The combined effect of bromadiolone and ivermectin (iBr) in controlling both rodents and their fleas
Abstract
Rodent pests not only cause severe agricultural loss but also spread zoonotic pathogens to human beings. Anticoagulant rodenticides are widely used to decrease the population densities of rodents but often lead to the spillover of ectoparasites because fleas and ticks may gather on surviving rodents. Therefore, it is necessary to kill fleas and ticks before culling rodents to minimize the risk of pathogen transmission. In this study, we used a mixture of ivermectin (an antiparasitic drug) and bromadiolone (an anticoagulant rodenticide) to control both rodent and flea/tick abundances. We found that in a laboratory test, 0.01% ivermectin bait was not lethal for greater long-tailed hamsters after 7 days of treatment, while 0.1% ivermectin bait was lethal for approximately 33% of treated rodents. In a field test, bait containing 0.001%, 0.005%, 0.01%, and 0.05% ivermectin decreased the number of fleas per vole of Brandt's voles to 0.42, 0.22, 0.12, and 0.2, respectively, compared with 0.77 in the control group, indicating that 0.01% ivermectin bait performed best in removing fleas. In another laboratory test, bait containing a 0.01% ivermectin and 0.005% bromadiolone mixture caused the death of all voles within 6–14 days after the intake of the bait. In the field test, the bait containing 0.01% ivermectin and 0.005% bromadiolone reduced the average number of fleas per vole to 0.35, which was significantly lower than the 0.77 of the control group. Our results indicate that a 0.01% ivermectin and 0.005% bromadiolone mixture could be used to control both rodents and fleas to minimize the spillover risk of disease transmission when using traditional rodenticides.
INTRODUCTION
Rodents cause serious damage to agricultural production and forest and grassland protection (Jurisic et al. 2022; Witmer 2022). They are also important reservoirs for many zoonotic pathogens that are transmitted to human beings and cause diseases, such as plague, yellow fever, and epidemic hemorrhagic fever, which impose a major threat to human health. More than 60% of known zoonotic viruses are from rodents (Johnson et al. 2020). Ectoparasites, such as fleas and ticks, play important roles in disease transmission from rodents to other animal hosts or people (Brouqui & Raoult 2006).
It is necessary to control rodent populations to reduce their damage or harm to people. Poisoning is widely applied to reduce the population density of rodents (Singleton et al. 2007). Currently, anticoagulant rodenticides such as bromadiolone, brodifacoum, and diphacinone are used in most countries throughout the world (McGee et al. 2020). Anticoagulant rodenticides generally cause the death of rodents within approximately one week after poisoning (Watt et al. 2005). Vitamin K1 is the specific antidote for anticoagulant rodenticides (Rizzoni et al. 1988; Watt et al. 2005), and vitamin K use could improve the safety of nontarget animals or people when anticoagulant rodenticides are used. Bromadiolone is a widely used second-generation anticoagulant rodenticide. Rodents consuming sufficient bromadiolone bait will die of multiple organ hemorrhage within a few days due to a reduction in vitamin K1 (Watt et al. 2005). Bromadiolone can kill laboratory rats within 7 days and mice within 13 days (Li et al. 2009).
Large-scale application of anticoagulant rodenticides would cause the release of the ectoparasites of rodents. Previous studies indicate that fleas can survive 5–15 days after their hosts die (Krasnov et al. 2002). They may live in the environment and seek new hosts, which increases the risk of spillover of zoonotic disease to people. Therefore, it is necessary to kill fleas and ticks before killing rodents to minimize the spillover risk after rodent control (Leirs et al. 2001; Hinds et al. 2021). Adding insecticides into the baits of rodenticides has been proposed to control both rodents and ectoparasites (Leirs et al. 2001; Rust 2020; Hinds et al. 2021). For example, fipronil and imidacloprid are effective in killing fleas after oral intake by animals (Leirs et al. 2001; Hinds et al. 2021; Jacob et al. 2021). The combination of fipronil and permethrin is able to kill fleas on dogs, and the effect lasts for approximately 1 month (Cvejic et al. 2017). The combined treatment of imidacloprid and moxidectin provides a 96.3% reduction in flea numbers on cats (Geurden et al. 2017). A recent study indicated that 0.005% fipronil bait feeding can decrease the survival of flea larvae in the feces of prairie dogs (Eads et al. 2023), explaining the long-lasting effect of fipronil. However, these compounds do not rapidly decompose in nature and may therefore remain harmful to wildlife and people (Tingle et al. 2003; Gibbons et al. 2015; Yu et al. 2021; Liu et al. 2022), which restricts their practical usage.
Ivermectin is an antiparasitic drug widely used in human and veterinary medicine (Geary 2005; Watt et al. 2005). Ivermectin has been shown to be effective against parasitic worms (Prichard 2021) and ticks (Cramer et al. 1988; Taylor & Kenny 1990). Recently, the roles of ivermectin in killing fleas and ticks in rats were also explored through oral delivery (Jacob et al. 2021). Ivermectin is considered easily decomposed under natural conditions (Halley et al. 1993; Boonstra et al. 2011), while some studies have shown that residual ivermectin is toxic to daphnids, dung organisms, and crustaceans (Liebig et al. 2010; Mancini et al. 2020). It is unclear whether it can be used in combination with rodenticides to control both rodents and fleas or ticks. In this study, we examined the combined effects of a bromadiolone and ivermectin mixture for controlling the abundance of both fleas and rodents, aiming to reduce spillover risks of flea-transmitted pathogens when controlling rodents.
MATERIALS AND METHODS
Experimental design
This study included three experiments that examined the toxic effects of ivermectin, the anti-flea effects of ivermectin, and the combined effect of a bromadiolone and ivermectin mixture (iBromadiolone, iBr) on reducing the abundance of fleas. All experiments were conducted by following the guidance of the Animal Care and Use Committee (ACUC), Institute of Zoology, Chinese Academy of Sciences. Dr. Ming Liu has the certificate of conducting animal experiments (1118091400 107) administered by the Beijing Association on Laboratory Animal Care and ACUC, Institute of Zoology, Chinese Academy of Sciences.
Preparation of the bait
Basal baits were made by using a manual granulator by adding 40% water (w/w, of basal baits) to produce wet chows. Basal baits contained 30% wheat powder, 30% maize powder, 39% grass powder, and 1% sucrose. The wet chows were dried at 50°C for 24 h and then stored at room temperature (avoiding light) until use. The granulated baits were cylindrical pellets with a diameter of 1.2 cm and a length of 1–5 cm.
The ivermectin powder (from Shenghuabaike Biotechnology Company) was dissolved by heating to 55°C in 100% ethanol, forming a 2–10 mg mL−1 solution. Then, the solution was added to water to form a milky white suspension. Then, we added the suspension into the wet basal baits. Bromadiolone was purchased as a concentrated storage solution (0.5% w/v, Longhua Company; LD50 = 1.75 mg kg−1 body weight on mice) (Vandenbroucke et al. 2008) and added to wet basal baits along with the ivermectin suspension. The final concentrations of ivermectin and bromadiolone in the bait were not measured.
EXP1: toxic effects of ivermectin on rodents in the laboratory
Greater long-tailed hamsters (Tscherskia triton), a rodent pest in farmland of China, were used to test the toxic effects of ivermectin under laboratory conditions. Adult male (n = 18) and female (n = 18) hamsters were used in this experiment, with body weights ranging from 125 to 135 g and from 120 to 130 g, respectively. The hamsters were divided into three groups: control (ctrl) group, 0.01% ivermectin (low Iv) group, and 0.1% ivermectin (high Iv) group. The dose of ivermectin in the low Iv group was selected based on a previous study (Jacob et al. 2021), in which bait containing 33.42 mg kg−1 ivermectin (milligrams of ivermectin per kilogram of bait) was effective in removing ticks but not fleas. Hence, we used 0.01% ivermectin bait to ensure that the bait was effective in killing fleas. Our recent study demonstrated that 0.01% ivermectin bait (100 milligrams of ivermectin per kilogram of bait) was effective in removing fleas and ticks from several rodent species (Liu et al. 2023). The dose of ivermectin in the high Iv group was selected as 0.3 × LD50 ivermectin in rats. In another previous study (Zashchepkina 2020), the oral LD50 of ivermectin was 165 mg kg−1 in rats (milligrams of ivermectin per kilogram of body weight). One greater long-tailed hamster consumed 6–10 g bait per day, and 0.1% bait approximately equaled 50–60 mg kg−1 body weight (approximately 0.3 × 165 mg kg−1).
Each group had six individuals (six males or six females). Before the experiment, individual greater long-tailed hamsters were held in individual plastic cages (L × W × H = 290 mm × 180 mm × 160 mm) at 23–25°C and 50–60% humidity under a 12:12 light:dark period and provided food and water ad libitum. Hamsters in the low Iv and high Iv groups were fed bait (in-house produced pellets, see the section “Preparation of the bait”) containing 0.01% and 0.1% ivermectin daily for 7 days. Hamsters in the control group were fed plain baits that did not contain ivermectin. After the 7-day experiment, the hamsters of all groups were fed plain baits for an additional 7 days. During the experiment (14 days), we recorded the survival status of hamsters daily.
EXP2: anti-flea effect of ivermectin and iBr on rodents in the field
The field test was performed at the East Ujimqin Banner, Xilinhot, Inner Mongolia, China, in September 2021. The experimental site is a steppe grassland of Inner Mongolia, and the dominant rodent is Brandt's vole (Lasiopodomys brandtii). The species is a social animal that is active in the early morning and at dusk. Each family occupies a burrow system composed of many holes that are easy to distinguish from each other. We selected 10 burrow systems of voles next to each other as one control or treatment group. Each burrow had two to five voles. The distance between any two burrow systems from different groups was no less than 400 m to avoid individual vole and flea immigration/emigration during the 4-day experiment. Counting fleas before treatment would lead to a disturbance of the fleas on the rodents; thus, we did not perform pretreatment counts of fleas on voles to minimize the disturbances to voles and fleas.
The experiment comprised two parts. The first part of the experiment was designed to determine the optimal concentration of ivermectin for killing fleas and included one control and four groups treated with bait containing 0.001% ivermectin (0.001% Iv), 0.005% ivermectin (0.005% Iv), 0.01% ivermectin (0.01% Iv), or 0.05% ivermectin (0.05% Iv). The control and each treatment had 10 burrow systems. The second part of the experiment was designed to detect the anti-flea effects of a bromadiolone and ivermectin mixture (iBr), including one control group (same site and captured individuals as the first part) and one treatment group provided with baits containing a 0.005% bromadiolone and 0.01% ivermectin mixture (iBr). The control and each treatment group had 10 burrow systems.
One hundred and fifty grams of bait was placed at the burrow entrance of each burrow system. The bait was delivered at 5:00 pm. At 9:00 am by the next day, all delivered baits had been consumed or stored by voles. Four days later, we captured the voles of all groups. Colored flags were placed in each burrow system to identify its position. We placed five snap traps for rodents at each of the burrow systems for a whole day (12 h) to capture the voles. The capture of the voles was only performed once, because most individuals living in these burrow systems were captured. To avoid the escape of the fleas, we checked snap traps for rodents every 30 min, and the captured vole was put into a sealed bag immediately. Each captured vole was placed in the sealed bag with a 0.5 g isoflurane cotton ball for 15 min to kill the fleas, which were collected with combs/forceps on a clean white enamel plate. The number of fleas on each individual was recorded, and the number of fleas per vole (total flea number/total number of voles captured) of each control or treatment group individual was used to measure the severity of flea parasitism. We previously tested the field effect of iBr in Ordos Inner Mongolia and found that all the rodents died 7 days later (unpublished). In this study, we captured voles for 4 days to count fleas after bait delivery to ensure a high sample size of voles (they all died within 14 days, see EXP3 below); thus, we were not able to calculate the killing rate of iBr.
EXP3: combined effects of a bromadiolone and ivermectin mixture in the laboratory
Brandt's voles with body weights of 45–90 g (males, n = 14) and 30–45 g (females, n = 14) were used in this experiment under laboratory conditions. The 14 male or female voles were divided into two groups: the control and ivermectin and bromadiolone mixture (iBr) groups, and each group had seven individuals (seven males or seven females). Individuals in the iBr group were fed baits containing 0.005% bromadiolone and 0.01% ivermectin daily for 7 days, while individuals in the control group were fed plain baits. One Brandt's vole consumed 5–8 g bait per day. After 7 days of the experiment, individuals of all groups were fed plain baits for an additional 8 days. Bait was made by following the protocol of Experiments 1 and 2.
Statistical analysis
Survival curves were used to describe the toxicity of ivermectin or the bromadiolone and ivermectin mixture (EXP1 and EXP3). In the field test, because we mixed the voles from different burrow systems within the same control or treated groups by mistake, there was no replication test. Considering that the flea load of most individual voles was 0 or 1 (Table S1, Supporting Information, nonnormal distribution), a chi-square test was used to detect significant differences in the number of fleas per vole between the control and treatment groups.
RESULTS
Toxicity of ivermectin for greater long-tailed hamsters in the laboratory
As shown in Fig. 1, bait containing 0.1% ivermectin (high Iv group) led to approximately 33% mortality (2 of 6) in male greater long-tailed hamsters. Two individuals in the high Iv group died on both days 6 and 7 (Fig. 1, left panel). In contrast, baits containing 0.01% ivermectin (low Iv group) did not cause the death of any individuals during the 14-day experiment (Fig. 1, left panel). No deaths of male hamsters were observed in the control group (Fig. 1, left panel).
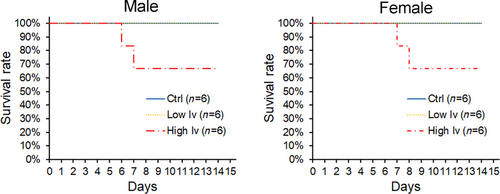
Female greater long-tailed hamsters showed a similar mortality response to ivermectin as males. Two individuals died in the 0.1% ivermectin bait treatment group (high Iv group) on days 7 and 8 (Fig. 1, right panel). There were no deaths in the control and 0.01% ivermectin bait (low Iv group) groups during the 14-day experiment (Fig. 1, right panel).
The anti-flea effect of ivermectin on Brandt's voles in field
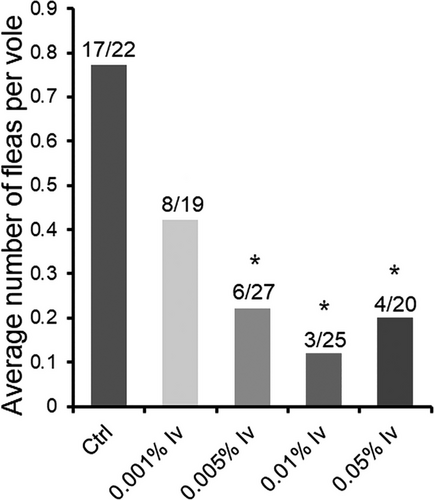
In the field test (first part of Experiment 2), the 0.005%, 0.01%, and 0.05% ivermectin bait groups all significantly reduced average numbers of fleas per Brandt's vole compared with the control group (Fig. 2). The number of fleas per vole of the 0.001% ivermectin group (0.001% Iv) was reduced to 0.42 (8 fleas/19 voles), equal to 54% of that of the control group (0.77, 17 fleas/22 voles). The number of fleas per vole of the 0.005% ivermectin group (0.005% Iv) was reduced to 0.22 (6 fleas/27 voles), equal to 29% of that of the control group. The number of fleas per vole of the 0.01% ivermectin group (0.01% Iv) was reduced to 0.12 (3 fleas/25 voles), approximately equal to 16% of that of the control group. The number of fleas per vole of the 0.05% ivermectin group (0.05% Iv) was reduced to 0.2 (4 fleas/20 voles), equal to 26% of that of the control group. The flea species in the field test comprised Leptopsylla pavlovskii (approximately 40%), Neopsylla pleskei orientalis (approximately 35%), Frontopsylla luculenta (approximately 15%), and Amphipsylla primaris mitis (approximately 10%).
The combined effects of the bromadiolone and ivermectin mixture (iBr) on Brandt's voles in the laboratory
The survival rates of Brandt's voles treated with a mixture of bromadiolone and ivermectin in the laboratory are shown in Fig. 3. For male voles, deaths occurred on days 6, 8, 10, and 11 after the delivery of the baits containing 0.005% bromadiolone and 0.01% ivermectin (iBr group, Fig. 3, left panel). Four individuals died on day 6; one individual died on each of days 8, 10, and 11 (Fig. 3, left panel). All seven individuals died during the 14-day experiment. In contrast, no animals in the control group died during the experiment (Fig. 3, left panel).
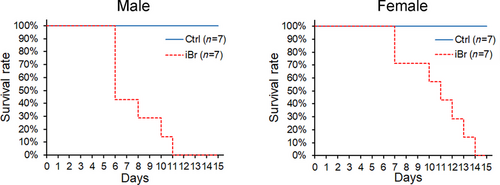
All female voles died during the 14-day experiment after treatment with bait containing 0.005% bromadiolone and 0.01% ivermectin (iBr group, Fig. 3, right panel) in the laboratory. Animals died on days 7 (n = 2), 10 (n = 1), 11 (n = 1), 12 (n = 1), 13 (n = 1), and 14 (n = 1) (Fig. 3, right panel). Individuals in the control group did not die (Fig. 3, right panel).
The anti-flea effects of the bromadiolone and ivermectin mixture (iBr) on Brandt's voles in the field
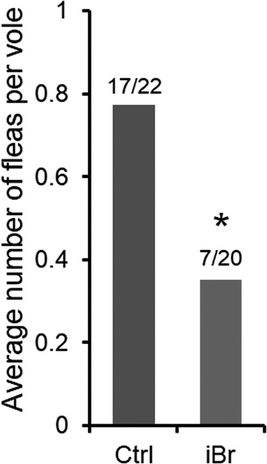
In the field test (second part of Experiment 2), the baits containing 0.005% bromadiolone and 0.01% ivermectin (iBr) significantly decreased the number of fleas per vole (Fig. 4). The number of fleas per vole was reduced to 0.35 (7 fleas/20 voles) in the 0.005% bromadiolone and 0.01% ivermectin treatment (iBr) group, approximately 45% of that of the control group (0.77, 17 fleas/22 voles).
The population of rodents was found to decrease to zero 7 days after iBr bait delivery (unpublished). In this study, to count the fleas and to have a larger sample size, we captured voles 4 days after bait delivery. Most of the individual voles were alive; however, two corpses of voles were observed in the iBr-treated site. No dead vole was found in the control group.
DISCUSSION
Our study demonstrated that 0.005%, 0.01%, and 0.05% ivermectin bait performed well in reducing fleas on rodents. Bait containing 0.005% bromadiolone and 0.01% ivermectin (iBr) significantly reduced the average number of fleas per rodent in the field and caused the death of all rodents within 6–14 days in the laboratory. Our results suggest that 0.005% bromadiolone and 0.005–0.05% ivermectin (iBr) may be effective in removing fleas, helping to minimize the spillover risk of fleas using traditional rodenticides.
Adding insecticides such as fipronil or imidacloprid to oral baits with bromadiolone has been tested to kill ectoparasites such as fleas (Leirs et al. 2001; Rust 2020; Hinds et al. 2021). A recent study indicated that baits containing 0.001% fipronil killed more than 90% of stickfast fleas (Echidnophaga gallinacea) and cat fleas (Ctenocephalides felis) on Norway rats (Jacob et al. 2021). However, fipronil and imidacloprid cause environmental problems. The residual imidacloprid in water showed adverse effects on nontarget animals (Li et al. 2009). Fipronil is hard for microorganisms to decompose and thus imposes a large threat to fish in water (Tingle et al. 2003; Gibbons et al. 2015). Thus, fipronil and imidacloprid are heavily restrained in field use.
Ivermectin is a macrolide antibiotic and is effective in eliminating many kinds of parasites, including fleas (Geary 2005). Currently, ivermectin is widely used to cure heartworm disease (Dirofilaria immitis) in cats and dogs (Wolstenholme et al. 2015), as well as in ticks for livestock (Soll et al. 1987; Davey et al. 2010). Some studies considered that residual ivermectin was very toxic to crustaceans and induced drug resistance (Liebig et al. 2010; Mancini et al. 2020), while others have found ivermectin to be safer to the environment (Halley et al. 1993; Boonstra et al. 2011). However, the anti-flea effect of ivermectin has not been fully investigated. In a recent study, it was found that oral intake of ivermectin was effective in controlling ticks on rats but not very effective in removing fleas on rats (Jacob et al. 2021), probably due to the very low dosage used. Pets or livestock are often treated with a low dose of ivermectin (usually 0.2–0.4 mg kg−1) to remove worms, ticks, or fleas (Sokol et al. 2015; Ozdemir et al. 2019). Heartworms and ticks are often exposed to ivermectin taken by hosts by direct contact with body fluid and by sucking a large amount of blood; thus, a low dose of ivermectin could be sufficient to kill them in a short time. In contrast to ticks and worms, fleas suck blood intermittently; thus, a high dose may be necessary to kill fleas in a short time period. Increasing the delivery dose of ivermectin can elongate the time window for the anti-flea effect of ivermectin (Gokbulut et al. 2011). In this study, we found that the use of oral bait containing 0.005%, 0.01%, and 0.05% ivermectin (equivalent to approximately 2.5–4, 5–8, and 25–40 mg kg−1 per day, respectively) for approximately 4 days performed well in reducing the number of fleas per vole effectively (Fig. 2), indicating that 0.005% ivermectin may be high enough to kill fleas in the field, so increasing the ivermectin concentration showed little extra effect. The mixture of 0.01% ivermectin and 0.005% bromadiolone (iBr) also performed well in controlling fleas in the field (Fig. 4).
Fleas will leave dead hosts and look for new hosts. Hence, a large number of fleas will be released into the environment when rodents are killed by using rodenticides such as bromadiolone (Hinds et al. 2021). Fleas can survive without sucking blood for 5–15 days (Krasnov et al. 2002). Thus, the traditional culling method would cause a rodent density decrease after killing, and the surviving fleas would move to a few rodents, which would result in an increase in flea load per rodent and the risk of disease transmission. Brandt's voles generally died within 6–14 days after bromadiolone treatment in the laboratory (Fig. 3), which provided a short time window for ivermectin to kill the fleas on the rodents before the rodents were killed.
Our results have implications for managing both rodents and fleas. Currently, plague is still a major threat to human health in many countries around the world. There are many plague foci covering a large area of the world where Yersina pestis circulates between rodents and fleas (Gao et al. 2021; Carlson et al. 2022; Rahelinirina et al. 2022). In plague foci where plague infection is found, rodenticides are used to kill rodents (Miarinjara et al. 2019; Rahelinirina et al. 2021), which causes the release of fleas (Hinds et al. 2021) and increases the spillover risk from rodents to people by these fleas. The mixture of 0.01% ivermectin and 0.005% bromadiolone (iBr) could be useful in terminating the circulation of Yersina pestis in plague foci and then to stop their prevalence in nature. Apart from bromadiolone, other anticoagulant rodenticides could also be used in combination with ivermectin to improve the management of both rodents and fleas. Furthermore, using a combination of a contraceptive and ivermectin could avoid the flea release caused when using rodenticides. Given that ivermectin has been suggested to decrease spermatogenesis in rats (Cordeiro et al. 2018; El-Maddawy & Abd El Naby 2018; Ahmed et al. 2020), a combination of ivermectin with a contraceptive could enhance the anti-fertility effect on rats, while reducing their flea/tick loads. This effect has been confirmed in our recent study using a combination of EP-1 (a contraceptive containing levonorgestrel and quinestrol) and ivermectin (Liu et al. 2023). However, because the anti-flea effects of ivermectin are not permanent, flea eggs in burrows may infect the infertile rodents or re-invasion may occur. The control area over which contraceptives and ivermectin are applied should be on a large scale to reduce re-invasion of rodents from outside the control area.
In summary, we found that oral baits containing 0.005% bromadiolone and 0.01% ivermectin (iBr) could be used to control both rodents and their fleas to minimize the spillover risk of zoonotic pathogens transmitted by vectors of rodents.
ACKNOWLEDGMENT
We are grateful to the grant supported by Science and Technology Service Network Initiative of the Chinese Academy of Sciences (KFJ-STS-ZDTP-2021-002).
CONFLICT OF INTEREST STATEMENT
The data of this manuscript have been used for Chinese patent application (ZL2022105022086.2).




