An expanded phylogeny of the genus Pseudamnicola (Gastropoda; Truncatelloidea; Hydrobiidae) across the Mediterranean Basin
Abstract
The genus Pseudamnicola Paulucci, 1878, is commonly found throughout the Mediterranean region. The genus displays considerable levels of endemism, accompanied by notable systematic and taxonomic ambiguity. However, the application of molecular data has proven highly effective in clarifying taxonomy and unveiling the diversity of cryptic species within the genus. Therefore, we employed all cytochrome c oxidase subunit I sequence data available and generated new ones from Greece to infer the phylogeny of the genus throughout its Mediterranean range and estimate the divergence times as well as the ancestral area of diversification. Our phylogenetic and time-estimate analyses demonstrate that with 36 to 38 extant Pseudamnicola species and genetic divergences across species ranging from 0.5% to 11.9% on average, the genus underwent relatively recent diversification during late Miocene (6.53 Ma), and the primary speciation events occurred during Plio-Pleistocene. The Italian Peninsula and Islands and the Ionian Drainages as defined by the Freshwater Ecoregions of the World are the ancestral regions of the genus following two different dispersal routes. Our study contributes to deepening our understanding of Pseudamnicola phylogeny by using data from throughout its range for the first time. This phylogeny provides evidence and confirms previous studies that relatively recent habitat isolation, followed by founder and dispersal events, has been one of the primary reasons for the evolution of the genus Pseudamnicola in the Mediterranean basin.
INTRODUCTION
Freshwater gastropods are a highly diverse group of molluscs that is often used for studying speciation and radiation processes. Among freshwater families, one takes the spotlight—the Hydrobiidae family, a species-rich truncatelloidean group. This family has been a focal point in numerous studies that delve into questions about evolutionary processes and the mechanisms that drive them. What makes the Hydrobiidae family so advantageous is the long evolutionary history, the large geographic distribution, and diverse ecological and morphological features, as well as the tremendous variety of habitats successfully colonized (Delicado et al. 2015).
The genus Pseudamnicola Paulucci, 1878 is one of the most speciose genera within Hydrobiidae, and it is widely distributed throughout the Mediterranean basin ranging from the Iberian Peninsula to Italy, Tunisia, the Balkan Peninsula, the Aegean Archipelago, and the Middle East (Glöer et al. 2015; Delicado et al. 2015, 2018). Species of Pseudamnicola have successfully colonized various freshwater habitats such as lakes, coastal streams, and springs, but mostly occur in aquatic systems of middle or low elevations (Delicado et al. 2018). Delicado et al. (2018) discovered that the rates of diversification in Pseudamnicola species increase from mid to lower elevations, indicating that the move to lower elevations may have had a role in their exceptional diversification. It is very likely that the greater presence of dispersal vectors at low elevations, such as birds and fish, as well as the higher connectivity among lower-reach sites may have aided in the colonization of various habitats (Delicado et al. 2018).
The genus displays high endemicity levels, but also systematic and taxonomic ambiguity (Haase et al. 2007; Delicado et al. 2013; Radea et al. 2016). The initial descriptions of numerous species were based almost entirely on shell morphology and, in some cases, also on the anatomy of reproductive systems. Shell features, though, have been acknowledged as insufficient for species discrimination, when used exclusively, due to weak sculpture and few distinguishing characteristics (Szarowska et al. 2006; Bunje & Lindberg 2007; Szarowska et al. 2015; Radea et al. 2016). However, despite that the anatomy is pretty simple and varies even within a single population due to miniaturization and similar adaptations for internal fertilization and development in freshwater systems (Delicado et al. 2013; Szarowska et al. 2015), there are anatomical characteristics that differ across species, such as the radula formula and size, nervous system traits, and the shape of female genitalia components that can assist with species discrimination (Radea et al. 2016).
Recent studies, however, have demonstrated that the use of molecular data has effectively managed to disentangle taxonomy and reveal the diversity of cryptic species within the genus (Delicado et al. 2012, 2015; Szarowska et al. 2015). The integration of shell morphology, anatomical attributes (especially from female genitalia and radulae), and molecular data have become a necessity to delineate species with confidence (Delicado et al. 2013, 2016; Szarowska et al. 2015; Radea et al. 2016). In the current study, we are aiming to: (i) infer the phylogenetic relationships of Pseudamnicola taxa distributed throughout the Mediterranean, (ii) investigate the underlying causes of the genus diversification within the area, (iii) estimate the time frame of Pseudamnicola species radiation, and (iv) evaluate the taxonomic status of previously described species.
MATERIAL AND METHODS
Data collection
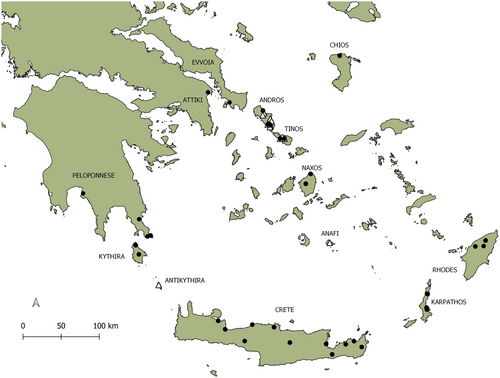
Specimens of Pseudamnicola from 11 freshwater localities distributed in the Aegean islands of Andros, Tinos, Anafi, and Antikythira and one locality from Cyprus were collected (Fig. 1). Some of the localities of Andros and all the localities from Tinos were previously known from Szarowska et al. (2015) and have been revisited. The specimens from Antikythira come from a newly discovered population recorded for the first time. All the specimens were collected by hand and preserved in 100% ethanol for further morphological and molecular analyses. Following the collection, morphological and anatomical assessment was performed to assign specimens to described species. Before dissection, the shells were removed by soaking in Perenyi solution (Clayden 1973). Shell and soft body features were photographed using a Canon EOS 1000D camera attached to a stereomicroscope (Stemi 2000-C, Zeiss, Germany). Anatomical terminology follows that of Hershler and Ponder (1998).
DNA data generation
For the extraction of DNA from specimens, we implemented the Macherey–Nagel NucleoSpin® Tissue kit following the manufacturer's protocol. Due to their small body size, the entire animal was used. Portions of the mitochondrial DNA (mtDNA) genes cytochrome c oxidase subunit I (COI), 16S rDNA, and cytochrome b were amplified from the specimens used. Especially for cytochrome b, custom-designed primers were used. However, both 16S rDNA and cytochrome b exhibited very low levels of sequence divergence and thus have not provided any support during the phylogenetic analyses. Thus, sequence data from these two gene fragments have not been used. Two different primer pairs were utilized in the PCR assays for the COI gene. The specimen being amplified determined the choice of primers to be used in the PCR. The universal COI primers, LCO1490 5′-GGTCAACAAATCATAAAGATATTGG-3′ and HCO2198 5′-TAAACTTCAGGGTGACCAAAAAAT-3′ (Folmer et al. 1994), were utilized as the first pair, while primer C1-J1718 5′-GGAGGATTTGGAAATTGATTAGTTCC-3′ (Simon et al. 1994) was used in place of LCO1490 in the second pair of primers. Each PCR was performed in a 25 µL volume, where 2 µL of template DNA was mixed with 0.2 mM dNTPs, 3.5 mM MgCl2, 0.4 µM of each primer, and 0.5 units of Taq Polymerase (Kappa). The PCR conditions were as follows: initial denaturation step of 3 min at 95°C, followed by 40 cycles of 15 s at 94°C, 1 min at 42°C, 1.5 min at 72°C, and a final extension of 10 min at 72°C. The PCR products were purified using Clean-Up columns (Macherey-NagelTM NucleoSpinTM Gel and PCR Clean-up Kit). Both strands of the purified PCR products were sequenced using the same pairs of primers as in the PCR amplification. The generated sequences were edited and aligned (Clustal algorithm) using the software CodonCode Aligner v. 2.06. The homology of the generated sequences to the targeted mtDNA gene was evaluated through a BLAST search.
In this study, 11 specimens—one from each sampling locality—were sequenced and pooled with 353 previously published sequences from Pseudamnicola populations across the Mediterranean region, out of which 124 are sequences from non-Greek populations (Table S1, Supporting Information). For species that have not been officially described and named yet, but are considered distinct species, the temporary names provided in previous studies were retained. The newly produced sequences have been deposited in GenBank under the Accession Numbers: OR490944–OR490955, OR490956 (Table S2, Supporting Information). The distribution of the Pseudamnicola populations across the Mediterranean and Greece is shown in Fig. 2. To better assess the phylogenetic relationships between the different Pseudamnicola species, three sequences of Mercuria emiliana (Paladilhe, 1869), which was considered to belong to the genus Pseudamnicola by Boeters and Falkner (2017), but contradicted later by Boulaassafer et al. (2018) and Miller et al. (2023), and three sequences of Erosiconcha geldiayana (Schütt & Bilgin 1970), which was also considered to be a Pseudamnicola species (Schütt & Bilgin 1970; Delicado & Gürlek 2021), were also included in the dataset (Table S1, Supporting Information). In addition, the species Corrosella bareai and Corrosella manueli (Delicado et al. 2012), were included in the analysis, since Corrosella is closely related to Pseudamnicola. Last, the species Peringia ulvae (Pennant, 1777) (AF118288) was used as an outgroup (Wilke & Davis 2000).
Genetic diversity and phylogenetic analysis
DnaSP v6.11.01 was used to calculate the unique haplotypes and haplotype and nucleotide diversity of the final data set (Rozas et al. 2017). The haplotypes from the Greek populations are presented in Table 1. MEGA v. 10.1.7 (Kumar et al. 2018) was used to calculate the uncorrected p genetic distances separating individual or grouped sequences, which are favored when sequences were short and came from sister taxa (Srivathsan & Meier 2012). Prior to the phylogenetic analysis, no saturation was found in any of the haplotypic sequences tested using the Iss statistic by Xia et al. (2003) in DAMBE (Xia 2018).
| Pseudamnicola spp. | Haplotype ID | Abbreviation | Locality | GenBank accession No. | References |
|---|---|---|---|---|---|
| P. sp. A | TINOS15 | TN15 | Komi | OR490954 | Present study |
| P. sp. A | TINOS16 | TN16 | Kardiani | OR490953 | Present study |
| P. sp. C | ANAFI1 | ANF1 | Vrysi | OR490945 | Present study |
| P. sp. B | ANDROS48 | AND48 | Vitali | MW751930 | Lampri et al. (2022) |
| P. sp. B | ANDROS41 | AND41 | Amoniakleon | OR490949 | Present study |
| P. sp. D | ANDROS43 | AND43 | Episkopio | MW751929 | Lampri et al. (2022) |
| P. sp. D | ANDROS46 | AND46 | Piso Meria | OR490946 | Present study |
| P. cf. chia | ANDROS22 | AND22 | Amoniakleon | KT710616 | Szarowska et al. (2015) |
| P. magdalenae | ANTIKYTHIRA1 | ANTIKYTH1 | Potamos | OR490955 | Present study |
| P. magdalenae | KYTHIRA1 | KYTH1 | Karavas | JF921906 | Falniowski (2016) |
| P. magdalenae | KYTHIRA2 | KYTH2 | Karavas | JF921907 | Falniowski (2016) |
| P. magdalenae | KYTHIRA3 | KYTH7 | Karavas | JF921912 | Falniowski (2016) |
| P. macrostoma | ATTIKI1 | ATT1 | Marathon, Kato Souli | KF758783 | Radea et al. (2016) |
| P. chia | CHIOS3 | CH3 | Leptopodha - Amadhes | KT710636 | Szarowska et al. (2015) |
| P. occultus | CRETE6 | CR6 | Fodele | MW732972 | Lampri et al. (2022) |
| P. occultus | CRETE17 | CR17 | Andravastoi | MW732979 | Lampri et al. (2022) |
| P. occultus | CRETE25 | CR25 | Sitia | MW732987 | Lampri et al. (2022) |
| P. occultus | CRETE26 | CR26 | Geropotamos | MW732988 | Lampri et al. (2022) |
| P. occultus | CRETE28 | CR28 | Geropotamos | MW732990 | Lampri et al. (2022) |
| P. cf. chia | CRETE33 | CR33 | Almiros, Chania | KT710660 | Szarowska et al. (2015) |
| P. negropontinus | EVVOIA1 | EVV1 | Marmari | KM668770 | Delicado et al. (2015) |
| P. negropontinus | EVVOIA3 | EVV3 | “Kato Souli”* | EF061915 | Szarowska et al. (2006) |
| P. pieperi | KARPATHOS1 | KAR1 | Prasteio | KT174487 | Radea et al. (2016) |
| P. pieperi | KARPATHOS2 | KAR2 | Aperi | KT174488 | Radea et al. (2016) |
| P. pieperi | KARPATHOS3 | KAR3 | Olympos | KT174489 | Radea et al. (2016) |
| P. pieperi | KARPATHOS4 | KAR4 | Agios Nikolaos temple | KT174490 | Radea et al. (2016) |
| P. pieperi | KARPATHOS7 | KAR7 | Aperi | KT710670 | Szarowska et al. (2015) |
| P. sp. E | NAXOS1 | NX1 | Aghio Kyriaki, Mesi Potamia | KT710679 | Szarowska et al. (2015) |
| P. sp E | NAXOS8 | NX8 | Aghia | KT710686 | Szarowska et al. (2015) |
| P. exilis | PELOP1 | PELOP1 | Agio Theodori, Taigetos Mt | JF921885 | Szarowska and Falniowski (2011) |
| P. sp. G | PELOP11 | PELOP11 | Ayios Niron, Sfondyli | JF921895 | Szarowska and Falniowski (2011) |
| P. sp. G | PELOP12 | PELOP12 | Ayios Niron, Sfondyli | JF921896 | Szarowska and Falniowski (2011) |
| P. sp. F | PELOP20 | PELOP20 | Nomia, Monvemvasia | JF921904 | Szarowska and Falniowski (2011) |
| P. sp. F | PELOP22 | PELOP22 | Maleas | MW751931 | Lampri et al. (2022) |
| P. ilione | RHODES1 | RH1 | Salakos | KT174492 | Radea et al. (2016) |
| P. ianthe | RHODES1 | RH1 | Petaloudes Valley | KT174493 | Radea et al. (2016) |
- * The site “Kato Souli” is probably an incorrect entry in Genbank. According to Genbank registration information, the origin of the sequence with Accession Number EF061915 is Kato Souli. The reference Szarowska et al. (2006) is linked to this accession number; the specimen of Pseudamnicola negropontinus is reported to be Marmaris (Marmari) on Evvoia Island.
The best-fit molecular evolution model of nucleotide substitution was investigated using PartitionFinder v2.1.1 (Lanfear et al. 2012). The models TrN + I + G and TRNEF + G were favored for the two partitions, codons 1 and 2 and codon 3, respectively. Maximum likelihood (ML) and Bayesian Inference (BI) methods were applied to infer the phylogeny using only the haplotypes. BI was conducted in MrBayes v 3.2.7 (Ronquist et al. 2012) implementing the parameters of the substitution models as suggested by PartitionFinder. Four simultaneous Monte-Carlo Markov chains and two separate runs with 2 × 106 generations each were used. The first 25% of trees were discarded as burn-in. Under the “allcompat” topology option, a consensus tree was created. The ML analysis was performed using the IQTREE2 web server (http://iqtree.cibiv.univie.ac.at/) under the Bayesian information criterion with 1000 ultrafast bootstraps (Trifinopoulos et al. 2016). The partitioned dataset was also used as input for model selection.
Assessing species boundaries
Although delineating species within the examined populations of Pseudamnicola was not among the goals of the current study, the preliminary differentiation between nominal species and local populations was considered an important prior step. Hence, we implemented the Automatic Barcode Gap Discovery (ABGD) method on the web server platform available at https://bioinfo.mnhn.fr/abi/public/abgd/abgdweb.html. This approach aims to characterize the position of the barcode gap separating intra-from interspecific distances (Puillandre et al. 2012). In ABGD, the process involves an initial partition and a recursive partition to identify putative species or groups within a dataset. The initial partition is the first step that broadly groups sequences based on a specified genetic distance threshold, while the recursive partition involves a more detailed analysis, breaking down these initial groups into finer partitions to better capture the diversity within each group. Prior to the analysis, a matrix of pairwise uncorrected p-distances between each pair of sequences was computed in MEGA X v. 10.1.7 (Kumar et al. 2018). The default settings of Pmin = 0.001, Pmax = 0.1, steps = 10, and Nb bins (for distance distribution) = 20 were kept. However, when the default value of X (relative gap width) = 1.5 was implemented, the analyses could not be performed, and a lower X value was used instead. Hence, we used a small relative gap width of X = 0.8 to obtain a wide range of partitions from strict to more relaxed species-delimitation partitions. The analysis was performed employing the Kimura K80 model (Kimura 1980).
Estimation of divergence times
To estimate the divergence times of the genus across its distribution range, a maximum clade credibility (MCC) tree using only haplotypic data was generated in BEAST v1.8.4 (Drummond et al. 2012). The final dataset comprised 89 COI haplotypic sequences of Pseudamnicola of a total of 702 bp with a haplotypic diversity (Hd) of 0.9743, 2 sequences of Corrosella, and the outgroup P. ulvae. The substitution rate for COI, determined by Delicado et al. (2013) to be 0.81% ± 0.24% per million years (percentage of substitutions per lineage Myr−1), was the one implemented in the time estimate analyses. Two independent runs were conducted with a chain length of 2 × 107 each. The convergence of the chains and the corresponding estimated sample sizes (ESSs > 200), which are two measures of convergence, were inspected with TRACER v. 1.7.1 (Rambaut et al. 2018). The runs were combined with LogCombiner v. 1.8.4, and TreeAnnotator v. 1.10.4 was used to summarize the information from the trees after discarding as burn-in the initial 10% of trees. The MCC tree was visualized using FigTree v. 1.4.3 (Rambaut 2013).
Ancestral range reconstruction
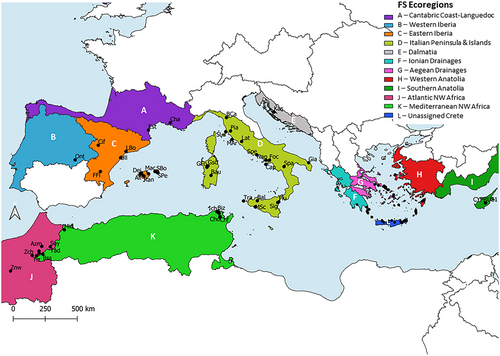
The distribution range of Pseudamnicola was divided into 12 areas, based on the worldwide freshwater ecoregions as shown in Fig. 2 (Abell et al. 2008). These areas are: Cantabric Coast—Languedoc (A), Western Iberia (B), Eastern Iberia (C), Italian Peninsula and Islands (D), Dalmatia (E), Ionian Drainages (F), Aegean Drainages (G), Western Anatolia (H), Southern Anatolia (I), Atlantic NW Africa (J), Mediterranean NW Africa (K), and an Unassigned region that corresponds to Crete Island (L). The BioGeoBEARS v. 1.1.2 package (Matzke 2013) in R v. 4.1.2 was used to infer ancestral ranges using the time-calibrated phylogenetic tree. The MCC tree generated in BEAST was used as input data and analysis parameters were set up following the tutorial at https://phylo.wikidot.com/biogeobears. Outgroups were excluded from the analyses, while each species was represented in the tree by a single lineage, and the maximum areas per lineage were set at three according to the maximum number of ecoregions that a single Pseudamnicola species is found. The models used for the analysis were the dispersal-extinction-cladogenesis model (DEC), the dispersal-vicariance analysis, and the BayArea model. These models include two free parameters (d = dispersion or range extension and e = extinction or range contraction). The founder effect parameter j, suitable for island systems (Matzke 2013), was also added to each of these models to evaluate whether it improves model fitting. AIC was used to select the models that best fit the data.
RESULTS
Phylogenetic inference and species boundaries
Most of the basal relationships and most of the terminal nodes that correspond to the current species are well resolved in ML and BI analyses; however, the in-between relationships of the main subgroups are unclear. Only the ML analysis is presented in Fig. 4, while the BI analysis can be viewed in Fig. S2, Supporting Information. The uncorrected p-distances between the main lineages are presented in Fig. S1, Supporting Information. The highest genetic distances estimated were between E. geldiayana and M. emiliana and the other Pseudamnicola species with an average of 17.5% and 16.4%, respectively. These high genetic divergences, along with their placement away from the other congeneric species, are suggestive of their assignment to two new genera.
The Corrosella species form a distinct group, which is a sister group to all the Pseudamnicola species, except E. geldiayana and P. emilianus, which are clustered outside the Corrosella—Pseudamnicola group. Within the Pseudamnicola group, most of the basal nodes are well-supported and many previously recognized relationships were recovered (Fig. 3). Within the Pseudamnicola group, P. sp. 2 from Tunisia is depicted as a sister taxon to all the other species followed by P. orsinii (Küster, 1852). The phylogenetic positions of P. conovula (Frauenfeld, 1863) from Italy and Croatia and P. calamensis Glöer, Bouzid & Boeters, 2010 from Italy are ambiguous. Within the remaining species, there are two apparent groups with low bootstrap support values, one hosting the Spanish, Moroccan, and Italian species and the second hosting the Spanish, Italian, Greek, and Cypriot species (Fig. 3).
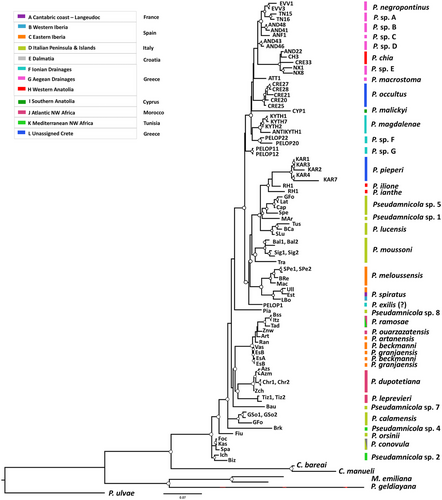
In the first group, species from Majorca Island and Morocco are clustered together with strong support, and they are closely related to P. sp. 7 from Sardinia Island in Italy (Fig. 3). Within the Majorca–Morocco clade, there are two sister groups with adequate support. The first one comprises P. ouarzazatensis Boulaassafer, Ghamizi, Machordom & Delicado, 2020 and P. ramosae Boulaassafer, Ghamizi, Machordom & Delicado, 2020 from Morocco and P. artanensis Altaba, 2007, P. beckmanni Glöer & Zettler, 2007, and P. granjaensis Glöer & Zettler, 2007 from Spain and the second one comprises P. dupotetianus (Forbes, 1838) and P. leprevieri (Pallary, 1928) from Morocco. The haplotypes of the two species, P. beckmanni and P. granjaensis, were not distinguished and exhibited the lowest genetic distances with an average genetic divergence of 0.5%.
In the second main group, there are two smaller clades with good support. P. sp. 8 appears as a sister taxon to them but with low bootstrap support values (Fig. 3). The first of the small clades encompasses P. exilis (Frauenfeld, 1863) from mainland Greece, which is the sister taxon to the remaining species of this clade. Sister relationships between P. spiratus (Paladilhe, 1869) (former P. subproductus) and P. meloussensis Altaba, 2007 from Spain, as well as between Pseudamnicola sp. 1 and Pseudamnicola sp. 5 from Italy are recovered with strong support. The phylogenetic position of P. moussonii Boeters & Glöer, 2015 within this clade is unclear. P. lucensis (Issel, 1866) is distinguished with good support, and last, P. pieperi Schütt, 1980, P. ianthe Radea & Parmakelis, 2016 and P. ilione Radea & Parmakelis, 2016 from southeast Aegean Archipelago in Greece were all grouped with high support. P. ianthe is strongly supported as a sister taxon to P. ilione and P. pieperi in both the ML and the BI analyses. Despite the monophyly of P. pieperi from Karpathos Island, high levels of intraspecific diversity were observed. The maximum genetic distance of 4.3% was observed between haplotypes that all originate from the type locality of the species.
The second small subclade includes the remaining of the Greek species and P. malickyi Schütt, 1980 from Cyprus. However, the phylogenetic placement of P. malickyi and P. macrostoma in relation to the other Greek species is ambiguous (Fig. 3). P. negropontinus from Evvoia Island, P. occultus from Crete Island, and P. magdalenae from Kythira Island are well separated. The new haplotype from Antikythira Island is grouped with the haplotypes of P. magdalenae and diverges at a maximum of 2.5%. The haplotypes from Andros Island split into three distinct groups. Specifically, haplotype AND22 from Andros is grouped with P. chia (E. von Martens, 1889) and a haplotype from Crete. The genetic divergences between the three haplotypes do not exceed 2.5%. Haplotypes from Naxos form a well-distinct group. The Anafi haplotype is distinct, while the haplotypes from Tinos share a sister relationship with P. negropontinus (Fig. 3). Last, the haplotypes from Peloponnese that were all assigned to P. exilis split into three groups separated by high genetic distances (3.6%).
In total, 36–37 Pseudamnicola species were retrieved according to the ML and the BI analyses. The single species difference in the total number of species is due to the over-split observed between P. beckmanni and P. granjaensis. In the ABGD method, the recursive partition supported 47 operational taxonomic units (OTUs), while the initial partition supported 46 OTUs (Fig. S3, Supporting Information). Overall, the ABGD method retrieved 16 out of the 37 species (43%) and displayed a tendency to split previously established species into two or more groups.
Divergence time estimation
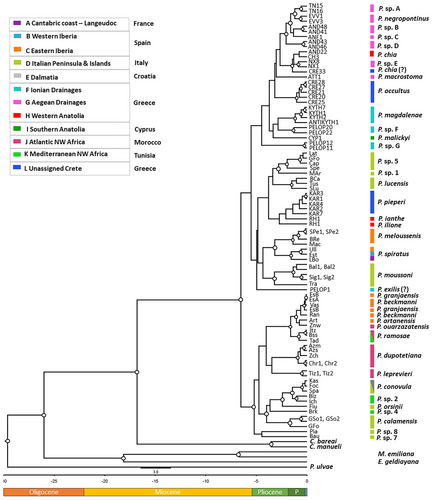
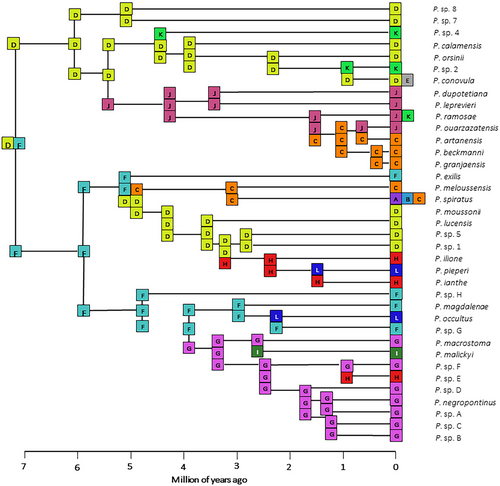
The chronogram generated in BEAST using an evolutionary rate of 0.81% ± 0.24% substitution/Myr for the COI fragment (Delicado et al. 2013) is presented in Fig. 4. The topology of the ultrametric tree exhibits similarities with the ML topology regarding the main groups. However, there is a lack of adequate support in the interior nodes of the two main subclades, which include the species from Spain, Italy, Greece, and Cyprus. According to the inferred time estimates, the diversification of Pseudamnicola is estimated to have occurred 6.53 Ma ago during the late Miocene, while the major speciation events leading to the present-day species are estimated to have occurred from 4 to 1 Myr during the late Pliocene with the highest rate observed during the Pleistocene (Fig. 4).
Ancestral range reconstruction
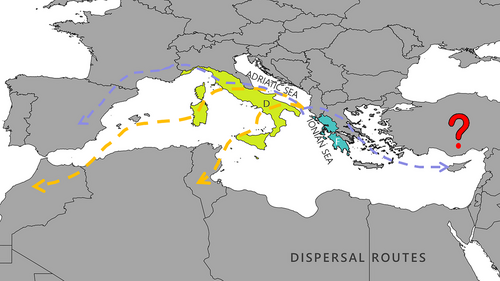
The unconstrained DEC model was identified as the best-fit model by ancestral range estimation using BioGeoBEARS, and adding the founder-event parameter (+j) increased the log-likelihood of this model for all analyses (P < 0.005). The DEC +J model obtained the highest score in model fitting (AIC = 171.60), which includes a long-distance dispersal (jump or founder effect event) parameter (Matzke 2013). The results are presented in Fig. 5. The analysis indicates the Italian Peninsula and Islands along with the Ionian Drainages, both members of the Peri-Mediterranean Region during the late Miocene (11.62–5.333 Myr) (Neubauer et al. 2015), as the ancestral area where the main cladogenetic events of the Pseudamnicola genus took place.
DISCUSSION
To date, this is the most comprehensive molecular study of the Pseudamnicola genus across its Mediterranean range. The dataset included all the COI sequences of Pseudamnicola available combined with newly generated ones and COI sequences from two Corrosella species and two species, namely E. geldiayana and P. emilianus that allegedly belong to Pseudamnicola as well. However, we should mention that 62 nominal Pseudamnicola species were not included in the current study due to the lack of molecular data for these species. The present study is in accordance with previous studies and demonstrates the distinction between the genera Pseudamnicola and Corrosella (Delicado et al. 2018, 2024). Furthermore, it confirms the misassignment of E. geldiayana and M. emiliana to the genus of Pseudamnicola in previous classifications (Delicado & Gürlek 2021; Miller et al. 2023).
The phylogenetic inferences and the estimation of the time of divergence showed that Pseudamnicola diversified relatively recently during the late Miocene (6.53 Ma). This aligns well with the diversification timeframe previously reported in other studies for the genus (Lampri et al. 2022; Delicado et al. 2024). Most speciation events took place during the Plio-Pleistocene, resulting in 36–38 extant Pseudamnicola species and genetic divergences that range from 0.5% to 11.9% between species with an average of 7.1%. The ABGD method supported only 43% of the OTUs that correspond to nominal species, possibly due to the wide range of species-specific divergence levels recorded in Pseudamnicola. Some species, such as P. calamensis, P. lucensis, P. meloussensis, P. moussonii, and P. pieperi were split, while other species such as P. artanensis, P. beckmanni, P. granjaensis, and P. ouarzazatensis were clustered as single species.
According to the ancestral range estimation, the ancestral region of Pseudamnicola is the Italian Peninsula and Islands (D) together with the Ionian Drainages (F). During the late Miocene when the diversification of the genus began, these two ecoregions were both part of the Peri-Mediterranean Dominion, which was also a part of the extended Peri-Mediterranean Region (Neubauer et al. 2015). At that time, the Messinian salinity crisis (MSC) occurred, resulting in massive desiccation of the Mediterranean basin, widespread evaporite precipitation, and the reconnection of many land masses that were previously isolated (Krijgsman et al. 1999; Anzidei et al. 2011). The series of deposits transitioning from freshwater to brackish and marine conditions are commonly known as “Lago-mare” and have been documented across the entire Mediterranean basin from mainland Italy, and a Peri-Mediterranean Dominion to Spain, France, Sicily, and Greece (Krijgsman et al. 1999; Neubauer et al. 2015). It is important to acknowledge, though, that these brackish water “Lago-Mare” basins likely provided a suitable habitat for brackish-water snails (Do Couto et al. 2014; Falniowski et al. 2021). These brackish environments along with the land corridors connecting Sicily and Sardinia to Africa and the Balearic Islands to the Iberian Peninsula (Delicado et al. 2015 and references therein) and the desiccation of the Adriatic Sea (Anzidei et al. 2011) have most likely facilitated dispersal events to remote areas throughout the Mediterranean basin. Two different dispersal routes are suggested for the gradual spread of Pseudamnicola within the Mediterranean (Fig. 6). One of the routes involved a westward dispersion toward the Iberian Peninsula and then to NW Africa. The second dispersal route involves a westward dispersion toward Italy and Spain and an eastward one toward the Aegean Archipelago and Anatolia (Figs 5, 6). The salinity crisis ended in 5.33 Ma with the Zanclean flood (Sondaar & Dermitzakis 1982; Neubauer et al. 2015) when the Gibraltar Strait reopened and there was a sea level rise leaving many areas isolated again. Ancestral range reconstruction would benefit greatly from genetic data from more Pseudamnicola species from the Western Balkans (between Croatia and Peloponnese), from Libya to Israel, and southeast Turkey, where the genus is also distributed (Bunje & Lindberg 2007). This would allow for a complete tracking of the dispersal pathways taken toward the east.
The two different dispersal routes depicted in the chronogram resulted in two large clades (Fig. 4). The first clade comprises the species from Croatia, Italy, Spain, Morocco, and Tunisia, and the second clade comprises the species from Greece, Cyprus, Italy, France, and Spain. Interestingly, there are no sister relationships found between the Moroccan species and the other northern African species originating from Tunisia showing independent colonization events. Within the first clade, the Majorca—Morocco subclade is recovered in both the ML and the BI analyses, but in the time-estimate analysis, this relationship was not strongly supported. However, a recent diversification of this group is plausible through dispersal processes resulting in speciation events. This conclusion agrees with the study of Boulaassafer et al. (2020). The lowest genetic distances separating Pseudamnicola species were estimated between P. artanensis, P. beckmanni, and P. granjaensis (min. 0.5% and max. 1.7%) from Majorca Island. In contrast to P. artanensis, which is more distant both genetically and geographically, P. beckmanni and P. granjaensis are recorded in habitats that are close to one another (Delicado et al. 2014). This is also reflected in the phylogenetic inference, where P. artanensis was recovered as a monophyletic haplotypic group, while the haplotypes of the other two species were split (Fig. 3). Such low genetic distances are an exception to the rule for the genetic distance between Pseudamnicola species. Even though species-specific morphological shell attributes and male genitalia were employed for delimiting P. beckmanni and P. granjaenesis (Glöer & Zettler 2007), additional data from female genitalia and radulae would be essential to validate the distinctiveness of the two species. As it concerns the Tunisian species, both ML and BI analyses showed that P. sp. 2 from Tunisia is a sister taxon to all the other Pseudamnicola species, while the time-estimate analysis placed it within the Italy–Tunisia group as a sister species to P. conovula from mainland Italy and Croatia (Figs 3, 4). P. sp. 4 from Tunisia seems close to P. orsinii and P. calamensis from Italy, but their in-between relationships remain unclear due to low support values in all analyses.
Concerning the second large clade, two subclades with adequate support are formed in both ML and BI analyses, which include species from Italy, Greece, and Spain in the first subclade and the remaining species from Greece and P. malickyi from Cyprus in the second (Fig. 4). These two subclades are also recovered in the ultrametric tree, but the divergence time of the split could not be estimated due to low posterior probability estimates (Fig. 4). The majority of the estimated speciation events within these subclades seem to have taken place during the Late Pliocene and Early Pleistocene.
Within the first subclade, our analyses recovered with strong support for the Minorca–Iberian clade. The divergence time between P. spiratus and P. meloussensis was estimated at 3.17 Myr (1.63–5.28 Myr; Fig. 4); a time estimate subsequent to the estimated time of the formation of the Balearic Islands at 30 Ma (Rosenbaum et al. 2002). This result is in agreement with other studies that suggest a more recent dispersal event followed by isolation leading to speciation in this area (Boulaassafer et al. 2020). It is interesting the clustering of the three species, P. pieperi, P. ianthe, and P. ilione from the SE Aegean Sea in the Western Anatolia ecoregion with Italian species and not with the other Greek ones with, however, poorly supported clades in the time-estimate analysis. In the Early Late Miocene (9 Ma), the western and eastern Aegean islands were separated by the Middle-Aegean Trench, located east of Crete and west of Kasos-Karpathos island system (Creutzburg 1963; Dermitzakis & Papanikolaou 1981; Fassoulas 2018). Subsequently, in the Early Pliocene, Karpathos became fully isolated from Rhodes island (Skourtanioti et al. 2016). A more complete taxon sampling that would allow the addition of molecular data from other Pseudamnicola species that occur in eastern Aegean islands and Turkey would shed more light on this unexpected relationship. It is worth noting that based on a phylogenetic analysis performed (not presented herein), the sequences of the study of Delicado et al. (2024) generated from Turkish specimens allegedly belonging to Pseudamnicola were found to be very distant apart to actual Pseudamnicola species. Therefore, these sequences were not included in the present study.
Within the second subclade, our results strongly support the existence of three distinct species in the Peloponnese region of mainland Greece based on tree topologies and genetic distances (Figs 3, 4). The genetic distances between the three populations from Peloponnese (mainland Greece) are quite high and along with their placement on the ML tree it is most likely that they correspond to three distinct species, P. exilis, P. sp. F, and P. sp. G., and not one as previously thought. Only the western population represented by haplotype PELOP1 should be considered as P. exilis. This population is the one closer to the type locality of the species (Fig. 1). This result agrees with the study of Szarowska et al. (2015). P. sp. G from SW Peloponnese is closely related to P. magdalenae from Kythira Island, which is very close to SW Peloponnese. Antikythira Island is most likely to also host P. magdalenae based on the low genetic distance estimated between the haplotype from Antikythira and P. magdalenae haplotypes as well as their phylogenetic position in all analyses. In all phylogenetic analyses, they form a distinct clade with good support and a recent divergence time (1.17 Ma; 0.46–2.22 Ma; Fig. 4).
Andros, Tinos, Naxos, and Anafi belong to the biogeographic region called Cyclades in Central Aegean Sea. There is a group with adequate support that encompasses P. negropontinus from Evvoia, P. sp. A from Tinos, P. sp. B from Andros, and P. sp. C. from Anafi in all analyses (Figs 3, 4). This group is closely related to P. sp. D from Andros. However, the relationships between P. sp. B and P. sp. C and the haplotypes from Tinos and P. negropontinus of the group remain unclear. P. sp. D also from Andros forms a well-supported distinct clade, and finally, there is one haplotype from Andros that is grouped with P. chia from Chios, one haplotype from Crete, and P. sp. E from Naxos with strong support in the ML and BI trees. On Crete, the presence of two species, the newly described island-endemic Pseudamnicola occultus Glöer & Hirschfelder, 2019 and a second undescribed species from NW Crete (Szarowska et al. 2015; Lampri et al. 2022) is also suspected. The haplotypes from NW Crete and P. sp. D from Andros differ genetically from P. chia by 2.5% and 1.3%, respectively. These estimates are much lower than the 3.6% COI threshold value for the Greek species (Radea et al. 2016); hence, it is very likely that these haplotypes also belong to P. chia. However, additional comparisons using morphological and anatomical data would further clarify this issue. Another intriguing result, which corroborates with the study of Szarowska et al. (2015), is the high levels of intraspecific diversity in Andros, Tinos, and Naxos and the relatively low genetic distances, based on the COI threshold for the genus, between P. negropontinus and some haplotypes from these islands (Table S2, Supporting Information), suggesting that these islands may also be home to P. negropontinus. In addition, relatively low genetic divergence is also observed between the haplotype from Anafi and a haplotype of P. negropontinus. Whether P. negropontinus is spread to these islands or not will be clarified with additional field sampling and the integration of morphological, anatomical, and molecular data.
The evolutionary mechanisms lying behind the speciation of Pseudamnicola in the Aegean Archipelago are associated with the complex paleogeography and history of this area. Crete has undergone several fragmentation and reconnection events before finally forming a single island at the end of the Pliocene or in the Early Pleistocene (Fassoulas 2018), but it was fully isolated from the mainland and Cyclades at 5.5–5.0 and 9.7–8.9 Myr, respectively (Parmakelis et al. 2005). In the Late Pliocene to Early Pleistocene (3.5–1.8 Myr), Evvoia was still a part of continental Greece. The Cyclades formed a central and independent shelf, which has been separated from the mainland since the Early Pleistocene (Foufopoulos & Ives 1999). Orogenetic and eustatic sea-level changes occurred throughout the Pleistocene in the Aegean Archipelago (Sfenthourakis & Triantis 2017). During the Last Glacial Maximum at ca. 20 ka, Andros, Tinos, and Naxos were all part of a “mega-island” (Kougioumoutzis et al. 2014) that was later divided into two smaller islands at ca. 12 ka separating Andros and Tinos from Naxos. Anafi, though, located in the southeastern part of Cyclades, is an oceanic type of island that remained isolated from other land masses since the time of the “mega-island” (Kougioumoutzis et al. 2014). By the end of the Pleistocene (14–10 kyr), the Cyclades began to be fragmented after the rising of the sea level resulting in their current formation at ca. 9 kyr (Lambeck 1996). Last but not least, it has been shown that Chios was previously connected to Asia Minor and only lately (late Pleistocene or Holocene) it became a fully isolated island (Dermitzakis 1990). The close relationship of Chios with Andros and Naxos could be attributed to nearly complete land corridors formed during the Middle Pleistocene due to sea level drops (Szarowska et al. 2015; Osikowski 2017). Consequently, according to the paleogeography of the Aegean and the recent divergence times estimated, Pseudamnicola is most likely to have colonized the Aegean islands via temporary land bridges that emerged after the sea level drops facilitated dispersal followed by vicariant events after the final formation of the islands. Several active and passive dispersal strategies for freshwater mollusks have been proposed (Kappes & Haase 2012), with the latter being quite common in freshwater gastropods via vectors such as fish and birds (Wada et al. 2012). According to personal observations of Szarowska et al. (2015), Pseudamnicola is a slightly amphibiotic organism, as it can be observed crawling and feeding above the water, offering an additional convincing explanation for why it is most likely to be passively transported by birds. The high levels of Pseudamnicola endemism in the Aegean Islands support the idea that climatic/tectonic events during the Pleistocene led to significant fluctuations in freshwater flows and sea level that eventually drove allopatric speciation phenomena in the genus.
Overall, the success and the high species richness of Pseudamnicola throughout the Mediterranean basin may be explained by the ability of the genus to inhabit a variety of habitat types (from headwaters to lowland freshwater systems) (Delicado et al. 2018). Our study provides evidence that dispersal and founder events followed by habitat isolation within a recent time frame have most certainly been among the dominant mechanisms for the evolution of the genus, a result that is in congruence with the findings of recent studies (Delicado et al. 2015; Szarowska et al. 2015; Boulaassafer et al. 2020; Lampri et al. 2022). Land corridors and Lago-Mare basins connecting several remote regions in the Mediterranean during MSC and Plio-Pleistocene glacial cycles had arguably facilitated colonization events (Delicado et al. 2015; Neubauer et al. 2015; Falniowski et al. 2021). Multiple cladogenetic events in Pseudamnicola occurred during the Pleistocene, where frequent interchange between glacial events and interglacials exerted pressure on the gastropod faunas (shifts in water flows) that scarcely had the chance to escape to climate-appropriate refuges (Strong et al. 2008; Neubauer et al. 2015). Therefore, this could explain why several Pliocene species became extinct in the Pleistocene and why most extant species only date back to the Pleistocene.
Unfortunately, numerous Pseudamnicola species from Greece lack molecular data and have been described based on shell morphology exclusively and on male genitalia in a few cases. To fully comprehend the evolutionary processes that shaped the diversity and distribution of this species-rich genus, future integrative investigations of Pseudamnicola populations from various Mediterranean regions using multiple molecular markers and morphological/anatomical data are required.
ACKNOWLEDGMENTS
We are grateful to Anastasios Porfyris and Simon Demetropoulos (Cyprus Wildlife Society) for providing snail specimens that were employed in this study.




