Environmental conditions influence host–parasite interactions and host fitness in a migratory passerine
Abstract
The study of host–parasite co-evolution is a central topic in evolutionary ecology. However, research is still fragmented and the extent to which parasites influence host life history is debated. One reason for this incomplete picture is the frequent omission of environmental conditions in studies analyzing host–parasite dynamics, which may influence the exposure to or effects of parasitism. To contribute to elucidating the largely unresolved question of how environmental conditions are related to the prevalence and intensity of infestation and their impact on hosts, we took advantage of 25 years of monitoring of a breeding population of pied flycatchers, Ficedula hypoleuca, in a Mediterranean area of central Spain. We investigated the influence of temperature and precipitation during the nestling stage at a local scale on the intensity of blowfly (Protocalliphora azurea) parasitism during the nestling stage. In addition, we explored the mediating effect of extrinsic and intrinsic factors and blowfly parasitism on breeding success (production of fledglings) and offspring quality (nestling mass on day 13). The prevalence and intensity of blowfly parasitism were associated with different intrinsic (host breeding date, brood size) and extrinsic (breeding habitat, mean temperature) factors. Specifically, higher average temperatures during the nestling phase were associated with lower intensities of parasitism, which may be explained by changes in blowflies’ activity or larval developmental success. In contrast, no relationship was found between the prevalence of parasitism and any of the environmental variables evaluated. Hosts that experienced high parasitism intensities in their broods produced more fledglings as temperature increased, suggesting that physiological responses to severe parasitism during nestling development might be enhanced in warmer conditions. The weight of fledglings was, however, unrelated to the interactive effect of parasitism intensity and environmental conditions. Overall, our results highlight the temperature dependence of parasite–host interactions and the importance of considering multiple fitness indicators and climate-mediated effects to understand their complex implications for avian fitness and population dynamics.
INTRODUCTION
Parasites are a crucial component of ecosystems, being a significant evolutionary force and influencing the population dynamics of their hosts (Price 1980). Understanding the co-evolution of parasites and their hosts is thus a central topic in evolutionary ecology (Schmid-Hempel 2009). Although parasitism and its ecological functions have been extensively studied (e.g. Wood & Johnson 2015; Loker & Hofkin 2022; Gardner et al. 2023), our understanding of the biology and ecology of parasites and host–parasite interactions is far from complete (Wobeser 2008; Bonneaud 2021).
In birds, parasites can have potential deleterious effects on phenotypic traits as well as influence the survival and fitness of their hosts (Møller 1997; Atkinson et al. 2009). These effects may be particularly important during early development, such as the nestling stage, because of the reduced capacity for defense and escape from parasitic infections (Merino 2010). In parasitized nestlings, parasites cause, among other effects, lower mass, smaller size (Allander 1998; Weddle 2000; Moreno et al. 2002), lower recruitment rates (Allander 1998), and restricted post-fledgling movements (Streby et al. 2009), as well as decreased blood levels (Richner et al. 1993; Hurtrez-Boussès et al. 1997a), delayed fledgling dates (Merino & Potti 1995a), and increased developmental time (Arendt 1985; Hurtrez-Boussès et al. 1997b; Reed et al. 2012) and mortality (Fessl et al. 2006; Streby et al. 2009; Antoniazzi et al. 2011; Dadam et al. 2019). However, these effects are highly variable, and research on them has often yielded inconsistent results (Poulin & Forbes 2012; Dunn et al. 2021). Possibly, this is because the potential effects of parasitic infections in their hosts depend on the direct and indirect effects of other (a)biotic factors that influence the risk of exposure to parasites or the severity of their effects (Hall 2021). However, the role of biotic and abiotic factors has been frequently neglected in studies of host–parasite interactions (Wolinska & King 2009).
The most important of these abiotic influences are climatic factors (Dunn et al. 2021; Maziarz et al. 2022). The consequences of current changes in climate trends are a reality for many organisms (Root et al. 2003; Rosenzweig et al. 2008; Weiskopf et al. 2020). Such consequences are assumed to be particularly severe for long-distance migratory birds (Lindström 2003; Carey 2009; Marra et al. 2015) due to phenological mismatches (Crick 2004; Both & Visser 2005), with potential effects on fitness (Mayor et al. 2017; Marrot et al. 2018; McLean et al. 2022; Mingozzi et al. 2022). Although an overall positive relationship between temperature and the intensity of parasitic infections has been reported (Antoniazzi et al. 2011; Hernandez et al. 2013; Loiseau et al. 2013; Dube et al. 2018), the effects of changes in climate variables on parasites are heterogeneous (Ogden & Lindsay 2016). Ectoparasites, which are the focus of this article, are expected to suffer significantly from changes in temperature and humidity regimes (Merino 2019), as abiotic environmental conditions may significantly affect their population dynamics and, therefore, the interactions with their hosts (Hudson et al. 2006; Antoniazzi et al. 2011; Castaño-Vázquez et al. 2018; Musgrave et al. 2019; Castaño-Vázquez et al. 2021). Accordingly, associations between the intensity of ectoparasitic infestations and precipitation, humidity, or temperature have often been reported, albeit in variable directions (Antoniazzi et al. 2011; Hernandez et al. 2013; Loiseau et al. 2013; Dube et al. 2018)
Environmental conditions and their variation are key determinants of host–parasite interactions as they can affect both the life cycles of bird hosts (Charmantier et al. 2008; Potti 2008a; Pautasso 2012) and their parasites (Hudson et al. 2006; Martínez-De La Puente et al. 2009; Ogden & Lindsay 2016) as well as host–parasite dynamics (Atkinson & Van Ripier III 1991; Møller et al. 2013; García del Río et al. 2020). The combination of more stressful climatic conditions and the increased incidence of parasitic infestations can have negative consequences on host fecundity and condition (Møller et al. 2013; Castaño-Vázquez et al. 2021) and ultimately on bird population dynamics (Antoniazzi et al. 2011). Nevertheless, the interactive effects between parasitism and environmental conditions on host fitness are not yet well understood (Dunn et al. 2021; Maziarz et al. 2022). Additionally, given the expected increase in parasite abundance in the current context of climate change (Møller et al. 2013; Castaño-Vázquez & Merino 2022), a largely unresolved question is how environmental conditions affect not only the prevalence and intensity of infestation but also the impact that these infestations have on hosts (Mouritsen et al. 2002; Hoover & Tylianakis 2012). Likewise, unraveling the responses of birds and their parasites to potential changes in multiple environmental variables is essential to predict the direction of changes in parasite–host co-evolution and dynamics in a climate change scenario and their complex and extensive consequences on host fitness (Hoover & Tylianakis 2012; Hall 2021; Maziarz et al. 2022).
Here, we used 25 years of data from a Mediterranean population of pied flycatchers (Ficedula hypoleuca), a small migratory, cavity-nesting passerine (Lundberg & Alatalo 1992) commonly parasitized by blowflies (Merino & Potti 1995a, 1998). Larvae of the blowfly Protocalliphora azurea are common obligate ectoparasites of birds that feed on the blood of nestlings (Hurtrez-Boussès et al. 1997a; Hurtrez-Boussès et al. 1998; Wesołowski 2001). This species infests a range of species of passerine birds in Europe (Moreno-Rueda 2021), in whose nests it may lay up to 75 eggs (Gold & Dahlsten 1989; Bennett & Whitworth 1991). Parasitism by Protocalliphora larvae negatively affects growth (Johnson & Albrecht 1993; Merino & Potti 1996; Hurtrez-Boussès et al. 1997b), mass (Hurtrez-Boussès et al. 1997a; Bańbura et al. 2004; Simon et al. 2004), blood levels (Whitworth & Bennett 1992; Hurtrez-Boussès et al. 1997b; Hannam 2006), and survival (Merino & Potti 1995a; Bańbura et al. 2004; Puchala 2004) during the early life of the bird hosts, as well as condition, fitness, and survival when nestlings reach adulthood (Hurtrez-Boussès et al. 1998; Moreno et al. 2002; Bańbura et al. 2004; Potti 2008a; Castaño-Vázquez & Merino 2022; Martínez-Padilla et al. 2022). Previous studies have often revealed higher prevalence and/or intensity of parasitism by larval blowflies under dry conditions and high ambient temperature (Heeb et al. 2000; Mennerat et al. 2021; Castaño-Vázquez & Merino 2022; Maziarz et al. 2022). However, the direction of the reported effects of climatic variables on these nest-dwelling ectoparasites was not consistent (Albert et al. 2023), and the joint effects of both on bird hosts remain unclear (Mennerat et al. 2021; Maziarz et al. 2022).
Our study aims were twofold: first, to identify the environmental variables underlying the prevalence and intensity of blowfly parasitism, and second, to investigate the interactive associations of environmental variables and parasitism on host fitness. Based on previous work on this and other species, we expect that: (1) warm and dry climates should favor the spread of nest ectoparasites; thus, we expect higher prevalence and/or intensity under these environmental conditions (e.g. Bennett & Whitworth 1991; Merino & Potti 1996; Heeb et al. 2000) and (2) under the aforementioned adverse conditions for nestling development, adverse effects of nest ectoparasites are increased (Howe 1992; Little 2008; Maziarz et al. 2022).
MATERIALS AND METHODS
Study species and population
The pied flycatcher is a small insectivorous passerine (11–13 g), trans-Saharan migrant that breeds in temperate forests across Eurasia (Lundberg & Alatalo 1992; Ouwehand et al. 2016), in both deciduous and coniferous woodlands, with a preference for the former (Mäntylä et al. 2015). The study area is located in the central Iberian Peninsula near La Hiruela (Madrid, 41°04′N, 3°27′W) and Colmenar de la Sierra (Castilla-La Mancha, 40°40′N, 4°8′W). The population occupies two different habitat patches that differ markedly in vegetation and structure: a 9.3-ha deciduous oakwood (Quercus pyrenaica, 1242 broods) and a 4.8-ha mixed coniferous plantation (primarily Pinus sylvestris, 686 broods).
Field data collection
Data for this study were collected between 1996 and 2022 (sample sizes in Table S1, Supporting Information). Sampling intensity was reduced in 2002 and 2003; therefore, these years were excluded from the analyses. Detailed field procedures have been previously described elsewhere (see Canal et al. 2011, 2021; Camacho et al. 2016). In summary, we conducted routine inspections (every 2–3 days) of all nest boxes throughout the breeding season (from the third week of April to the first half of July), to ascertain their occupation by pied flycatchers and to record laying date (first egg laid), clutch size (typically 5–6 eggs), hatching date (of the first egg), and the number of nestlings at 13 days of age (brood size). Adult birds were captured while feeding nestlings 8–10 days post-hatching. Many individuals were of known age since they had been ringed as nestlings (Potti & Montalvo 1991), and the local recruitment rate is among the highest recorded for the species (reaching up to 22%, averaging 14%) (Potti & Montalvo 1991; Potti et al. 2013, 2014). Unringed individuals were sexed and aged as either 1 year old or older based on the criteria outlined by Karlsson et al. (1986) and Svensson (1992). We took standard morphological measurements for all nestlings that survived to the ringing age (13 days), including tarsus length (±0.01 mm) and body mass (±0.1 g).
Blowfly quantification
P. azurea is a blowfly (family Calliphoridae) in which adults are free-living organisms while the larvae are obligatory nest-dwelling hematophagous ectoparasites of birds. Blowfly larval development starts after nestlings hatch, and pupation is completed in about 13–14 days when the adult flies emerge. Adults overwinter near the nesting sites of avian hosts until the following spring when the females lay their eggs (Zumpt 1965; Bennett & Whitworth 1991). Infestation by blowflies was assessed by disassembling nest contents just after the young fledged (15–19 days after hatching). We defined prevalence as the proportion of infested nests (those with the presence of blowfly larvae and/or pupae buried in the nest material; Moreno-Rueda et al. 2016). Infestation intensity was assessed by counting the number of blowflies in infested nests. Only P. azurea was considered since no other blowfly species have been found in our study area (Potti 2008b; Garrido-Bautista et al. 2020).
Environmental variables
Meteorological data were obtained from the only nearby station (50 km away) having a complete record for the entire study period (Colmenar Viejo AEMET station—40°39′N, 3°45′W; https://opendata.aemet.es/centrodedescargas/productosAEMET). Average temperatures and precipitation did not differ from the data available for the meteorological station closest to the study area but with an incomplete temporal record (Buitrago de Lozoya—41°00′N, 3°36′W). Average temperature and precipitation in the years when both stations were active were highly correlated, confirming the validity of the long-term climate data used in the analyses (see Le Vaillant et al. 2021 for further details).
Initially, we considered average temperatures and precipitation in different periods for each year: April, May, June, and spring (which includes the averages of April, May, and June), in addition to those calculated for each brood for the incubation period (from the laying of the last egg until hatching) and the nestling stage (from hatching date until day 13 of nestling age) of each brood. After checking the correlations among all climatic variables (Table S2, Supporting Information), we decided to use the mean temperatures and precipitations during the nestling stage for three reasons: (1) these two indexes are correlated with those from other periods (Table S2, Supporting Information); (2) considering temperature and precipitation during the nestling stage makes the most biological sense given the developmental cycle of the blowfly (Zumpt 1965; Bennett & Whitworth 1991) and that the nestling stage is a very sensitive period in altricial birds, including pied flycatchers; (3) these indexes are explicitly calculated for each brood (unlike, e.g. monthly indexes), thus reflecting more accurately the conditions faced by the blowflies and nestlings in each nest box. Considering relative time windows affecting trait expression is crucial when those traits are expressed asynchronously between individuals, as is the case in our study system (Gienapp et al. 2005; van de Pol & Cockburn 2011; van de Pol et al. 2016). It is worth noting that adult blowflies seem to oviposit in avian nests typically after the eggs hatch (Gold & Dahlsten 1989; Sabrosky et al. 1989; Bennett & Whitworth 1991; Maziarz et al. 2022), but the exact timing of blowfly infestation of pied flycatcher nests is unknown, and the environmental conditions before hatching could affect survival and behavior of adult blowflies. For completeness and full comparison, we explored the effect of mean temperatures and precipitation during both the nestling and incubation stages in the models analyzing the probability and intensity of blowfly parasitization, considering brood size and clutch size, respectively. The relevant variables in this model were similar to those in the model with environmental variables during the nestling period (Table S3, Supporting Information); for simplicity and the reasons given above, only the results of the analyses involving the nestling stage are reported in the main text.
Statistical analyses
Different statistical analyses were used for each analysis block described below (see Table S1, Supporting Information, for details of variables and sample sizes for each analysis).
(A) Probability and intensity of blowfly parasitization: First, we simultaneously investigated the environmental factors affecting the prevalence (presence/absence) and intensity (larvae/pupae count per nest in infested broods) of blowfly parasitization with a hurdle generalized linear mixed model (GLMM). Because of the large number of zeros in parasite counts, we fitted a truncated negative binomial error distribution with the blowfly count per nest as the response variable. Hurdle regression models have two components (models) for which estimates are calculated: (i) a binomial component for the zero versus positive counts conceptually reflecting the prevalence of parasites (Checchi et al. 2012; Niu et al. 2024) by analyzing the factors associated with the probability of not being parasitized (zero hurdle model coefficients), thus allowing to account for data overdispersion (Cameron & Trivedi 1998; Dalrymple et al. 2003; Brooks et al. 2017; Hacking et al. 2018; Álvarez-Ruiz et al. 2021); and (ii) a truncated negative binomial component only for the non-zero counts that analyzes the factors related to the parasitization intensity (count model coefficients). As fixed factors, we included laying date, brood size, breeding habitat (oak wood vs. pine plantation), relative ages of female and male parents (as a proxy for their breeding experience, two-level class variable: 1-year old [young] or older [adult]), and mean temperature and precipitation during the nestling stage. We included temperatures and precipitation with their linear and quadratic terms, as bell-shaped effects between environmental variables and blowfly populations have been previously reported (Dawson et al. 2005; Ogden & Lindsay 2016). Finally, we included the year and the nest box ID as random factor intercepts but excluded the female and male IDs because their inclusion resulted in model singularity. To check whether there was any relationship between the two dependent variables at the year level, we tested the relation between the annual prevalence of infested nests (% of nests with blowflies) and the intensity of infestation (average number of blowflies in infested nests) with a Pearson correlation, using the average annual data from 1996 to 2022 (see Results).
(B) Interactive effects of environmental factors and blowfly parasitization on fitness: Second, we investigated the interactive effects of environmental conditions and intensity of parasitization during the nestling stage on breeding success and nestling mass. To model breeding success, we used a GLMM (binomial error structure and logit link function). Our response variable was a 2-column matrix that combined the number of chicks that fledged and the number of eggs that failed to produce fledglings (e.g. due to hatching failure or early chick loss; Grueber et al. 2011). Using this matrix as the response variable rather than the ratio of chicks fledged to eggs laid accounts for differences in the absolute number of chicks produced. That is, the breeding success of an individual producing, for example, three chicks from six eggs is considered higher than that of a bird producing two chicks from four eggs, despite the chick-to-egg ration being 50% in both cases. We investigated the influence being 50% in both cases. As covariates, we included breeding habitat (oak wood vs. pine plantation), parental ages (young vs. adult), and the two-way interactions between the number of blowflies and: (i) laying date, (ii) mean temperature during the nestling stage, and (iii) mean precipitation during the nestling stage. We included the year, the nest box ID, and the female and male IDs as random intercept effects. The GLMM analyzing the effect of environmental conditions and the intensity of blowfly parasitization on nestling mass (which is a good proxy of survival in our study population, Potti et al. 2002) was fitted with a Student's t error distribution (Lange et al. 1989; Brooks et al. 2017). The random and fixed factors in this model were the same as in the previous one, except that tarsus length (to control for allometric effects) and brood size were also included as covariates.
Statistical analyses were done using R-4.2.2 (R Core Team 2022, http://cran.r-project.org/). For the mixed modeling, we used the package “lme4” (Bates et al. 2015), while the package “glmmTMB” was used for the hurdle GLMM (Brooks et al. 2017). In the GLMM built to analyze the breeding success, the bivariate response variable was calculated using the “cbind” function in R software (R Core Team 2022). We checked the fit of all models through visual examination of residuals and using the packages “DHARMa” (Hartig 2020) and “performance” (Lüdecke et al. 2021), and we discarded collinearity (all VIFs <3, Zuur et al. 2010) using the package “car” (Fox & Weisberg 2011). Model diagnostics suggested no deviations from the model assumptions. We compared error distributions and random structures of the models described above by fitting them with restricted maximum likelihood and comparing Akaike's information criterion (AIC; Akaike 1973; Zuur et al. 2009). The goodness-of-fit was assessed using the conditional R2 (R2c), which estimates the proportion of variance in the response variable explained by both fixed and random factors (i.e. the entire model; Nakagawa & Schielzeth 2013). AICs were estimated using the R library “MuMIn” (Barton 2020), while R2 was calculated with the “r2_zeroinflated” function from the “performance” package for the hurdle GLMM and with the “r.squaredGLMM” function from the “MuMIn” package in the GLMMs analyzing breeding success and nestling mass. Numeric variables were standardized to mean = 0 and standard deviation = 1 to make the estimates comparable (Schielzeth 2010). For data visualization, we used the “ggplot2” package (Wickham et al. 2016). We assessed the statistical significance of fixed effects, verifying that the 95% confidence intervals of the estimates did not contain 0 (computed using the “car” package; Fox & Weisberg 2011).
Ethical note
All applicable international, national, and/or institutional guidelines for capturing and ringing birds were adhered to, and the study received approval from the Spanish institutional authorities, most recently under license no. 530293. The CSIC Ethical Committee approved field procedures (refs. PAC05-006-2, CGL2006-07481/BOS, CGL2009-10652, CGL2011-29694, CGL2014-55969-P, CGL2015-70639-P, PGC2018-099685-B-I00, PID2019-104835GB-I00), along with the Andalusian Committee of Animal Experimentation (ref. 2011_03), to comply with Spanish and European legislation regarding the protection of animals used for scientific purposes. The time required for field procedures was minimized, and all activities were conducted with utmost care and efficiency.
RESULTS
The prevalence of blowfly parasitism was 44% (n = 848 out of 1928 broods). Among infested nests, the mean intensity (±SD) of blowfly parasitism was 7.4 (±7.2) blowflies per infested nest (3.27 ± 6.05 blowflies per nest considering all nests, range 0–55). The number of occupied nest boxes in each year was unrelated to either the annual prevalence of blowfly parasitism (r = −0.27, 95% CI = −0.598–0.144) or its intensity (r = 0.08, 95% CI = −0.328–0.458).
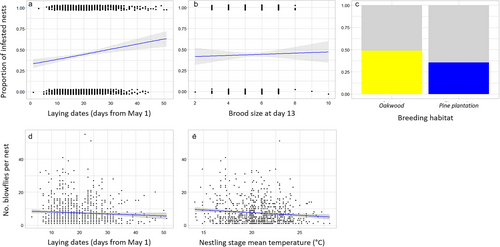
(A) Probability and intensity of blowfly parasitization: Yearly prevalences and mean intensities of blowfly parasitism were not correlated over time (r = 0.35, 95% CI = −0.055–0.653). On the one hand, the hurdle model (R2 = 0.778) showed that the probability of being parasitized by blowflies was higher in the pine plantation than in the oakwood, and increased with the breeding date and the brood size (Table 1a; Fig. 1a,b,d). No association was detected between the prevalence of blowfly infection and either mean temperature or precipitation during the nestling stage. On the other hand, the intensity of parasitism increased with brood size and decreased linearly with higher mean temperatures during the nestling stage (Table 1b; Fig. 1d,e) but was not related to mean precipitation during the nestling stage.
| Model coefficients and confidence intervals | ||||
|---|---|---|---|---|
| (a) Probability of non-parasitization (zero-inflation model) | ||||
| Fixed effects | β | SE | z | CI |
| Intercept | 0.099 | 0.256 | 0.385 | −0.403–0.601 |
| Laying date (days) | −0.411 | 0.090 | −4.567 | −0.587–(−0.235) |
| No. fledglings | −0.165 | 0.059 | −2.814 | −0.280–(−0.050) |
| Nestling stage mean temperature, NMT (°C) | −0.199 | 0.1059 | −1.892 | −0.405–0.007 |
| Nestling stage mean temperature, NMT2 (°C), quadratic | 0.102 | 0.070 | 1.465 | −0.034–0.239 |
| Nestling stage mean precipitation, NMP (mm) | 0.087 | 0.090 | 0.964 | −0.090–0.265 |
| Nestling stage mean precipitation, NMP2 (mm), quadratic | −0.084 | 0.065 | −1.299 | −0.212–0.043 |
| Habitat | 0.628 | 0.136 | 4.617 | 0.362–0.894 |
| Female age | −0.077 | 0.149 | −0.520 | −0.369–0.214 |
| Male age | 0.026 | 0.181 | 0.145 | −0.328–0.380 |
| Random effects | σ2 | SD | ||
| Year | 0.479 | 0.692 | ||
| Nest box ID | 0.306 | 0.553 | ||
| (b) Intensity of parasitization (count model) | ||||
| Fixed effects | β | SE | z | CI |
| Intercept | 1.947 | 0.150 | 12.963 | 1.653–2.242 |
| Laying date (days) | 0.008 | 0.053 | 0.152 | −0.097–0.113 |
| No. fledglings | 0.100 | 0.042 | 2.356 | 0.017–0.183 |
| Nestling stage mean temperature, NMT (°C) | −0.135 | 0.063 | −2.145 | −0.258–(−0.012) |
| Nestling stage mean temperature, NMT2 (°C), quadratic | 0.023 | 0.049 | 0.475 | −0.073–0.120 |
| Nestling stage mean precipitation, NMP (mm) | 0.016 | 0.059 | 0.261 | −0.101–0.132 |
| Nestling stage mean precipitation, NMP2 (mm), quadratic | −0.030 | 0.046 | −0.647 | −0.121–0.061 |
| Habitat | −0.120 | 0.092 | −1.311 | −0.299–0.059 |
| Female age | −0.136 | 0.107 | −1.267 | −0.345–0.074 |
| Male age | 0.046 | 0.125 | 0.367 | −0.199–0.290 |
| Random effects | σ2 | SD | ||
| Year | 0.042 | 0.206 | ||
| Nest box ID | 0.017 | 0.131 | ||
- Here, we provide parameters corresponding to both the zero hurdle model (a), analyzing the probability of non-infestation, and the count model (b), analyzing the intensity of blowfly parasitization. For each fixed effect, the estimate (β), standard error (SE), z value (z), and 95% confidence interval (CI) are given. For each random effect, variance (σ2) and standard deviation (SD) are shown. The reference levels for factors Habitat, Female age, and Male age are “Oakwood,” “Young,” and “Young,” respectively. The most important parameters are highlighted in bold.
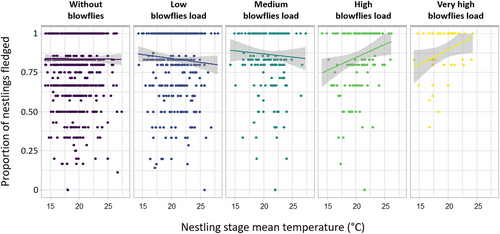
(B) Interactive effects of environmental factors and blowfly parasitization on host fitness: Pied flycatcher breeding success (GLMM: R2c = 0.595, R2m = 0.048) was related to the interaction between the number of blowflies infesting their nests and the mean temperatures experienced during the nestling stage (Table 2 and Fig. 2; see Fig. S1, Supporting Information, for a three-dimensional visualization of the interaction). Specifically, mean temperature during the nestling stage was positively linked to the breeding success at high parasitism intensities, but not at low intensities. In addition, the breeding success decreased with advancing laying date and was lower in the pine plantation than in the oak wood (Table 2).
| Breeding success | Model coefficients and confidence intervals | |||
|---|---|---|---|---|
| Fixed effects | β | SE | z | CI |
| Intercept | 1.775 | 0.163 | 10.916 | 1.456–2.093 |
| No. of blowflies per nest (NPUP) | 0.084 | 0.035 | 2.378 | 0.015–0.153 |
| Laying date (days) | −0.202 | 0.051 | −3.959 | −0.303–(−0.102) |
| Nestling stage mean temperature (NMT, °C) | 0.024 | 0.060 | 0.396 | −0.093–0.141 |
| Nestling stage mean precipitation (NMP, mm) | 0.020 | 0.053 | 0.368 | −0.085–0.124 |
| Habitat | −0.177 | 0.085 | −2.082 | −0.343–(−0.010) |
| Female age | −0.115 | 0.093 | −1.239 | −0.297–0.067 |
| Male age | 0.187 | 0.109 | 1.727 | −0.025–0.400 |
| NPUP × laying date | −0.013 | 0.035 | −0.374 | −0.081–0.055 |
| NPUP × NMT | 0.090 | 0.036 | 2.473 | 0.019–0.161 |
| NPUP × NMP | 0.007 | 0.036 | 0.187 | −0.065–0.078 |
| Random effects | σ2 | SD | ||
| Year | 0.229 | 0.478 | ||
| Female ID | 0.389 | 0.624 | ||
| Male ID | 0.121 | 0.349 | ||
| Nest box ID | 0.062 | 0.249 | ||
- For each fixed effect, we report estimate (β), standard error (SE), z value (z), and 95% confidence interval (CI). For each random effect, we report variance (σ2) and standard deviation (SD). The reference levels for factors Habitat, Female age, and Male age are “Oakwood,” “Young,” and “Young,” respectively. The most important parameters are highlighted in bold.
Nestling mass (GLMM: R2c = 0.722, R2m = 0. 178) was unrelated to the interactions between parasitism and mean temperature as well as between parasitism and precipitation. However, nestling mass decreased with advancing laying date and larger brood sizes as well as with mean temperature and precipitation during the nestling stage (Table 3). In addition, fledglings weighed more on average in the pine plantation than in the oak wood (Table 3).
| Nestling mass | Model coefficients and confidence intervals | |||
|---|---|---|---|---|
| Fixed effects | β | SE | z | CI |
| Intercept | 14.196 | 0.120 | 118.510 | 13.961–14.431 |
| No. of blowflies per nest (NPUP) | −0.022 | 0.018 | −1.220 | −0.058–0.014 |
| Laying date (days) | −0.120 | 0.029 | −4.150 | −0.176–(−0.063) |
| Brood size | −0.043 | 0.017 | −2.600 | −0.075–(−0.010) |
| Tarsus length (mm) | 0.504 | 0.012 | 41.700 | 0.480–0.527 |
| Nestling stage mean temperature (NMT, °C) | −0.239 | 0.033 | −7.310 | −0.303–(−0.175) |
| Nestling stage mean precipitation (NMP, mm) | −0.065 | 0.030 | −2.210 | −0.123–(−0.007) |
| Habitat | 0.139 | 0.070 | 1.990 | 0.002–0.276 |
| Female age | −0.060 | 0.050 | −1.210 | −0.157–0.037 |
| Male age | 0.007 | 0.061 | 0.120 | −0.112–0.127 |
| NPUP × laying date | 0.013 | 0.018 | 0.740 | −0.022–0.049 |
| NPUP × NMT | 0.020 | 0.019 | 1.060 | −0.039–0.030 |
| NPUP × NMP | −0.004 | 0.018 | −0.250 | −0.017–0.057 |
| Random effects | σ2 | SD | ||
| Year | 0.203 | 0.451 | ||
| Female ID | 0.368 | 0.606 | ||
| Male ID | 0.345 | 0.587 | ||
| Nest box ID | 0.099 | 0.315 | ||
- For each fixed effect, we report estimate (β), standard error (SE), z value (z) and 95% confidence interval (CI). For each random effect, we report variance (σ2) and standard deviation (SD). The reference levels for factors Habitat, Female age, and Male age are “Oakwood,” “Young,” and “Young,” respectively. The most important parameters are highlighted in bold.
DISCUSSION
Using a 25-year data set collected in a songbird population, we investigated how environmental conditions modulate the probability and intensity of blowfly infestation and their impact on host fitness. We showed that a combination of factors both extrinsic and intrinsic to the nest influenced the prevalence of blowflies (host breeding date, brood size, and habitat) and the intensity of parasitism (brood size and temperature). In addition, hosts’ breeding success showed a temperature-mediated dependence on parasite load, while nestling mass was unrelated to blowfly parasitism.
(A) Probability and intensity of blowfly parasitization: Annual prevalences and intensities of blowflies’ parasitism were not correlated over time. In other host–parasite systems, a positive relationship between prevalence and either abundance of parasites (Morand & Guégan 2000; Šimková et al. 2002) or intensity of parasitism (Poulin 1999) has been reported, although the latter is highly variable and seems to be contingent on parasite/host groups (Shaw & Dobson 1995; Poulin 1999). Our results may be explained if adult blowflies distribute their eggs among several nests rather than deposit all/most of their eggs in a single bird nest, although more information on the blowflies’ ecology would be needed to verify this hypothesis.
In line with what was explained in the previous paragraph, blowfly prevalence and intensity of infestation were differently associated with extrinsic factors. Blowfly prevalence differed between the two types of habitats in our study. Habitat differences in prevalence have been detected in other locations and attributed to differences in habitat quality, with a lower prevalence of blowfly parasitism in lower-quality habitats, where the absence or scarcity of understory plants limited food sources and survival of adult blowflies (Eeva et al. 1994; Eeva & Klemola 2013). The two habitats of the study population differ markedly in the composition and structure of vegetation, and following the above idea, we found a higher prevalence of blowfly parasitism in the more luxuriant natural oak forest than in the relatively barren pine plantation (Mäntylä et al. 2015; Camacho et al. 2018, 2019). Thus, the pine plantation would be a less attractive habitat for blowflies than the oakwood. In addition, we detected a negative effect of ambient temperature on the intensity of blowfly parasitism (but no association with prevalence). Protocalliphora species, being ectotherms, depend on environmental temperature for their development and activity (Bennett & Whitworth 1991). The negative relationship between temperature and parasitism intensity contrasts with some previous studies describing that cold conditions can decrease blowfly infestations or that blowfly abundances increase with temperature (Gold & Dahlsten 1989; Bennett & Whitworth 1991; Merino & Potti 1996; Dawson et al. 2005; Mennerat et al. 2021; Castaño-Vázquez et al. 2022). However, this relationship is host-specific, suggesting an indirect relationship of temperature with the parasite through a direct effect on hosts (reviewed by Albert et al. 2023). This may be the case in our population, where a positive relationship between temperature and breeding success dependent on parasitism intensity was detected, pointing to a better response of hosts to parasitism or an attenuated effect of infestation under warmer conditions (see section (B) Interactive effects of environmental factors and blowfly parasitization on host fitness). Also, it has been reported that temperature increases above certain thresholds could become detrimental to blowfly populations (Dawson et al. 2005; Ogden & Lindsay 2016; Moreno-Rueda 2021). However, we have not found quadratic effects of temperature, so we rule out a non-linear relationship between temperature and parasitism and suggest the indirect host-mediated relationship between temperature and blowflies mentioned above. Unlike temperature and contrary to predictions, there was no association between rainfall and the prevalence or intensity of blowfly parasitism, supporting previous research in flycatchers (Eeva et al. 1994, but see Merino & Potti 1996). Other studies in different passerines reported mixed evidence (Heeb et al. 2000; Wesołowski 2001), including a recent study with blue tits in a study area close to ours, where Merino et al. (2024) found a positive relationship with rainfall in interaction with temperature. In view of this, the effects of rainfall on blowfly parasitism appear to be host-dependent, as is the case with temperature (Albert et al. 2023).
Blowfly prevalence and intensity were also related to the intrinsic characteristics of the brood. Thus, the probability of parasitization, but not its intensity, increased as the season advanced. In other passerines, variable relationships between blowfly prevalence or abundance and breeding date have been reported (positive: Roby et al. 1992; Maziarz et al. 2022; no significant relationship: Hurtrez-Boussès et al. 1999). The higher prevalence of blowflies in late nests in our study may be due to better conditions for parasite development as spring progresses (e.g. availability of flowers for blowfly adults; Eeva et al. 1994; Eeva & Klemola 2013) or due to infestation by adult blowflies hatched earlier in the same year, which is more likely later in the season (Bennett & Whitworth 1991). Nests with larger brood sizes also suffered from a higher prevalence and intensity of parasitism. The relationship between brood size and blowfly parasitism intensity is also inconsistent across passerine species (Hurtrez-Boussès et al. 1999; Wesołowski 2001; Dawson et al. 2005; Maziarz et al. 2022), suggesting that the relationship is host-specific or environmentally mediated. Large host broods are probably more attractive to adult blowflies and/or favor larvae developmental success given that they provide more resources for the larvae.
(B) Interactive effects of environmental factors and blowfly parasitization on host fitness: Parasitism by blowflies has been found to increase nestling mortality in several passerine species (Bańbura et al. 2004; Puchala 2004; Simon et al. 2004), but a lack of effect has also been reported in several studies (Roby et al. 1992; Johnson & Albrecht 1993; Wesołowski 2001; Dawson et al. 2005; Hannam 2006; Streby et al. 2009; Garrido-Bautista et al. 2023), including those focused on other flycatcher populations (Eeva et al. 1994; Moreno et al. 2002). In a previous study in our population, an effect of blowflies on nestling mortality was found, but only in one of the three studied years, suggesting that the effect may be year-dependent (Merino & Potti 1995a, 1996). Discrepancies between studies suggest that other factors may play a role in parasite–host relationships. For example, it may be expected that the detrimental effects of parasites would be enhanced in the presence of additional stressors, such as adverse environmental conditions (Antoniazzi et al. 2011; Møller et al. 2013; Castaño-Vázquez et al. 2021; Dunn et al. 2021; Hall 2021). Accordingly, high temperatures during the nestling stage were associated with increased fledging success in nests with high parasite loads. A possible explanation for this temperature-mediated effect might be an enhanced immune response against parasitism favored by warm temperatures (or an attenuated response with decreasing temperature), as already described in other birds (Lifjeld et al. 2002; Ardia 2005; Garvin et al. 2006; Butler et al. 2009). This effect may be particularly relevant if the heightened response extends to other pathogens, as blowfly parasitism is associated with higher risks of bacterial and viral infections in nestlings (Warren 1994; Mennerat et al. 2009). A non-mutually exclusive explanation for the interactive effect of temperature and parasitism on host fitness might be that favorable environmental conditions during the nestling phase directly or indirectly reduce the adverse effects of ectoparasites on survival, for example, due to increased food availability (Hurtrez-Boussès et al. 1998; Simon et al. 2004; Little 2008), which may translate into faster growth or a better condition to implement physiological responses to ectoparasites (Simon et al. 2004; O'Brien & Dawson 2008; Knutie 2020). However, this last idea does not seem to explain the temperature-dependent effect of parasitism on the breeding success, insomuch as nestling mass was not affected similarly.
Nestling mass was not associated with the intensity of parasite infestation either on its own or in interaction with environmental conditions. This may initially seem surprising as these climatic variables influenced both the nestling mass and the intensity of blowfly infestation. The effect of environmental variables on nestling mass in altricial birds is highly variable in direction (Sauve et al. 2021) and varies geographically (Both et al. 2010), though it has been suggested that parasitism may affect host fitness and condition through the indirect effect of temperature (Møller et al. 2013). The detrimental effects of blowflies on the growth of flycatcher nestlings may be buffered by an early brood reduction due to parasitism (Merino & Potti 1995a). This is a possible explanation for the observed differences between both fitness traits and may affect the lighter nestlings so that surviving fledglings are in similar physical condition. Finally, although the fitness consequences investigated here are a priori restricted to the nestling period, it cannot be ruled out that there may be subsequent detrimental effects related to blowfly parasitism interacting with climatic variables, as has been shown for both factors separately (Potti 2008a,b). Regardless of the underlying mechanisms, our findings highlight the need to analyze the effects of parasites on different proxies of fitness to understand parasite–host dynamics comprehensively.
In response to blowfly infestations of their nests, pied flycatchers may remove some larvae from their nests by nest sanitation behavior (Hurtrez-Boussés et al. 2000; Cantarero et al. 2013), as well as increasing their feeding rates and/or changing the composition of the food delivered to nestlings (Bouslama et al. 2002; Bańbura et al. 2004). This suggests a cost of parasitism shared between adults and nestlings that may be partially compensated (Hurtrez-Boussés et al. 2000; Cantarero et al. 2013). We cannot rule out that nest sanitization in our population may counteract increases in blowfly abundance in nest boxes, although we discount extrinsic factors such as climatic variables as having a significant effect on this behavior. Another possible defense against nest ectoparasites may be the removal of old nest material that may attract or contain more ectoparasites (Merino & Potti 1995b; López-Arrabé et al. 2012). In this regard, our nest boxes are thoroughly cleaned by researchers between breeding seasons, so we ruled out this situation that could generate noise in our results.
In summary, our study highlights the context-dependent nature of host–parasite relationships. The relationship between the intensity of parasitism and temperature and their combined effects on breeding success emphasizes the importance of considering multiple climate-mediated effects in the context of ongoing climate change and its impact on avian populations (Brooks & Hoberg 2007; Pautasso 2012; Mennerat et al. 2021). Our findings contribute to understanding the multifaceted relationships between parasitism and environmental factors and their potential implications for the population dynamics of hosts (Antoniazzi et al. 2011; Møller et al. 2013; García del Río et al. 2020).
ACKNOWLEDGMENTS
This research received support from projects CGL2006-07481/BOS (to J.C. Senar), CGL2009-10652 (to J.C. Senar), CGL2011-29694 (to J. Potti), and CGL2014-55969-P (to F. Valera) from the Spanish Ministry of Education, as well as project PAC05-006-2 (to J.A. Dávila) from the Universidad de Castilla-La Mancha (Spain). During the writing process, E.G.B. was supported by a Margarita Salas Contract financed by the European Union-NextGenerationEU and the Recovery, Transformation, and Resilience Plan (Spanish Ministry of Universities). D.C. received support through a Talent Attraction fellowship from the Autonomous Community of Madrid (CAM), Spain (2022-T1_AMB-24025), and the projects PID2022-141763NA-I00, CGL2015-70639-P, and PID2019-104835GB-I00, funded by MCIN/AEI (doi: 10.13039/501100011033). C.C. was supported by the grant ref. RYC2021-033977-I funded by MCIN/AEI/10.13039/501100011033 and the European Union NextGenerationEU/PRTR. J.M.P. was supported by the ARAID Foundation. Funding for open access charge: Universidad de Granada/CBUA.




