The male's scent triggered a neural response in females despite ambiguous behavioral response in Asian house rats
Abstract
Pheromones and olfactory communication play vital roles in sex recognition and mate choice in rodents. Asian house rats (Rattus tanezumi) (RT) often startle easily, making behavioral measurements difficult to carry out accurately in the laboratory. Here, the behavioral and olfactory preferences of the female RT between males and females were not observed using a conventional two-choice device; we then explored the neuro-immunohistochemical evidence in the brains of RT females. We found that male urine elicited significantly higher c-fos expression in the accessory olfactory system and sex-related brain regions in females than female urine did. On the other hand, the differences of volatile compounds and major urinary proteins (MUPs) in both voided urine and preputial glands (PGs) of the RT were detected using gas chromatography–mass spectrometer, sodium dodecyl sulfate-polyacrylamide gel electrophoresis, isoelectric focusing electrophoresis, and liquid chromatography–electrospray ionization mass spectrometry. We found that PG-derived 1-(4,5-dihydro-2-thiazolyl)-ethanone and total MUPs were more abundant in males versus females, suggesting these sexually divergent components might activate the female's accessory olfactory system. In conclusion, the neuro-immunohistochemical evidence indicated that potential sex pheromones might exist in RT; however, the strength of the chemical signal might be too weak to cause behavioral responses in females.
INTRODUCTION
Pheromones are bioactive substances that facilitate intra-species interactions (Novotny 2003; Brennan 2010). In 1959, this concept was first proposed by Peter Karlson and Martin Lüscher (Liberles 2014). Bombykol was initially identified as an insect sex pheromone, and the pheromone has been known throughout the whole animal kingdom up to now (Liberles 2014). The sources of mammalian pheromones primarily include urine, sweat, saliva, tear, and specialized sebum gland secretion, producing chemical substances covarying with species, sex, age, genotype, endocrine status, and so on in mammals (Ben-Shaul et al. 2010; Ferrero et al. 2013; Liberles 2014). The structural composition of mammalian pheromones shows great diversity, including volatile small molecules, steroid derivatives, peptides, and major urinary proteins (MUPs) (Symonds & Elgar 2008; Brennan 2010; Liberles 2014; Wyatt 2014). MUPs are of low molecular weight (approximately 19 kDa) and highly polymorphic, which are synthesized by the liver and excreted in the urine of some rodent species (Gomez-Baena et al. 2014). The MUPs not only bind to and slow the release of volatile compounds but also act as pheromones to convey sociosexual information to receivers of conspecific and thereby regulate sociosexual interactions (Novotny 2003; Liberles 2014). Pheromones have been shown to play a key role in almost all aspects of mammalian behaviors such as individual discrimination, sexual attractiveness, social status, and mate choice (Firestein 2001; Brennan & Zufall 2006; Munger et al. 2009). A limited number of mammalian species have been studied for pheromone chemical identification, with the mouse and rat being the most detailed (Novotny 2003; Brennan & Zufall 2006; Wyatt 2014; Zhang & Zhang 2014; Guo et al. 2017; Rowe et al. 2020).
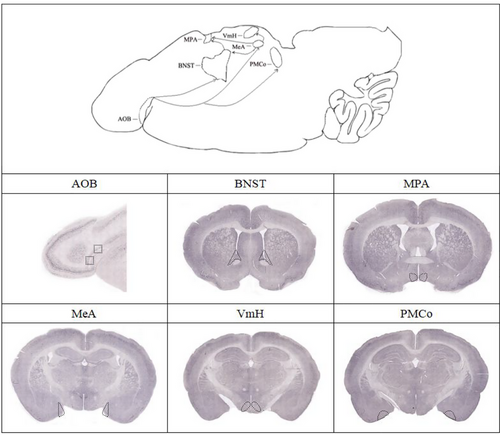
The mammalian olfactory system consists of the main olfactory system (a broadly tuned odor sensor) and the accessory olfactory system which perceives pheromones to regulate sociosexual behaviors, kinship, endocrine status, and so on (Firestein 2001; Luo & Katz 2004; Munger et al. 2009). The accessory olfactory bulb (AOB) receives sensory inputs from the vomeronasal organ (VNO) and projects to the medial amygdaloid nucleus (MeA) and the posteromedial amygdaloid cortical nucleus (PMCo) with additional connections to the bed nucleus of the stria terminalis (BNST) (Dulac & Wagner 2006). Neurons from these nuclei project further to the hypothalamic nuclei, including the medial preoptic area (MPA) and ventromedial hypothalamus (VmH), which send out the output signals to direct intraspecies physiological and behavioral responses (Dulac & Wagner 2006; Munger et al. 2009; Liu et al. 2019; Tirindelli 2021).
The voided urine of murine rodents is the most common source of pheromones, including volatile organic compounds (VOCs) and major urine proteins (MUPs) (Beynon & Hurst 2003; Zhang et al. 2008b; Roberts et al. 2010; Guo et al. 2019). The voided urine carries the chemical metabolites from both the bladder urine and the preputial glands (PGs) (Novotny 2003; Zhang et al. 2008a,b). In mice and rats, the bladder urine itself contains both VOC and MUP pheromones (Novotny 2003; Brennan & Zufall 2006; Roberts et al. 2010; Guo et al. 2019). The PGs are specialized paired sebaceous glands located in the subcutaneous tissue lateral to the penis and have ducts leading into the urethra to excrete with urine (Novotny 2003; Zhang et al. 2008a,b; Wyatt 2014). PG, as the significant source of pheromones, is ubiquitous in Murinae such as mice and rats and plays an important role in chemical communication such as the information about species, sex, kinship, physiological condition, and social status (Brennan & Zufall 2006; Zhang et al. 2007; Brennan 2010). The PGs are usually better developed in males than in females under androgenic control (Zhang et al. 2008a). In addition to excreting volatile pheromones in mice and rats, the PGs also excrete MUPs in rats (Held & Gallagher 1985; Liberles 2014). Sexual dimorphism in the chemical composition of the urine and PG secretion is an essential precondition for sexual communication and the screening of sex pheromones; for example, well-known male pheromones such as (s)-2-sec-butyl-4,5-dihydrothiazole, R,R-3,4-dehydro-exo-brevicomin, E-β-farnesene, E,E-ɑ-farnesene, 1-hexadecanol, and darcin in mice and 2-heptanone and MUP13 in rats are all increased in amount in males (Novotny 2003; Zhang et al. 2008a,b; Guo et al. 2019). Thus, screening for chemical components with sex differences is an effective means of identifying potential sex pheromones.
Pheromones in wild rat species have rarely been investigated (Selvaraj & Archunan 2002; Hurst & Beynon 2013; Zhang et al. 2019b; Rowe et al. 2020; Zhang et al. 2021). The Asian house rat (Rattus tanezumi) (RT) is mainly native to Southeast Asia and South Asia and its physical and behavioral traits are very similar to those of its sister species, the black rat (R. rattus); for example, they are both good climbers and build their nest on roofs and high places (Musser & Carleton 2005; Chen et al. 2021). However, it appears difficult to determine the olfactory preferences between male and female odors with previous methods that were effective in other assays, likely due to the specificity of these rat species (Selvaraj & Archunan 2002; Zhang et al. 2008a,b; Guo et al. 2017; Guo et al. 2019).
In the current study, to investigate possible pheromone-based sexual recognition of RT, using immunohistochemical staining for c-fos protein in the brain, we determined differences in the vomeronasal pathways of females that responded to urine from males and females. On the other hand, we analyzed sexual differences in volatile organic composition with gas chromatography–mass spectrometer (GC-MS) and MUPs with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), isoelectric focusing electrophoresis (IEF), and liquid chromatography–electrospray ionization mass spectrometry (LC-ESI-MS) in respective voided urine and PG secretion.
MATERIALS AND METHODS
Subjects
RT used were more than ten generations of ancestors captured from the wild population. For breeding, RT were caged in groups of same-sex siblings after being weaned at 4 weeks of age and kept in plastic rat cages (37 × 26 × 17 cm) (Suzhou Fengshi Laboratory Animal Equipment Co., Ltd., Suzhou, China) (14:10 h light: dark photoperiod, lights on at 19:00) with wood shavings for bedding (Beijing Keao Xieli Feed Co., Ltd., Beijing, China) at a temperature of 23 ± 2°C. Standard rat chow and tap water were provided ad libitum. All RT were 5 to 12 months of age, sexually naive, and caged individually for 2 weeks prior to initial urine collection. Eight males and eight females served as urine donors. All the females were sexually naïve and had a 4- or 5-day estrous cycle. The phase of the estrous cycle was determined by vaginal smear cytology, and only females with regular estrous cycles were used in this study (Marcondes et al. 2002). The procedure of animal handling complied with the guidelines of the Animal Use Committee of the Institute of Zoology, Chinese Academy of Sciences (IOZ 2017).
Two-way choice tests
Forty-seven male RT and 54 female RT were used in the two-way choice tests.
Experiment 1: Assessment of the attractiveness of urinary volatile compounds produced by males and females to RT. The behavior experiments were conducted in a three-chamber testing apparatus constructed from three plastic rat cages (37 × 26 × 17 cm). Two cages served as the choice cages and were symmetrically connected to the long side of the neutral cage by two Plexiglas choice tubes (internal diameter, 7 cm; length, 50 cm). Each choice cage was partitioned by perforated galvanized iron sheets. Each tube had a removable perforated galvanized iron sheet partition placed 10 cm away from the neutral cage to control RT access (Fig. 1a). One male and one female were placed in either of the two choice cages, respectively, for 30 min for them to leave scent substances, especially fresh urine (urine marks), and then were removed. One RT (male or female) was placed in the neutral cage and acclimated in the neutral cage for 10 min. We then opened the door to allow RT to freely sniff the urine marks, but it could not touch and lick the urine. We recorded the investigation time (the time spent in each choice cage) for 30 min immediately after the focal rat initially entered either of the choice arms.
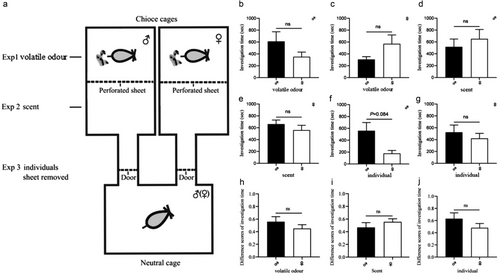
Experiment 2: Assessment of the attractiveness of urinary volatile compounds and MUPs of males and females to RT. The three-chamber testing apparatus is the same as described in Experiment 1. One male and one female were placed in either of the two choice cages for 30 min and then removed from the choice cages. One RT (male or female) was placed in the neutral cage and acclimated in the neutral cage for 10 min. Then, we opened the door and removed the perforated partition of each choice cage to allow RT to freely sniff and lick the urine marks. We recorded the investigation time (the time spent in each choice cage) for 30 min immediately after the focal rat initially entered either of the choice arms.
Experiment 3: Assessment of the attractiveness of males and females to RT. The three-chamber testing apparatus is the same as described in Experiment 1. One male and one female were placed in either of the two choice cages for 30 min. One RT (male or female) was placed in the neutral cage for 10 min of acclimation and then opened the door to allow RT to freely respond to the RT after the perforated partition sheet. The perforated sheet remained in the choice cages to prevent free interactions between the subjects and the wandering of stimulus animals around the test apparatus. We recorded the investigation time (the time spent in each choice cage) for 30 min immediately after the focal rat initially entered either of the choice arms.
The three-chamber testing apparatus was cleaned thoroughly with 75% ethanol and water between trials. If a rat did not enter either choice tube within 30 min, it was excluded from the experiment (Guo et al. 2017; Zhang et al. 2019b). The difference score of investigation time referred to time spent sniffing males and was calculated through [investigation time on males/total investigation time on both males and females].
Urine collection and tissue samples
Seven male and 7 female RT were used as urine and PG donors. Urine collection was conducted in the clean metabolic cages and continued for 8 h during the dark phase of the light cycle. Standard rat chow and water were freely available. The urine from the metabolic cage flowed into a collection tube immersed in the ice box. The urine samples were stored at −80°C prior to use. The metabolic cages were cleaned thoroughly with water and 75% alcohol between urine collections.
RT were killed by decapitation and their PGs were dissected and frozen with liquid nitrogen immediately. All the procedures were conducted within 10 min, and the samples were stored at −80°C for use.
Odor stimulation
Female subjects were first placed in a separate room with good ventilation for 2 days. When experimenting, 100 μL of urine of either males or females was applied to the nose of subjects. After the experiment lasted an entire 90 min, the subjects were anesthetized for immunohistochemistry.
Immunohistochemistry
Eight females were used in immunohistochemistry. Urine from RT males was applied to four females. Urine from RT females was applied to four females. Pentobarbital sodium solution (40 mg kg−1) was injected intraperitoneally, and the brain and olfactory bulb were obtained after cardiac perfusion. The brain and olfactory bulb of subjects were fixed with 4% paraformaldehyde (PFA) at 4°C for 24 h, followed by 10%, 20%, and 30% sucrose gradient dehydration for 1 day, until the tissues completely sank to the bottom. The thickness of the sagittal section of AOB was 20 μm, and the coronal section of the brain was 40 μm. The slices were washed with 1 × PBS three times for 10 min each time. The slices were incubated in 0.3% H2O2 for 30 min in the dark, then washed with 1 × PBS three times, 10 min each time. TBS with 10% NGS was used to block the slice for 1 h. Anti-c-fos antibody (Abcam, ab190289) (1:2000) was diluted with primary antibody diluent, and the sections were incubated on a shaking table overnight at 4°C. The slices were rewarmed for 45 min, and the slices washed with 1 × PBS three times for 10 min each time. Anti-rabbit IgG (H&L) (Vectorlabs, BA1000) was diluted in TBS at 1:300 and incubated for 1 h; washed with 1 × PBS three times for 10 min each time. ABC complex (A:B:1 × PBS = 1:1:100) was prepared and mixed evenly 40 min in advance, then washed with 1 × PBS three times, 10 min each time. DAB dye solution was used to dye the sections for 3–5 min until the color became darker. The tissue sections were pasted on the adhesive glass slides and prepared for sealing, followed by 70%, 80%, and 90% ethanol for 2 min and 100% ethanol for 5 min, respectively. Then, the tissue was soaked in xylene I and xylene II for 5 min, respectively. The slides were sealed with neutral gum and imaged by Aperio VESA8 (Leica, Germany). Unless otherwise specified, all operations were carried out at room temperature. The regions of interest were a standardized subsection of MPA (200 μm × 500 μm) and other brain regions (500 μm × 500 μm) which are outlined in Figs 3 and 4 during fos cell counting. The five slices with the same coordinates were chosen from different RT. For each RT, five slices were collected from the AOB and each brain region, and the mean value was taken as the number of immunoreactive c-fos cells. ImageJ software (NIH, Bethesda, MD, USA) was used to count positively stained cells (Liu et al. 2019).
Extraction proteins of preputial glands
The crude extract of PGs was homogenized by a tissue grinder (Tiangen, China) in phosphate-buffered saline (pH 7.2) (100 mg mL−1), followed by centrifugation at 13 000 rpm for 15 min at 4°C. The supernatant was immediately collected for use (Rajkumar et al. 2010).
Gas chromatography–mass spectrometer assay of urinary and preputial glands' volatile compounds
To extract compounds from the urine of RT, we mixed 150 μL of dichloromethane (purity > 99.5%; DIMA Technology, Inc.) with 150 μL of urine, stirred thoroughly, stored at 0°C for 12 h, and then used the bottom phase (the dichloromethane layer) for chemical analysis. We weighed the PGs, added dichloromethane (0.2 mg μL−1) into the vial, and kept it at 0°C for 12 h. Then, we discarded the tissue and utilized the remaining solution for chemical analysis. Chemical analysis was performed on an Agilent Technologies Network 6890N GC system coupled with 5973 Mass Selective Detector with the NIST/EPA/NIH Mass Spectral Library (2008 version, Agilent Technologies, Inc., USA). The conditions and parameters of GC-MS were the same as the previous literature (Zhang et al. 2008a,b). We injected 4 μL in a splitless mode for urine and 2 μL in a split mode (1:5) for PG extract. Tentative identifications were done by comparing the mass spectra of GC peaks with those in the MS library (NIST 2008). For a particular compound, the abundance was quantified by GC peak area, and the relative abundance was a percentage of the summed peak areas from all targeted GC peaks.
Seven of the tentatively identified compounds, 2-heptanone, dimethyl sulfone, benzaldehyde, 4-methyl-phenol, 4-ethyl-phenol, n-hexadecanoic acid, and squalene were further confirmed by matching retention time and mass spectra with the authentic analogs (all purity >98%; ACROS Organics). (S)-2-sec-butyl-4,5-dihydrothiazole was compared with the same component in the urine of male mice (Novotny 2003; Zhang et al. 2007).
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of MUPs
The relative abundances of MUPs were qualified by SDS-PAGE using the Mini P-4 system (Cavoy, China). Sixteen microliters of each urine sample were mixed with 4 μL of 5 × SDS-PAGE loading buffer (Solarbio, China). A 2.5-fold dilution was performed on the supernatant of each individual's PD extract, combining it with 4 μL of 5 × SDS-PAGE loading buffer and 8 μL of sterile water. The mixed protein samples were heated at 100°C for 5 min. 8 μL of each mixed urine sample, 4 μL of the supernatant of PGs of each individual, and 6 μL of protein molecular weight marker (low) (Takara, Japan) were fractionated on 15% SDS-PAGE gels at a constant voltage of 150 V for 1 h. The protein gels were stained with Coomassie brilliant blue (Sigma-Aldrich) dye for 1 h and then imaged by the ChemiDoc MP system (BioRad, USA). The relative abundance of MUPs was quantified with Image J software (National Institutes of Health, USA). One sample was selected as a standard control and fractionated on each gel to correct for bias between runs and to normalize the relative abundance of other samples (Guo et al. 2019).
Isoelectric focusing electrophoresis
Six samples each of male and female RT urine (supernatant of PG extract) were randomly selected, and two samples were equally mixed into one for IEF. IEF was performed to separate proteins based on MUPs’ isoelectric point by Mini Cell equipment (Bio-Rad, USA). The polyacrylamide gel included sterile water (2.75 mL), monomer concentrate (25% T, 3% C, 1 mL), 25% glycerol (w/v, 1 mL), ampholyte (250 μL) with 3–10 PH, 10% ammonium persulfate (w/v, 7.5 μL), 0.1% FMN (w/v, 25 μL), and TEMED (1.5 μL). The desalted and precipitated protein solutions from 130 μL of urine and 70 μL of PG homogenate were obtained by desalted columns (Thermo, USA), vacuum freeze drying, and dissolution with 10 μL of ddH2O. 1 μL of protein solutions and 2 μL of markers (SERVA, Germany) with isoelectric point fractions (3–10) were applied to the gel and allowed to diffuse for 5 min. The gel was run under the conditions of 100 V for 15 min and 450 V for 1 h. Targeted bands of the gel were imaged by the ChemiDoc MP instrument (Bio-Rad, USA) followed by fixing, staining, and destaining (Guo et al. 2019).
Liquid chromatography–electrospray ionization mass spectrometry (LC-ESI-MS) assay of MUPs
Six samples each of male and female RT urine (supernatant of PG extract) were randomly selected and equally mixed into one for LC-ESI-MS. All analyses were performed on the liquid chromatography (Agilent 1290, USA) coupled with Q-TOF mass spectrometer (Agilent 6530, USA) and fitted with an AJS ESI ion source. Samples were injected into the AdvanceBio RP-mAb C4 (2.1 × 50 mm, C4, 3.5 μm, 300 Å, Agilent) desalting column before elution at a flow rate of 200 μL min−1 over stepwise acetonitrile gradient (0–100%), acquiring spectra between m/z 600 and 2400. Raw data were processed and transformed to a neutral average mass using Qualitative Analysis B.06.00 (MassHunter Workstation Software, Agilent). The intensity was quantified by peak height for a particular protein. ESI-MS allowed a semi-quantitative assessment of the relative amount of each MUP. The complete quality of Mups matched the mature protein which was removed the predicted signal peptide, and subtracted of 2 Da over a mass range of 18–20 KDa, predicted by the genome or cDNA sequence (Gomez-Baena et al. 2019).
Data analysis
Kolmogorov–Smirnov tests were used to examine the distribution of raw data, and nonparametric or parametric analyses were subsequently performed as appropriate based on the outcome of Shapiro–Wilks test. The investigation time was examined for differences using Wilcoxon signed-rank test. Independent t-test or Mann–Whitney U-test was used to compare the difference scores of investigation time, the abundance of urine and PG volatiles of RT males and females, and determine the differences in the density of c-fos-ir cells in AOB and pheromone-related brain areas of female RT stimulated with respective male urine and female urine. Bonferroni corrected P-values were used in multiple comparisons. All statistical analyses were conducted using SPSS (Version 18.0). Significance was set to P < 0.05.
RESULTS
Two-way choice
Experiment 1 was designed to test the olfactory preferences of either male or female RT for volatile odor produced by both male and female RT. We found that RT males had no preference for volatile odor produced by males or females (z = 0.628, P = 0.53, n = 12) (Fig. 1b), and neither did RT females (z = 1.083, P = 0.279, n = 13) (Fig. 1c).
The purpose of experiment 2 was to test the olfactory preference of male or female RT for scent (including urine volatile compounds and MUPs) produced by male and female RT. The results indicated that RT males had no preference for scent produced by males or females (z = 0.14, P = 0.889, n = 13) (Fig. 1d) and neither did RT females (z = 0.594, P = 0.552, n = 13) (Fig. 1e).
The aim of experiment 3 was to test the attractiveness of both male and female RT rats themselves to either male rats or female RT rats. The results suggested that RT males approached females to a much higher proportion than males (z = 1.726, P = 0.084, n = 12, marginal significant) (Fig. 1f). RT females had no preference for male or female (z = 0.345, P = 0.73, n = 14) (Fig. 1g).
RT females and RT males did not differ in difference scores of investigation time in response to volatile and scent from RT males (Fig. 1h,i). The difference scores of investigation time between RT females and RT males did not differ in response to male individuals (Fig. 1j).
The expression of c-fos in the brain of females responding to male and female urine
Male urine induced higher levels of c-fos expression in anterior AOB (aAOB) than female urine did (t = 7.367, P = 0.000, n = 4), so did male urine in the posterior AOB (pAOB) (t = 5.344, P = 0.002, n = 4). However, there was no difference in the expression of c-fos between the anterior and posterior of the AOB (t = 0.709, P = 0.505, n = 4) following exposure to the urine of the male. There was no difference in c-fos response between aAOB and pAOB of the female RT (t = 1.67, P = 0.146, n = 4) after exposure to the urine of females (Figs 2, 3).
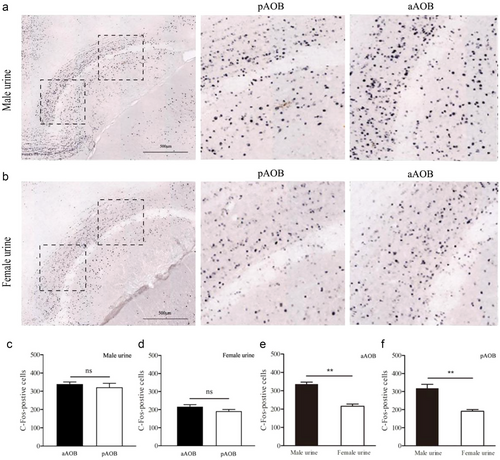
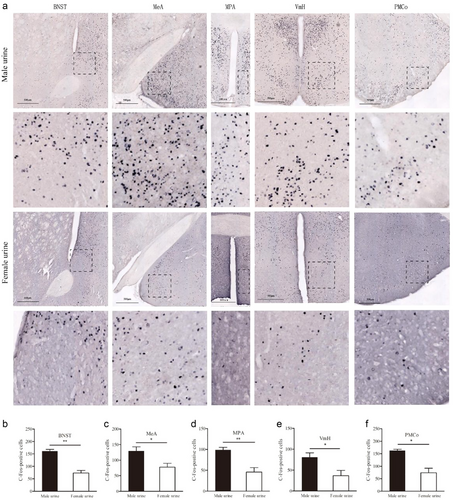
Male urine also induced higher levels of c-fos expression in the BNST than female urine did (t = 6.797, P = 0.000, n = 4), so did male urine in the MeA (t = 2.844, P = 0.029, n = 4), MPA (t = 4.247, P = 0.005, n = 4), VmH (t = 2.669, P = 0.037, n = 4), and PMCo (t = 4.654, P = 0.012, n = 4) (Figs 2, 4).
Volatile compounds in the urine and PG secretion
We detected and temporally characterized 10 main compounds from the voided urine, including ketones, sulfones, phenols, esters, and amines via GC-MS (Fig. 5; Table S1, Supporting Information). Di-n-octyl phthalate is the most abundant volatile compound in the urine of male and female RT, accounting for about 50% of the total volatile compounds. The relative abundance of dimethyl phthalate was significantly higher in female urine than in male urine (t = 4.506, P = 0.000, n = 8) (Fig. 5a,b).
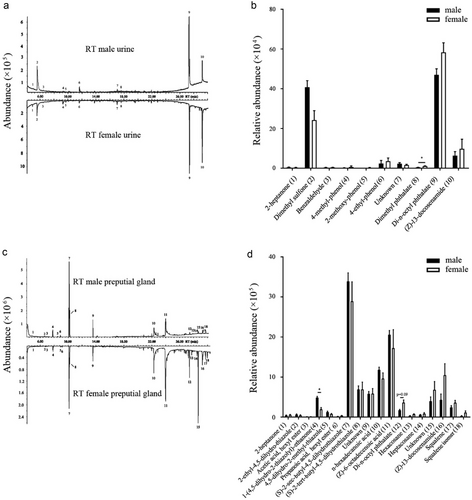
We detected and temporally identified 18 main compounds from the PG secretion including ketones, fatty acids, thiazoles, esters, and terpenes (Fig. 5; Table S2, Supporting Information), where (s)-2-sec-butyl-4,5-dihydrothiazole was the most abundant compound, accounting for about 30% of all volatile compounds. 1-(4,5-dihydro-2-thiazolyl)-ethanone was more abundant in males than in females (t = 5.511, P = 0.000, n = 8 for males, n = 7 for females). In contrast, hexacosane (t = 3.413, P = 0.09, n = 8 for males, n = 7 for females, marginal significance) showed a tendency to be more abundant in females than in males (Fig. 5c,d).
MUPs in the urine and PGs
The weight of male RT was significantly higher than that of female (t = 2.66, P = 0.019, n = 8) (Fig. 6a). The relative weight of PGs of male RT was significantly higher than that of female (t = 3.617, P = 0.003, n = 8) (Fig. 6e).
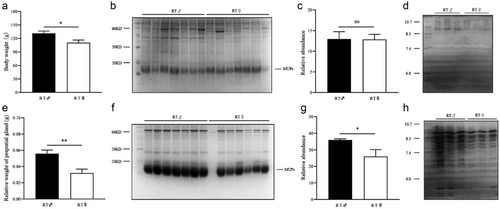
SDS-PAGE analysis revealed that there was no significant difference in the abundance of urine MUPs between males and females (t = 0.039, P = 0.969, n = 7) (Fig. 6b,c), while the MUPs in PGs were more abundant in males than in females (t = 3.145, P = 0.019, n = 7) (Fig. 6f,g).
IEF analysis indicated that the abundance and type of protein at different isoelectric points in the urine of females and males were similar in RT (Fig. 6d). The abundance and type of protein at different isoelectric points in the PGs of males were more than in females in RT (Fig. 6h).
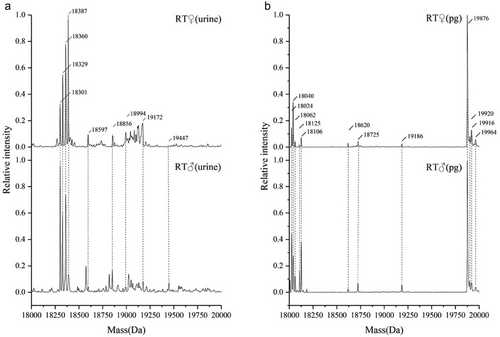
LC-ESI-MS was used to profile the isoforms of the MUPs both in the urine and in the PGs of RT. The LC peak profiles of MUPs in the urine and PG were different between males and females, and the degree of polymorphism in PGs was low (Fig. 7). The MUPs dominated the deconvoluted mass spectra, and several proteins and multiple discrete masses in the 18–20 kDa mass range were evident. The protein at 18 301 was the most intense in the urine of male RT. The protein at 18 387 was the most intense in the urine of female RT. The protein at 19 876 was the most intense in the PGs of male and female RT.
DISCUSSION
In the two-choice tests, we did not observe the olfactory preferences of RT females for living rats or their scents between males and females. Such a two-choice method has been successfully used for olfactory preferences of females of brown rats between conspecific males with different pheromones and heterospecific males (Guo et al. 2017; Zhang et al. 2019b). Rattus norvegicus (RN) that have long been domesticated for laboratory rats are relatively tame and have been widely used in various experiments, whereas RT rats are as timid and hyperactive as R. rattus and difficult to be experimented using conventional behavioral tests (Appleby 1973; Zohar & Terkel 1991). In RT, the olfactory preferences of females between males and females were not demonstrated through the two-way choice tests (Selvaraj & Archunan 2002).
Our chemical data showed that the volatile constituents and MUP profiles of either voided urine or PG secretion exhibited detectable sexual dimorphism in RT, indicating the possible presence of sex pheromones. Sexual dimorphism refers to the fact that the two sexes of a species differ in their external appearance (size, shape, color, the development of appendages, scent, vocalizations, or other features), which is widespread in mammals (Ralls & Mesnick 2009). Some of the appearances are usually sexual traits such as feather ornaments and the song complexity of physical communication in birds to mediate sexual behavior and mate choice (Andersson & Simmons 2006). As the most important sensory modality of rodents, murine rodents rely primarily on chemical communication of sex differences in odor components for sex recognition (Novotny 2003; Zhang et al. 2008a,b; Roberts et al. 2010; Guo et al. 2019). Now that the chemical differences between male and female scents were detectable in RT, the differences in the behavioral responses of RT females to male and female scents could be detected through improved behavioral approaches. However, the two-way testing device used here was also used in testing the species-between preferences by females between males of RT rats and brown rats, revealing that RT females showed clear preferences for living rats or contactable urine of conspecific males to heterospecific males (Wang et al. 2023). Alternatively, the differences in chemical signals might be not enough to cause differences in behavioral responses in RT, as in some RN rats. We have exemplified in some RN rats that even if there are statistically significant differences in chemical signals between male individuals, the degree of difference is not enough to convey reliable information, and it is not used as an honest signal by animals to mediate female mate choice (Zhang et al. 2021). In fact, the content and profiles of total MUPs in the urine were less abundant and diverse in RT than in RN (Guo 2016; Guo et al. 2019). In addition, sexual differences in ultrasonic vocalizations have been detected in RT other than RN (unpublished data) and might be used in sexual attractiveness alone or together with olfactory signals in RT.
However, we found that male urine versus female urine activated more Fos-immunoreactive neurons in sex-specific neural circuits in aAOB, pAOB, amygdala, hypothalamus, and BNST of RT females, suggesting that the voided urine did bear male pheromones to activate classical pheromone sensing-related neural nuclei (i.e. MeA, BNST, MPA, and VmH) underlying the generation of the sexual behavior of the female RT (Paredes 2003; Bargmann 2006). Male urine elicited similar responses of aAOB and pAOB, suggesting that RT urine might contain both male-enhanced volatile pheromones to activate aAOB and MUP pheromones to activate pAOB (Novotny 2003; Brennan & Zufall 2006; McCarthy 2008; Zhang et al. 2019a).
The pheromone signals are detected by vomeronasal receptors in families 1 and 2 and then transmitted either to aAOB for the volatile pheromones or to pAOB for the MUP pheromones (Keverne 2004; Luo & Katz 2004). The MeA plays a critical role in species and sex-specific signal processing and is also connected to many other brain regions that mediate social and sexual behavior (Li et al. 2017; Raam & Hong 2021). Accurate pheromone-sexual behavior responses are analyzed by MeA and then controlled by the VmH (Choi & Anderson 2005). VmH plays a role in sexual behavior in females (lordosis), which stimulates their sexual arousal (Kow & Pfaff 1998). Precopulatory behavior involves multiple brain areas, including the MPA as well as the MeA and the BNST (Paredes 2003). Here, we also performed an immunohistochemical assay and unveiled that c-fos gene expression in the AOB and sex-related brain regions of females was increased by male urine versus female urine in the brown rat with five known male pheromones (see Figs S1,S2, Supporting Information) (Zhang et al. 2008b; Zhang & Zhang 2014; Guo et al. 2019). Therefore, males might have urine-borne sex pheromones to cause a response in the olfactory system of females in RT.
Previous studies have shown that several urinary volatile pheromones (2-heptanone, 4-heptanone, and 9-hydroxy-2-nonanone) have been identified as male pheromones, exhibiting androgen-dependency, sexual dimorphism, and sexual attractiveness to females in RN (Zhang et al. 2008b; Zhang et al. 2019b). The volatile compounds in the urine of RT males were similar to those in the urine of RN males of their closely related species, but in different proportions (Guo 2016). In RT, dimethyl phthalate was more abundant in females than males, but it might not be evolutionarily stable and honest signals, because the phthalates are widely present in the environment as plasticizers and their sex differences might be attributed to sex differences in the metabolism of phthalates in RT (Navarro et al. 1987; Kannan et al. 1998; Silva et al. 2005; Ito et al. 2014).
The PG secretion can be excreted with urine to transmit chemical signals via urine marking (Novotny 2003; Zhang et al. 2008a,b; Gomez-Baena et al. 2014). Seven thiazoline compounds were identified from PGs of two Rattus rat species, R. fuscipes and R. leucopus (Rowe et al. 2020). It has been exemplified that urine metabolized (s)-2-sec-butyl-4,5-dihydrothiazole (SBT) is a male pheromone involved in female attraction in mice (Novotny 2003; Liberles 2014; Liu et al. 2017). Here, (s)-2-sec-butyl-4,5-dihydrothiazole was the most abundant volatile compound of the PGs of RT with no sex difference in quantity. However, 1-(4,5-dihydro-2-thiazolyl)-ethanone was more abundant in males than in females, implying a possible male pheromone.
The PG-secreted volatiles exhibited a great difference in the types of compounds between RT rats and brown rats (Zhang & Zhang 2014; Guo et al. 2017; Wang et al. 2022). In brown rats, PG secretion is sexually attractive and contains rich squalene and aliphatic acids but is poor in esters (Burgess & Wilson 1963; Albro & Moore 1974; Natynczuk et al. 1995; Zhang & Zhang 2014). PG-secreted squalene is a major component and potential male pheromone in RN as reported previously, but it was a minor component with no significant difference in quantity in RT (Zhang et al. 2008b; Zhang & Zhang 2014). PG-secreted hexacosan was detected to be more abundant in females than in males, implying that it might be related to different effects of male and female scents on the olfactory system of females in RT (Burgess & Wilson 1963; Albro & Moore 1974; Natynczuk et al. 1995).
MUPs in urine were polymorphic and sexually dimorphic, indicating potential nonvolatile pheromones in RT (Beynon & Hurst 2004). SDS-PAGE and IEF analysis revealed that total MUPs were more abundant and diverse in males than in females in RT. Coincidently, LC-ESI-MS analysis showed that MUP profiles were sexually different in both urine and PGs, implying that MUPs might play a role in chemical communication for sex recognition. Sexual dimorphism was also observed for total MUPs in the urine of house mice and brown rats (Knopf et al. 1983; Roberts et al. 2010; Guo et al. 2019), but MUPs are only present in the PGs of Rattus rats (Held & Gallagher 1985; Liberles 2014). In addition, the PGs are often better developed in males than in females which can be enhanced by androgen in some murine rodents such as house mice, wood mice (Apodemus sylvaticus), and black rats, although such sexual differences disappeared in brown rats, Brandt's voles (Lasiopodomys brandtii), and musk rats (Ondatra zibethicus) (Mallick 1991; Natynczuk et al. 1995; Zhang et al. 2008a). Here, the PGs of males were larger than females, enlarging the intensity of the difference of volatile components and MUPs of PGs in RT.
In conclusion, Asian house rats had significant chemical differences between male and female scents, and male scent had stronger effects on the olfactory system of females than female scent, suggesting the existence of potential sex pheromones. However, the strength of the chemical signal might be weak and thus could not cause behavioral responses in females and might need to coordinate with other cues such as ultrasonic vocalizations to mediate sexual behavior in the Asian house rat.
ACKNOWLEDGMENTS
This work was supported by grants from the National Natural Science Foundation of China (31872227 to JXZ and 32070451 to YHZ).




