Santarelli et al. 2017). One review summarized many previous studies and suggested that early stressful experiences can induce both vulnerability and resilience to later stress-related psychopathology in primates depending on the type, duration, and frequency of stress, as well as ecological validity, sensory modality, and developmental timing (Parker & Maestripieri 2011). However, whether tail-pinching and back-brushing during early life can alter vulnerability to stress remains unclear.
The neuropeptide oxytocin (OXT), synthesized in the supraoptic nucleus (SON) and paraventricular nucleus (PVN) (Gundlach & Burazin 1998), plays a role in the modulation of a number of behaviors. Research has demonstrated that skin-to-skin contact during parent–offspring interactions can enhance offspring plasma OXT levels and foster a secure parent–child relationship (Scatliffe et al. 2019). Additionally, massage and gentle tactile stimulation have been found to increase plasma OXT levels and c-Fos expression in OXT neurons (Okabe et al. 2015). OXT shows the effects of the facilitation of social interactions, the counteraction of the stress response, and the stimulation of processes related to growth and recovery (Moberg et al. 2020). Furthermore, OXT is capable of mediating the effects of early experiences on cognitive performance in adulthood (Boccia et al. 2021). The manipulation of OXT and its receptors in the rat brain may influence susceptibility to maternal separation (MS) (Shi et al. 2021). When administered intranasally, OXT was observed to overcome MS-induced anxiety-like behavior and promote social competence, spatial learning, and memory in maternally separated rats (Joushi et al. 2021). In addition, plasma OXT concentrations have been demonstrated to significantly and positively correlate with cerebrospinal fluid OXT concentrations (Carson et al. 2015). Thus, we proposed that back-brushing may increase plasma OXT levels and subsequently reverses tail-pinching-induced abnormalities in emotion and sociality.
Glucocorticoids (GCs), such as cortisol in humans and corticosterone (CORT) in rodents, are steroid hormones synthesized by the adrenal glands in response to stress (Moraitis et al. 2017). Mice exposed to early-life stress showed elevated plasma CORT levels (Dallé et al. 2020). Another study found that pups experiencing maltreatment during development had increased CORT levels and altered social behavior toward their mothers (Raineki et al. 2019). However, whether tail-pinching can increase levels of CORT and whether back-brushing can reverse this increase remains unclear.
Here, we investigated whether positive tactile stimulation (back-brushing) can reverse the negative effects of maltreatment (tail-pinching) on emotional and social behaviors. We established a 2-week model of early life stress and tail-pinching and subsequently used a soft-bristled brush to simulate early maternal grooming of pups. Mandarin voles with high sociality were used to characterize these effects in the present study. These experiments could shed light on the effects of positive and negative early experiences on behaviors and susceptibility to stress in adulthood.
MATERIALS AND METHODS
Animals
The mandarin voles (Microtus mandarinus) were housed in Shaanxi Normal University's animal care facility under 12-h day-night cycles and standardized conditions (24°C ± 2°C), and the humidity was between 40% and 60%. The mandarin voles were provided with conventional feed and carrots freely. One female and one male mandarin vole were housed in each breeding cage. After weaning, four to five mandarin voles of the same sex were housed per cage until behavioral testing. The behavioral tests were performed when the voles were 8 weeks old. All experimental designs and procedures were approved by the Animal Protection and Utilization Committee of Shaanxi Normal University and the study followed the Guidelines for the Care and Use of Experimental Animals in China.
Early stimulation and stress paradigms at adult
After adult males and females were paired and gave birth, littermates were randomly assigned to different treatment groups for within-litter controls. For males, the groups and sample size were Con (control, n = 9), Con+BB (Control+Back-brushing, n = 8), TP (Tail-pinching, n = 12), and TP+BB (Tail-pinching+Back-brushing, n = 9). For females, the groups and sample size were Con (n = 8), Con+BB (n = 8), TP (n = 11), and TP+BB (n = 11). Tactile stimulation was applied for 2 weeks starting from postnatal day 8 of the pups. A modified method of tail-pinching was used (Wong et al. 2022): Tail-pinches were applied for 1 min per day using a plastic clamp approximately 1 cm from the end of the tail to induce pain without damaging it. Afterward, pup cages were placed on a 32°C heating pad, and pups were brushed from the neck to the tail base with a soft-bristled brush (6 cm wide, soft fan-shaped brush) at a rate of 3 cm per second. Brushing was repeated three times a day (one trial consisting of 100 s of brushing followed by a 5-min rest) (Liu et al. 2022a). After 21 days from birth, male and female pups were weaned and separated. Behavioral experiments were performed 56 days after birth (Fig. 1a). To avoid the effects of different estrous stages on behaviors, only anestrous females were used in the behavioral testing.
To test susceptibility to stress, we subjected mandarin voles that had experienced early tactile stimulation or tail pinching to a sub-threshold variable stress stimulus that does not normally result in depression-like behavior for standard-reared mandarin voles (Peña et al. 2019). At postnatal 56–58 days, the following stresses were applied to mandarin voles: 100 random mild foot shocks at 0.45 mA for 1 h (2 voles per chamber), tail suspension stress for 1 h, and restraint stress in a 50-mL conical tube for 1 h (Peña et al. 2019) (Fig. 4a). Then, their behaviors were tested (for males, Con, n = 12; Con+BB, n = 10; TP, n = 11, TP+BB, n = 8. For females, Con, n = 11, Con+BB, n = 10, TP, n = 11, TP+BB, n = 10).
Behavioral testing
The tests were performed in the following sequence: open field test (OFT), elevated plus-maze test (EPM), light–dark box test (LD), three-chamber sociability test (TCS), and tail suspension test (TST).
Open field test
The mandarin voles were placed in the OFT experimental setup (dimensions: length × width × height = 50 cm × 50 cm × 50 cm, light intensity 200 lux) for 5 min. Tracker systems were used to analyze the activities of the mandarin voles recorded by a camera. The video was recorded using a video camera and behavioral analysis was conducted using a video tracking system (SuperMaze software, Shanghai Xinruan Information Technology Co., Ltd, China). The percentage of time spent in the central area (i.e., the time spent in the central area/total test time × 100%) and the number of visits to the center area were recorded. After each animal trial, the experimental setup was wiped down with 75% ethanol to prevent influencing the behavior of future experimental animals.
Elevated plus-maze test
The EPM consisted of two closed arms (dimensions: length × width × height = 30 cm × 7 cm × 0.5 cm) and two open arms (dimensions: length × width × height = 30 cm × 7 cm × 10 cm) with a central zone (dimensions: length × width = 7 cm × 7 cm). The mandarin voles were placed in the central area facing the open arms, videotaped, and recorded with a video tracking system for 5 min. The experiment was performed under a light intensity of 300 lux. The percentage of time spent in the open arm (i.e. time spent in the open arm/total test time × 100%) was recorded. During each experimental break interval, the setup was cleaned with 75% ethanol.
Light–dark box test
The LD consists of a light (dimensions: length × width × height = 27 cm × 27 cm × 27 cm, 350 lux) and a dark (dimensions: length × width × height = 27 cm × 18 cm × 27 cm) compartment separated by an opening for passage (dimensions: width × height = 6 cm × 6 cm). The mandarin voles were placed in the center of the light compartment facing the opening, videotaped, and recorded with a video tracking system for 5 min. The percentage of time spent in the light compartment (i.e. time spent in the light compartment/total test time × 100%) was recorded. During each experimental break interval, the setup was cleaned with 75% ethanol.
Tail suspension test
The mandarin voles were suspended in the tail suspension box (dimensions: length × width × height = 45 cm ×31 cm × 37 cm) with the tape 1 cm proximal to the tail tip, and videotaped with a camera for 6 min. The duration of immobility in the last 4 min was analyzed by a blinded experimenter using J-Watcher software. The percentage of immobile time (i.e. the duration of immobility in the last 4 min per total test time × 100%) was recorded.
Three-chamber sociability tests
The TCS was performed as described in Hung et al. (Hung et al. 2017). The three-chamber setup (dimensions: length × width × height = 60 cm×40 cm×20 cm) was divided into three small compartments (dimensions: length × width × height = 20 cm × 40 cm× 20 cm). In the habituation stage, the wire-mesh cages in the side chambers were empty, and the mandarin voles were allowed to explore the chamber for 10 min to habituate the apparatus. In the sociability test stage, a wire-mesh cage containing an unfamiliar mandarin vole (same sex, 3–5 weeks) was placed on one side of the chamber, and an empty wire-mesh cage was placed on the other side. Each test mandarin vole was placed in the middle chamber and allowed to freely explore both side chambers for 10 min. In the social recognition memory test stage, a novel mandarin vole was placed into the previously empty cage. Then the test mandarin vole was moved to the test apparatus and allowed to freely explore both side chambers for 10 min. It was assumed that the 5-cm-wide area in front of a wire mesh cage, and the 8-cm-wide area on the left and right of the wire mesh cage were the socialization areas between voles. Sociability was evaluated by the sociability index (SI), which was defined as: [(time in the unfamiliar side − time in the empty side)/(time in the unfamiliar side + time in the empty side) × 100%]. The social novelty preference index was defined as: [(time in the novel side − time in the familiar juvenile side)/(time in the novel side + time in the familiar juvenile side) × 100%] (Zhang et al. 2016; Liu et al. 2022b).
ELISA
To assess the effects of early tactile stimulation on OT and CORT in plasma, quantification of plasma OT and CORT was performed using ELISA (enzyme-linked immunosorbent assay) (n = 6 for all groups). Following the behavioral test, experimental mandarin voles were anesthetized with an intraperitoneal injection of Ulatan (0.2 g mL−1). Mandarin vole orbital whole blood was collected into anticoagulation tubes, and plasma was obtained by centrifugation at 3000 rpm for 10 min at 4°C. Plasma OT (ADI-900-153A, Enzo Life Sciences, America) and CORT (ml037564, Enzyme-linked Biotechnology, Shanghai, China) levels were measured using the mouse enzyme-linked immunosorbent assay (Shanghai Xitang Biotechnology, Shanghai, China), according to the manufacturer's instructions. The resultant absorbance was measured using a Metertech microplate reader (BioTek Instruments, Winooski, USA).
Data analysis
Parametric tests were used to analyze normally distributed data; nonparametric tests were used for all other data. One-way ANOVA was used to analyze the OFT, EPM, LD, TST, OT, and CORT in different groups. Two-way ANOVA was used to analyze the three-chamber sociability test (treatment × target), followed by a Tukey post hoc test. All data were presented as mean ± SEM. Statistical analyses of data were performed using SPSS 20.0 software.
RESULTS
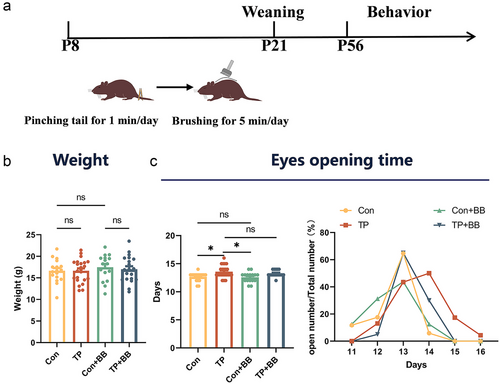
Effects of early tail-pinching and back-brushing on body weight and eye opening
Early tail-pinching and back-brushing did not affect body weight (F3,72 = 0.33, P = 0.804, Fig. 1b). However, a significant difference was observed on the day of eye opening between the different groups (Welch F = 4.512, P = 0.008). Games–Howell tests indicate a significant delay in the eyes-opening days of the pups in the TP group compared to the other groups (Con vs TP, P = 0.018, Con+BB vs TP, P = 0.016, Fig. 1c left). Additionally, the line chart shows a tendency to advance eye opening after brushing. The peak of eye opening occurred on day 14 in the TP group, while in the other groups, the peak of eye opening occurred on day 13 (Fig. 1c right).
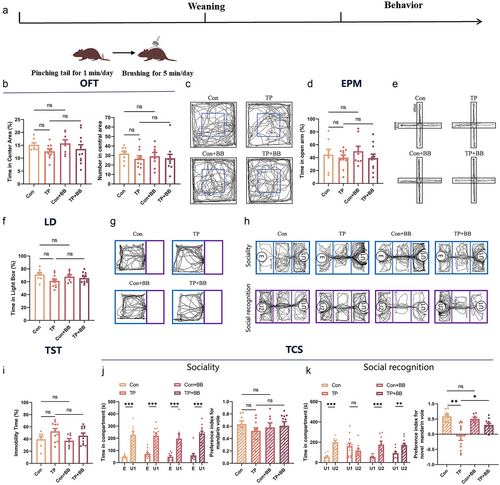
Positive tactile stimulation (back-brushing) reversed the increase of anxiety and decrease in sociability induced by tail-pinching in male mandarin voles
The results of the OFT indicate that there were no significant differences in the percentage of time spent in the central area (F3,34 = 2.698, P = 0.061, Fig. 2b left, c) and entries into the central area (F3,34 = 0.776, P = 0.515, Fig. 2b right, c) between the different groups. However, in the EPM, there was a significant difference in the percentage of time spent in the open arm between groups (F3,34 = 6.239, P = 0.002, Fig. 2d,e). Tukey tests revealed that tail-pinching decreased the percentage of time spent in the open arm, and back-brushing reversed this change (Con vs TP, P = 0.012; Con+BB vs TP, P = 0.003, Fig. 2d left). The percentage of time spent in the light side in the LD test (F3,34 = 1.802, P = 0.165, Fig. 2f,g) and the percentage of immobile time in TST (F3,43 = 0.255, P = 0.857, Fig. 2i) did not differ significantly between the different groups. In the three-chamber sociability test, a significant interaction between treatment and target was observed in the time spent exploring U1 and E in males (F3,68 = 6.821, P < 0.001). Separate-effect analysis revealed that the Con (P < 0.001), Con+BB (P < 0.001), and TP+BB (P < 0.001) groups spent significantly more time investigating an unfamiliar mandarin vole. However, the TP group spent a similar amount of time on U1 and E (P = 0.622, Fig. 2h, j left). According to the Games–Howell tests, back-brushing alone did not affect the sociability index in U1 (P = 0.99), tail-pinching reduced the sociability index in U1 (P = 0.043), while back-brushing after tail-pinching increased the sociability index in U1 (P = 0.038, Fig. 2j right). In the subsequent social novelty recognition test, a significant interaction between treatment and target was found in the time spent exploring U2 and U1 (F3,68 = 3.67, P = 0.048). Separate-effect analysis revealed that the Con (P < 0.001) and Con+BB (P < 0.001) groups spent significantly more time in U2. The TP group spent a similar amount of time in both U1 and U2 (P = 0.143). Back-brushing after tail-pinching did not improve social recognition impairment (P = 0.263, Fig. 2k left,h). There was no significant difference in the social novelty preference index (Fig. 2k right).

Positive tactile stimulation ameliorates the impairment of social recognition induced by tail-pinching in female mandarin voles
Effects of early tail-pinching and back-brushing on anxiety and depressive-like behaviors in females were found to be similar to those observed in male mandarin voles (Fig. 3b,d,f,i). The percentage of time spent in the central area (Welch F = 2.076, P = 0.14, Fig. 3b left) and entries into the central area in the open field test (F3,34 = 0.424, P = 0.737, Fig. 3b right,c), the open arm in the EPM test (F3,34 = 0.567, P = 0.641, Fig. 3d,e), the percentage of time spent in the light side in the LD test (F3,34 = 2.205, P = 0.105, Fig. 3f,g), and the percentage of immobile time in the TST (F3,34 = 2.431, P = 0.082, Fig. 3i) were not significantly different between different groups. No significant interaction between treatment and target was found in the three-chamber sociability test regarding time spent exploring U1 and E (F3,68 = 0.346, P = 0.792). However, targets showed a significant main effect (F1,28 = 122.633, P < 0.001). Pairwise comparison analysis revealed that all groups spent significantly more time investigating U1 (P < 0.001, Fig. 3j left,h). The sociability index was not significantly different between groups (Fig. 3j right). In the subsequent social novelty recognition test, a significant treatment × target interaction was found in time spent exploring U2 and U1 (F3,68 = 8.225, P < 0.001). Post hoc multiple comparisons revealed that the control (P < 0.001), Con+BB (P < 0.001), and TP+BB (P = 0.006) groups spent significantly longer time investigating U2. However, the TP group spent a similar time on U1 and U2 (P = 0.127, Fig. 3k left,h). Games–Howell tests indicated that back-brushing alone did not affect the social novelty preference index in U2 (P = 0.808), tail-pinching reduced the social novelty preference index in U2 (P = 0.003), whereas back-brushing after tail-pinching increased the sociability index in U2 (P = 0.02, Fig. 3k right).
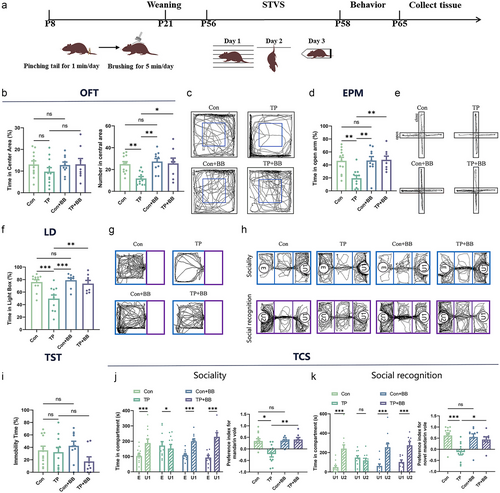
Effects of early tail-pinching and back-brushing on stress susceptibility in male mandarin voles
The results of OFT showed that the percentage of time spent in the central area (F3,68 = 8.225, P < 0.001, Fig. 4b left,c) was not significantly different between different groups. However, a significant difference was found in the number of entries into the central area between different groups (F3,37 = 6.522, P = 0.001). Compared with the control group, tail-pinching reduced the number of entries into the central area (P = 0.008), whereas back-brushing after tail-pinching increased the number of entries into the central area (P = 0.012, Fig. 4b right,c). In the EPM test, the tail-pinching group of male mandarin voles exhibited a significant reduction in the percentage of time spent in the open arm (P = 0.003), whereas brushing after tail-pinching increased the percentage of time spent in the open arm (P = 0.006, Fig. 4d,e). The results of the LD test showed that tail-pinching reduced the percentage of time spent in the light side (P = 0.001), while the brushing after the tail-pinching group increased (P = 0.006, Fig. 4f,g). Back-brushing alone did not affect anxiety behavior in male mandarin voles in OFT (P = 0.928), EPM (P = 1), and LD (P = 0.965). The results of TST showed that the percentage of immobile time was not significantly different (F3,37 = 1.782, P = 0.167, Fig. 4i). In the TCS, significant treatment × target interaction was found on time spent in exploring U1 and E (F3,74 = 10.347, P < 0.001). Separate-effect analysis revealed that the Con (P < 0.001), Con+BB (P < 0.001), and TP+BB (P < 0.001) groups exhibited a significantly higher time spent in investigation U1, while the TP group spent a significantly more time in investigating E (P = 0.029, Fig. 4j left,h). Games–Howell tests indicated that back-brushing alone did not affect the sociability index in U1 (P = 0.999), tail-pinching decreased the sociability index in U1 (P = 0.011), whereas back-brushing after tail-pinching increased the sociability index in U1 (P = 0.005, Fig. 4j right). In the subsequent social novelty recognition test, significant treatment × target interaction was found in time spent exploring U2 and U1 (F3,74 = 7.244, P < 0.001). Separate-effect analysis revealed that Con (P < 0.001), Con+BB (P < 0.001), and TP+BB (P < 0.001) groups exhibited a significantly higher time spent in investigation U2, but the TP group spent similar time on U1 and U2 (P = 0.472, Fig. 4k left,h). Back-brushing alone did not affect the social novelty preference index in U2 (P = 0.981), tail-pinching reduced the social novelty preference index in U2 (P = 0.001), whereas brushing after tail-pinching increased the index during social recognition testing (P = 0.027, Fig. 4k left).
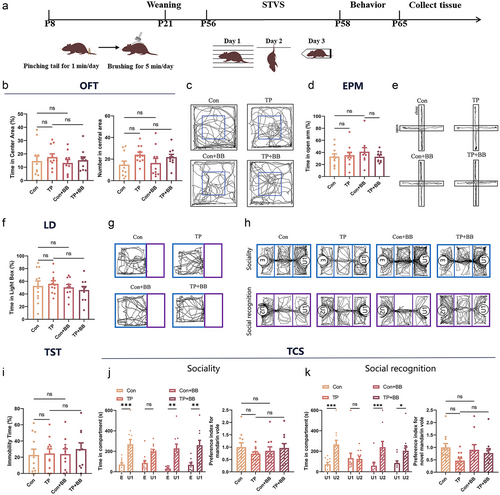
Early positive tactile stimulation ameliorates the stress-induced effects on sociability and social recognition in female mandarin voles in adulthood
The parameters in OFT (F3,38 = 0.416, P = 0.742, Fig. 5b,c), EPM (F3,38 = 0.584, P = 0.629, Fig. 5d,e), LD (F3,38 = 0.473, P = 0.703, Fig. 5f,g), and TST (F3,38 = 0.227, P = 0.877, Fig. 5i) were not significantly different between different groups. In the TCS, no significant treatment × target interaction was found on time spent in exploring U1 and E (F3,76 = 0.922, P = 0.434), while targets showed a significant main effect (F1,76 = 60.667, P < 0.001). Pairwise comparison analysis revealed that the Con (P < 0.001), Con+BB (P = 0.0013), and TP+BB (P = 0.0023) groups exhibited a significantly higher time spent in investigating U1, whereas the TP group did not differ significantly in time spent on U1 and E (P = 3068, Fig. 5j left,h). The sociability index was not significantly different (F3,38 = 0.652, P = 0.587, Fig. 4j right). In the subsequent social novelty recognition test, significant treatment × target interaction was found on time spent exploring U2 and U1 (F3,76 = 3.069, P = 0.033). Separate-effect analysis revealed that Con (P < 0.001), Con+BB (P = 0.001), and TP+BB (P = 0.031) groups exhibited a significantly higher time spent investigating U2, but the TP group spent similar time on U1 and U2 (P = 0.872, Fig. 5k left,h). The social novelty preference index was not significantly different (Fig. 5k right).
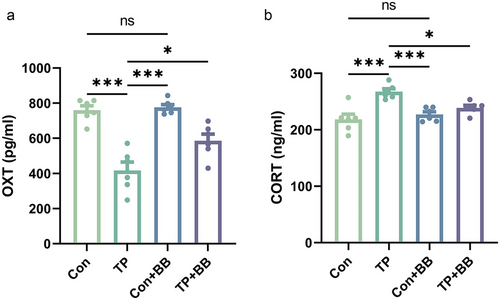
Effects of early tail-pinching and back-brushing on levels of plasma OXT and CORT in male mandarin voles
Based on the results presented above, it is found that early tail-pinching and back-brushing produced significant effects on emotional and social behavior in male mandarin voles. Effects of early tail-pinching and back-brushing on levels of plasma OXT and CORT were only investigated in male mandarin voles. A significant difference was found in plasma OXT levels between different groups (F3,20 = 23.606, P < 0.001). Back-brushing alone did not affect the levels of plasma OXT (P = 0.985). The reduction in plasma OXT levels was observed in tail-pinching group (P < 0.001, Fig. 6a). Nevertheless, brushing after tail-pinching increased the OXT plasma levels (P = 0.012, Fig. 6a). Compared with control group, back-brushing alone did not affect the levels of plasma CORT (P = 0.757), tail-pinching increased the level of plasma CORT (P < 0.001), whereas brushing after tail-pinching reduced the level of plasma CORT (P = 0.019, Fig. 6b).
DISCUSSION
The present study found that early negative stimulation, tail-pinching, retarded the eye opening of pups, increased levels of anxiety and serum CORT, reduced levels of sociality and serum OT, and especially increased susceptibility to stress in males. Positive stimulation, back-brushing, could reverse these effects. This study shows a protective effect of tactile stimulation to stress such as mechanical pain that is possibly caused by pup-retrieval using an incisor by parents or attack (infanticide) from other individuals. The back-brushing can simulate tactile stimulation, such as licking and grooming from parents. Thus, the result can also be used to explain why the absence of parents or separation from parents can lead to abnormalities in emotional and social behaviors. Our findings also suggest that OXT and CORT may play a significant role in this process.
One interesting finding is that tail pinching retarded the eye opening of pups and back-brushing reversed this effect. It has been found that neonatal stress and maternal separation treatment slowed early growth (McPherson et al. 2007). This finding is also consistent with Schanberg and Field's work with preterm infants that massage promoted faster weight gain and lower levels of cortisol in massaged infants. The researchers attempted to replicate the mother's licking and grooming behavior by stroking the rat pups with a soft, wet paintbrush. This type of stimulation, provided during the first few weeks after birth, was able to reverse the adverse effects of stress caused by separation from the mother on both growth and growth hormone secretion (Schanberg & Field 1987). According to Guzzetta et al., enriching the environment with body massage accelerates brain development in infants. The study found that massage speeds up visual maturation in rat pups (Guzzetta et al. 2009). These studies further support the present finding that positive tactile stimulation can reverse adverse effects on physical development induced by negative stimulation.
Our results indicate that early tail-pinching, a form of maltreatment stimulation, increased levels of anxiety-like behavior and reduced levels of sociability in adult mandarin voles. Especially, these stimulations significantly altered the behavioral outcome of stress susceptibility. These effects could be reversed by tactile stimulation. These findings are consistent with Peña et al.’s study using mice (Peña et al. 2019) that the postnatal ELS that occurs later in development increases vulnerability to stress in adulthood. These effects may be due to a sensitive period of catecholamine circuitry development occurring later (Suri et al. 2015), as well as the later development of threat circuitry (Opendak & Sullivan 2019). Early life stress can alter brain development and lead to persistent sensitization of limbic circuits, making individuals more susceptible to mood and anxiety disorders even in response to mild stress in adulthood. It is hypothesized that milder manipulations may promote neural plasticity, while chronic stressful conditions may sensitize limbic circuits to stress, decrease brain plasticity, and lead to greater susceptibility to psychopathology (Cirulli et al. 2003). Moreover, neonatal tactile stimulation and deep touch pressure reduced levels of depression-like behavior in adult animals (Balikci et al. 2020). This treatment also reversed increases in anxiety-like behavior and pain sensitivity induced by neonatal isolation (Imanaka et al. 2008). All these reports support our findings that neonatal back-brushing as positive tactile stimulation can reverse the effects of tail-pinching as a negative stimulation on emotional and social behaviors.
However, the present study found that back-brushing did not alter the levels of anxiety, depression, and sociability of individuals without tail-pinching. It is consistent with one previous study that tactile stimulation did not reduce levels of anxiety in normal individuals (Richards et al. 2012). It is possible that there are ceiling effects that normal individuals are in better emotional and neuroendocrine conditions. The treatment that is used to improve emotional and neuroendocrine conditions may produce no significant effects on normal individuals. Thus, in many behavioral tasks, achieving better scores than normal/good can be difficult.
In line with previous studies, the present study revealed different behavioral outcomes between female and male mandarin voles induced by tail-pinching. Male mandarin voles may be more susceptible to anxiety than females in the present study. This result is supported by some evidence that males display greater phenotypic variation and higher susceptibility to environmental factors (Del Giudice et al. 2018). A number of brain regions involved in stress and CRF-mediated regulation of the HPA axis, including the prefrontal cortex, amygdala, hippocampus, and hypothalamus, exhibit significant sex differences in structure and function in adulthood. Furthermore, gonadal hormones influence the rate of maturation of these brain regions between males and females, which ultimately determines GC stress sensitivity in adulthood (Zuloaga et al. 2020). The transient rise in testosterone begins to affect the male brain differently from the female brain during the prenatal period (Moisan 2021). In males, higher levels of androgens during prenatal and early postnatal life should enhance brain plasticity and consequently result in greater variability than in females (Del Giudice et al. 2018). According to statistics, more males were affected by ASD, with a ratio of 4:1 between males and females (Genovese & Butler 2023). This could be a reason why male mandarin voles are more vulnerable to stress than females. Additionally, female mandarin voles may be more susceptible to their own hormones. Female rodents exhibit varying behavioral characteristics during different stages of the estrous cycle (Inoue 2022). For instance, female rodents exhibit improved cognitive performance and reduced anxiety levels prior to their estrous cycle (Jaric et al. 2019). According to Bullmore, the reason for the higher prevalence of autism in boys than girls might be because girls tend to exhibit less obvious symptoms and are better at concealing their condition (Lai et al. 2019). In summary, it was observed that the negative early stimulation produced more pronounced effects in male mandarin voles than in females. Therefore, the focus of the following experiment would be on male mandarin voles.
Another interesting finding is that tail-pinching increased serum CORT levels, but reduced serum OT levels, and back-brushing could reverse these effects. Tail-pinching, like other stressors, can activate the HPA axis and result in increased CORT levels (de Kloet et al. 2008). It is similar to one previous study that neonatal pain (needle pokes the first four days of life) produced an increase in serum corticosterone (Mooney-Leber et al. 2018). Numerous studies in animal models have consistently reported that emotional touch significantly increases cerebrospinal fluid and peripheral OXT concentrations (Uvnäs-Moberg et al. 2014; Vittner et al. 2018). The OXT neurons in the paraventricular nucleus receive projections from the insula (McGlone et al. 2014), which may be the main pathway by which CT fiber activation during emotional touch initially affects the brain's OXT system. Increased activity of the OXT system may buffer the stress response during the postnatal period, similar to its effects in adults, and consequently reduce CORT levels (Hou et al. 2023).
The present study did not measure OXT levels within the central nervous system. Nevertheless, it is demonstrated that a history of childhood trauma or stressors is consistently associated with lower OXT levels, measured in cerebrospinal fluid, plasma, or urine (Wismer Fries et al. 2005; Heim et al. 2009; Opacka-Juffry & Mohiyeddini 2012). It is possible that changes in peripheral OXT levels may be indicative of variations in central OXT production and individual behavioral patterns. Plasma OXT concentrations have been demonstrated to be significantly and positively correlated with cerebrospinal fluid OXT concentrations (Carson et al. 2015). These reports support our experiments to measure blood OXT levels as a surrogate for central OXT activity.
Central oxytocin acts primarily within the brain and indirectly influences corticosterone secretion by modulating the HPA axis and emotional state. Within the central nervous system, oxytocin neurons within the SON and PVN project to GABAergic interneurons located outside the PVN. These GABAergic neurons then provide negative feedback by projecting back to the PVN, thereby inhibiting CRH secretion in a process that is mediated by GABA A receptors (Legros et al. 1984; Bülbül et al. 2011; Takahashi 2020). Furthermore, OXT inhibits the secretion of ACTH in two ways, via axon collaterals projecting into the median eminence from the magnocellular neurons in the SON and PVN, which project to the posterior pituitary as well as by a bundle of parvocellular oxytocinergic neurons from the PVN projecting into the median eminence. Oxytocin released into the median eminence then reaches the ACTH-producing cells in the anterior pituitary, where they inhibit ACTH secretion and consequently the levels of cortisol (Uvnäs-Moberg et al. 2024). Peripheral OXT has been demonstrated to exert a direct inhibitory effect on CORT release through its action on the adrenal cortex (Legros et al. 1988). In addition, many lines of evidence have supported that OXT promotes social behavior (Marsh et al. 2021) and reduces levels of anxiety-like behavior (Li et al. 2021), while CORT increases levels of anxiety-like behavior (Peng et al. 2021). Thus, we conclude that positive tactile stimulation reversed increases in anxiety and impairments of social behavior induced by negative stimulation in male mandarin voles possibly via alteration of OT and corticosterone levels.
Overall, it was found that positive tactile stimulation, such as licking and grooming, can reverse adverse effects on emotional and social behaviors induced by negative stimulation such as mechanical pain during developmental periods. This finding can help us understand the important roles of parents in the development of normal emotional and social behaviors and their underlying mechanisms. It also suggests that positive tactile stimulation shows protective effects against stress during early periods and can be used to prevent or treat abnormalities in emotional and social behaviors induced by early stress.
ACKNOWLEDGMENTS
This research was funded by the STI2030-Majior Projects grant number 2022ZD0205101, the National Natural Science Foundation of China grants numbers 32270510 and 31901082, the Natural Science Foundation of Shaanxi Province, China grant number 2020JQ-412, the China Postdoctoral Science Foundation grant number 2019M653534, and the Fundamental Research Funds for Central University grants number GK202301012.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.




