Parasitic flies alter the dietary preference of grasshoppers
Abstract
Parasitism is known to affect the behavior of host species to enhance parasite dispersal and transmission. However, host behavioral responses to parasitism unrelated to parasite dispersal and transmission have been much less studied. The objective of this study was to determine whether grasshopper hosts infected and uninfected with a parasitic fly (Blaesoxipha sp.) differ in terms of the nutrient content of the diet they consume. We investigated the dietary preferences of two grasshopper species (i.e. Asulconotus chinghaiensis and Chorthippus fallax) in terms of the C/N composition of plant species consumed, and determined whether this affected the egg production of unparasitized and parasitized grasshoppers by flies in a Tibetan alpine meadow. The composition of plants consumed differed significantly between the unparasitized and parasitized grasshoppers. Specifically, the abundance of N-rich legumes was lower and that of high C/N grasses was higher in the diet of the parasitized compared to the unparasitized grasshoppers. Diet N content was higher and C/N was lower in the diet of unparasitized grasshoppers, and parasitized females produced fewer eggs than their unparasitized conspecifics. Future enquiries are needed to understand the specific mechanisms underlying these dietary differences. The effects of parasites on the fitness–associated behavior of hosts should be studied more broadly to better understand parasite evolution and adaptation.
INTRODUCTION
By their very nature, parasites rely on their hosts for the nutritional needs of their growth and development (Lefevre et al. 2009; Zanette & Clinchy 2010; Labaude et al. 2015; Narr & Frost 2016), and data show that parasites may change their hosts in ways that can potentially control their host's life history, morphology, or physiological characteristics (Horwitz & Wilcox 2005; Pérez-Jvostov et al. 2017). For example, host behavior can be altered to complete the life cycle of the parasite to increase its selective advantage (e.g. Thomas et al. 2005, 2007, 2011). Studying such parasite-induced change in host behavior is important to understanding the evolution and adaptation of parasites (Frost et al. 2008; Vickery & Poulin 2009; Cezilly et al. 2010).
Studies addressing the effect of parasites on host performance are largely focused on behaviors affecting parasite dispersal and transmission. For example, the infectious fungus Ophiocordyceps unilateralis induces zombie ants to climb plants before death, thereby releasing fungal spores high above the ground (de Bekker et al. 2015; Hughes & Libersat 2019). Parasitic nematomorph worms induce crickets to jump into moving water, facilitating the dispersal of worms (Libersat et al. 2009; Ponton et al. 2011; Sato et al. 2012).
However, equally important host behaviors other than parasite dispersal have been largely neglected. For example, host feeding behavior is essential to the growth and development of both the host and the parasite, and parasites may somehow change the biochemistry and physiology of the host (e.g. by altering protein synthesis pathways). For example, the developing larvae of parasitic dipteran can excrete digestive enzymes and additional enteric components into the hemocoel of hosts, thereby modifying the internal environment of the host to facilitate their growth (Casu et al. 1996). Such physiological changes may cause the host to alter its dietary preferences (as postulated by the physiology disrupt hypothesis; Heil 2016). The growth and development of a parasite critically depends on the resources provided by its host (Harvey et al. 2009), and when parasite load is low, the parasite may alter the host's dietary preferences to its advantage even to the extent of having negative consequences on the host (Lim et al. 2010). For example, some parasitoid wasp species increase the food consumption of their herbivorous hosts to fuel their rapid development (Xi et al. 2015). Thus, parasitized herbivores may have to spend more time feeding, and hence have less time searching and finding food items with low abundance, compared to unparasitized conspecifics. Moreover, parasitized herbivores are found to be less active and sluggish than unparasitized ones (Howitt 1951; H. Guan, personal observation), once again suggesting that they are less likely to feed on low-abundance plants.
Such a dietary shift may incur a negative effect on hosts such as reducing the survival and reproductive success of either male or female conspecific hosts (Hurd 1998; Wamiti et al. 2018). Many N-rich species (e.g. legumes) are often rare in communities (as in the current study site), such that parasitized hosts cannot find them to feed on them, resulting in a lower N concentration and higher C/N in their host's diet. Low N uptake often limits animal reproductive investment (Le Gall et al. 2020) such that parasitized hosts produce fewer high-nutrient gametes (eggs or sperm). For example, some parasitic flies significantly impair the egg production and fecundity of their host grasshoppers (Prescott 1960; Laws & Joern 2012). Similarly, the trematode Trichobilharzia ocellata alters the behavior of its host Lymnaea stagnalis to increase its host carbohydrate but not nutrient reserve to provide the energy for the parasite's dispersal at a cost to its host reproduction (Plaistow et al. 2001).
In this study, we investigated the difference between the diet composition of unparasitized and fly parasitized herbivorous hosts in an alpine meadow. The study system included the parasitic fly Blaesoxipha sp. (Diptera Sarcophagidae) and its two host grasshopper species Asulconotus chinghaiensis and Chorthippus fallax. We determined the dietary composition of the parasitized and unparasitized conspecifics for two grasshopper species using DNA metabarcoding, and we measured the leaf C and N content of the plant species within the meadow that compose the diet of the grasshoppers. We also calculated the weighted average N content and C/N of the grasshopper diets. We hypothesized that: (1) the diet composition would differ between parasitized and unparasitized conspecifics, (2) the diet of the parasitized hosts would have a lower N content and a higher C/N, and (3) parasitized females would produce fewer eggs compared to their unparasitized conspecifics.
MATERIALS AND METHODS
Study site
This study was carried out in the Hongyuan Alpine Meadow Station (32°48′N, 102°33′E) situated on the eastern Qinghai–Tibetan Plateau, at an altitude of 3500 m. The mean annual temperature was 1.7°C, with a maximum and minimum monthly means of 13.4°C and −15.1°C occurring in July and January between 1961 and 2022, according to the Hongyuan County meteorological station (∼ 5.0 km distance to our study site). The mean annual precipitation is about 763 mm, fluctuating between 508 and 1032 mm among years, 80% of which occurs from May to September (Hu et al. 2020).
The alpine meadow has a >90% vegetation cover, with an average plant height of ∼30 cm. The vegetation is dominated by forbs (e.g. Saussurea nigrescens, Anaphalis flavescens, Polygonum viviparum, Anemone rivularis, and Potentilla anserine), sedges (e.g. Kobresia myosuroides, and Carex spp.), and grasses (e.g. Deschampsia caespitosa, Koeleria litvinowii, Festuca ovina, Poa glauca, and Elymus nutans). The N-rich legumes include Hedysarum sikkimense, Oxytropis kansuensis, and Lathyrus quinquenervius, and are rare in the meadow, comprising ∼2% of plant cover (Hu et al. 2021).
Study species
The two grasshopper species used in our study were A. chinghaiensis and C. fallax, both of which give rise to new generations every year that emerge as nymphs in mid and late June, and lay eggs from late August to mid–September. The eggs overwinter in the soil.
Parasitoid flies (Blaesoxipha sp.; Diptera Sarcophagidae) infect both grasshopper species, except for the males of A. chinghaiensis for reasons that require future research. Over 200 males of A. chinghaiensis were dissected and none were found to be parasitized. Adult female flies lay eggs on the surface of grasshoppers, after which the eggs hatch as larvae. Typically, one to four larvae burrow into the grasshopper, feeding on its thoracic and abdominal fat and hemolymph. In late September, only one larva successfully achieves the last instar and emerges from each parasitized host and pupates. The parasitized grasshopper dies after larval emergence, and the pupae overwinter in the soil. A survey from 2016 to 2020 (data not shown) indicated that the parasitism rate is between 5% and 30% in the field, fluctuating among years. The grasshopper density is about 8000 per ha.
Grasshopper collection and dissection
More than 800 adult grasshoppers were randomly collected using sweeping nets in the study site from mid-August to mid-September, before laying their eggs. After initial dissection, 60 females of A. chinghaiensis, 70 females and 60 males of C. fallax, half of which were parasitized and the other half unparasitized, were randomly chosen. Each individual was immediately placed in a 5-mL centrifuge tube filled with 99% ethanol for euthanasia and refrigerated at −20°C for further dissection.
Individuals were moved from the refrigerator for further dissection and immediately rinsed with 75% ethanol to remove all possible external non-host plant debris. The stomach and intestines (foregut, midgut, and hindgut) of each individual were dissected and the contents were rinsed into a 5-mL cryogenic tube. The gut contents of five randomly selected conspecifics were combined and mixed to reduce the diet variability of each sex with different parasitized states. The gut contents were stored in a −20°C refrigerator until processing.
During dissection, all parasitic larvae were preserved, and their DNA sequenced to identify them to the species level. We used the TIANcombi DNA Lyse and Det PCR Kit to extract mitochondrial DNA CO1 fragment. The DNA CO1 region (∼600-bp) was amplified by the universal primers LCO1490 and HCO2198. The details of PCR process and sequences alignments are provided by Dong et al. (2019). In addition, about 300 adult parasitic flies were collected and identified by taxonomists familiar with the parasitic flies in the study area. The adult flies were sequenced in the same way as the larvae. All larvae were identified to the level of species belonging to the genus Blaesoxipha.
During the dissection, we recorded the number of eggs per female grasshopper. In addition, we collected an additional 10 adults of each species and sex (except for male A. chinghaiensis) of both parasitized and unparasitized grasshoppers during the same period. After starvation for 24 h, we measured their body weight.
Plant barcode database to determine grasshopper diet
To determine the taxonomic identity of the plant species in the gut samples, we developed a database of plant ITS2 (internal transcribed spacer 2) barcode after extensive sampling of plants in the study site. Green leaves were collected from at least three individuals for each plant species, and then were placed in plastic bags with silica gel. We used the DNAsecure Plant Kit to extract DNA, and the ITS2 region (∼300-bp) was amplified by the primers S2F 5′ATGCGATACTTGGTGTG AAT-3′ and S3R 5′-GACGCTTCTCCAGACTACAAT-3′. The details of PCR process and sequences alignments are provided by Lan et al. (2021). As in a previous study (Lan et al. 2021), the ITS2 sequences database includes 266 plant specimens representing 130 species from our local collection.
DNA meta–barcoding for grasshopper diet
DNA meta-barcoding for the grasshopper diets was carried out using four procedures. First, we used the DNAsecure Plant Kit to extract DNA from each sample of the homogenized gut contents (20–30 mg wet weight). Second, next-generation sequencing library preparation and Illumina MiSeq sequencing were conducted by GENEWIZ, Inc. (Suzhou, China). Third, the raw sequence data (Pass Filter Data) was constructed as a FASTQ format file, and fourth, the dietary plant identification was performed by comparing diet sequencing reads with the local plant barcode database (see above) using Blast 2.7.1. Details were described for each procedure in Lan et al. (2021). In addition, to avoid interference, we removed species with less than a 1% sequence number. A total of 34 plant species in the diet of the grasshopper species were examined in this study by sequence comparisons.
Plant nutrient contents
Based on field observations, we collected a total of 80 plant species that could potentially provide a diet for the grasshoppers examined in this study. Mature leaves were taken from five individuals of each plant from each species and dried to constant weight. The carbon (C) and nitrogen (N) concentrations of each collected leaf were measured using German Elementar Vario ELIII. Accordingly, the leaf C/N was calculated for each plant species.
Data analysis
We used the sequence relative read abundance (RRA) method to determine grasshopper diet composition. RRA is the proportion of the sequence number of a certain plant species in the total sequence number of the grasshopper diet. The results showed that 80% of the total observation period captured more than 91% of the dietary plant species richness of each grasshopper species in different parasitized states (Fig. S1, Supporting Information), indicating that the sampling was sufficient. Using the leaf average N concentration and C/N of each plant, we calculated the weighted N (NW) and the weighted C/N (C/NW) in the diet (weighted by the relative abundance [%] of each plant species) for each grasshopper individual.
The results of the Shapiro–Wilk test showed that the data from the RRA of four plant functional groups (grasses, sedges, legumes, and forbs) and the female grasshopper egg number were not normally distributed. We therefore used the Wilcoxon test to determine the difference of these plant functional groups and egg number between the unparasitized and parasitized grasshoppers. Because these data for grasshopper body mass and nutritional composition (NW and C/NW) were consistent with normality and homogeneity of variance among treatments, we used the Student's t-test to determine the differences in grasshopper body mass and the differences in nutritional composition (NW and C/NW) of each species and each sex (except the male of A. chinghaiensis) between unparasitized and parasitized grasshoppers. In addition, a linear model was used to determine the relationships between egg production and NW and C/NW.
The dietary differences between parasitized and unparasitized individuals for the same sex of each of the two grasshopper species were calculated using Bray–Curtis dissimilarities, which was performed using the vegan package. Non-metric multidimensional scaling (NMDS) based on sequence data was used to visualize dietary differences between parasitized and unparasitized grasshoppers among the same species and sex. Differences in dietary composition were analyzed as independent functions of parasitized states using perMANOVA (permutational multivariate analysis of variance); perMANOVA was performed with adonis for 9999 permutations in the vegan package to obtain P-values. All of these data analyses were performed in R 4.0.3.
RESULTS
Dietary differences between parasitized and unparasitized grasshoppers
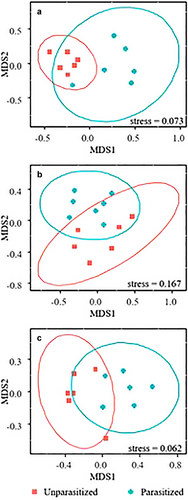
The diet of the two grasshopper species included 34 plant species, belonging to 28 plant genera across 13 families. Each grasshopper species had a broad diet, including between 15 and 20 plant species (Fig. 1). PerMANOVA and NMDS analyses showed that the diets of the parasitized and unparasitized of ♀A. chinghaiensis and ♀C. fallax were significantly different (F1,10 = 5.200, R2 = 0.342, P = 0.002; Fig. 2a) and (F1,12 = 2.794, R2 = 0.189, P = 0.019; Fig. 2b), respectively, as was that of ♂ C. fallax (F1,10 = 6.101, R2 = 0.379, P = 0.004; Fig. 2c).
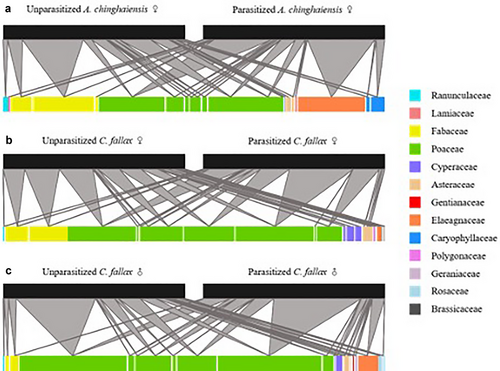
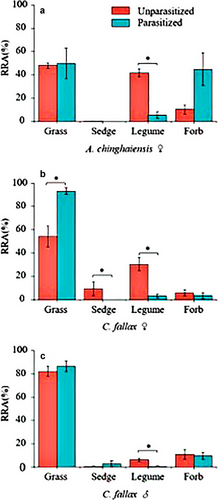
The RRA of each plant functional group differed between parasitized and unparasitized grasshopper conspecifics (Fig. 3). Specifically, the diets of parasitized grasshoppers had a lower proportional abundance of legumes relative to the diets of unparasitized grasshoppers (♀A. chinghaiensis: w = 0, P = 0.005; ♀C. fallax: w = 0, P = 0.002; ♂ C. fallax: w = 3.5, P = 0.017; Fig. 3a–c). The RRA of sedges was lower in parasitized ♀ C. fallax compared to unparasitized conspecifics (w = 10.5, P = 0.031; Fig. 3b). In contrast, the RRA of grass was significantly higher in the diet of parasitized ♀C. fallax than that of unparasitized conspecifics (w = 47, P = 0.005; Fig. 3b). Moreover, the diet of parasitized ♀A. chinghaiensis included a higher proportion of forbs than that of unparasitized ones (w = 31, P = 0.044; Fig. 3a).
Diet nutritional composition
The different functional plant species groups had different leaf nutrient contents (Table S1, Supporting Information). The leaf N content of legumes (38.56 mg g−1) was significantly higher than in monocots (18.87 mg g−1) and forbs (21.97 mg g−1), and the leaf C/N of legumes (11.97) was significantly lower than in monocots (25.09) and forbs (23.88).
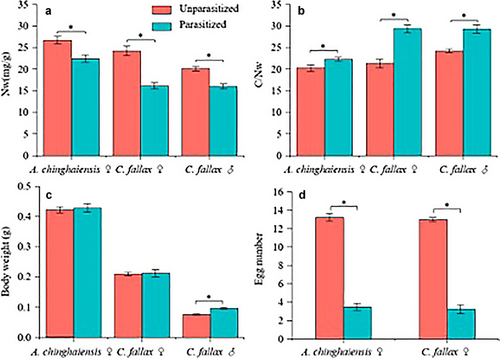
NW was significantly higher in the diets of the unparasitized grasshoppers than in those of the parasitized conspecifics (♀A. chinghaiensis: t = −3.285, df = 9.935, P = 0.008; ♀C. fallax: t = −5.612, df = 9.918, P < 0.001; ♂ C. fallax: t = −4.872, df = 9.607, P < 0.001; Fig. 4a). Consequently, the C/NW was higher in the diets of the parasitized grasshoppers compared to the unparasitized conspecifics (♀A. chinghaiensis: t = 2.234, df = 8.599, P = 0.054; ♀C. fallax: t = 5.506, df = 11.953, P < 0.001; ♂ C. fallax: t = 4.545, df = 6.695, P = 0.003; Fig. 4b).
Grasshopper traits
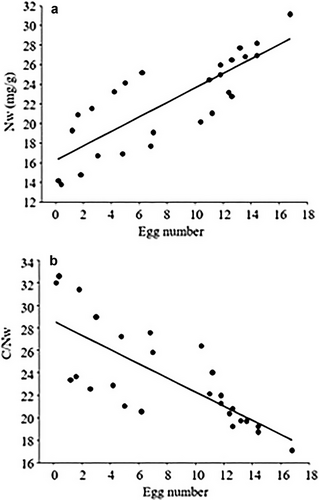
The body mass of the parasitized ♂ C. fallax (t = 5.905, df = 17.999, P < 0.001; Fig. 4c) was greater than that of the unparasitized counterparts. Moreover, the egg numbers of the unparasitized ♀ A. chinghaiensis and C. fallax were significantly greater than that of the parasitized counterparts (♀A. chinghaiensis: w = 0, P < 0.001; ♀C. fallax: w = 0, P < 0.001; Fig. 4d). NW was positively correlated with egg number per female (F = 48.259, R2 = 0.654, P < 0.001; Fig. 5a), and C/NW was positively correlated with egg number per female (F = 32.056, R2 = 0.554, P < 0.001; Fig. 5b) across parasitized and unparasitized individuals of the two species.
DISCUSSION
This study is one of the few instances where parasitism is shown to alter host diet and nutrient status. These data show clearly that statistically significant differences exist in the diets of the two species of parasitized and unparasitized grasshopper hosts. The diets of the parasitized hosts contain fewer N-rich legumes and more carbohydrate-rich grasses, compared to those of the unparasitized conspecifics. The diet of the parasitized hosts is also higher in weight averaged C/N and low in N content. In addition, the parasitized female hosts produce fewer eggs than their unparasitized counterparts.
These differences in the dietary composition between parasitized and unparasitized grasshoppers can be explained both at the family level and the plant functional level. The major plant species in the diet of both unparasitized and parasitized grasshoppers belong to the Fabaceae and Poaceae. Analyses indicate that the legume contents are much greater and that the grass contents are much smaller in the diet of unparasitized grasshoppers than in the diet of their parasitized counterparts. In the study meadow, legume species are extremely rare with a relative abundance of <3% in terms of aboveground plant biomass. However, the diet of unparasitized grasshoppers includes >10% RRA of legumes. This is interpreted to indicate that unparasitized grasshoppers are selective and deliberately feed on legumes, whereas parasitism decreases the foraging preference of grasshoppers for legume species or simply increases the consumption of monocot species.
The mechanism(s) responsible for changing the dietary preferences of parasitized grasshoppers are unclear. However, we speculate it involves the disruption of host physiology. In our study, each grasshopper can support only one larval fly to the last instar, and the growth and development of the parasite depend exclusively on its host's diet. We observed that parasitized female grasshoppers have a slightly greater body mass compared to their unparasitized counterparts. This suggests that parasitism tends to increase the consumption of plants, given that the nutrient and energy transfer from the host to parasitic flies should on average be less than one. Moreover, we observed that parasitized grasshoppers are less active (e.g. reflected by smaller leaping frequency; H. Guan, personal observation) than their unparasitized counterparts, and hence, they feed more intensively on dominant monocot species (e.g. K. litvinowii and Festuca rubra), which are more abundant. This is consistent with several studies that report reduced activity and feeding rates of parasitized hosts (Pasternak et al. 1995; de Bekker et al. 2015), although few studies have examined dietary differences.
The effects of the dietary differences between parasitized and noninfected grasshoppers resulted in an increased diet C/N and might further reduce N uptake of the parasitized hosts. However, the proximate cause for the N reduction is unclear because there are two possibilities: (1) fly parasitism may have directly or indirectly caused a reduction in the consumption of N-rich food by the host, or (2) parasitic fly larvae may have consumed N-rich resources within the host. For example, it is reported that developing parasitic larval insects can disrupt the normal hormonal processes accompanying vitellogenesis within their hosts (Beckage 2003). These two possibilities can be viewed as either parasite manipulation or physiological disruption depending on the mechanism by which parasitic larvae affect their hosts. Unfortunately, the available data do not allow us to distinguish between these two equally plausible explanations. Future research is planned to clarify this issue.
Regardless of the mechanism(s) underlying the differences in the diets of parasitized and uninfected grasshoppers, it is not surprising that the fecundity of parasitized hosts is reduced. Specifically, our data indicate that the total egg number of the parasitized hosts is ∼26% (A. chinghaiensis) and ∼28% (C. fallax) less than that of the unparasitized conspecifics. A similar phenomenology has been reported in other case studies. For example, female Melanoplus sanguinipes grasshoppers that are parasitized by Blaesoxipha sp. are reported to have a 59% lower egg production (Sanchez & Onsager 1994). Likewise, snail egg laying is reported to be significantly suppressed by the trematode T. ocellata (de Jong-Brink et al. 2001). This phenomenology is understandable because egg production requires comparatively large quantities of nutrients (Maklakov et al. 2008; Ng et al. 2019). In this regard, our data show that the N intake of parasitized C. fallax males is also reduced. Although the reproductive tissues (e.g. testes, accessory glands, thoracic muscle) of parasitized males involve a significantly small proportion of the total body mass of males (Kolluru et al. 2002), the main component of the secretion of the male accessory glands is nutrient-rich (Gillott 2003).
In summary, by examining the changes in egg production and dietary composition of parasitized and unparasitized grasshoppers, this study provides one of the few examples of parasitism altering host diet and nutrient composition. Such behavioral changes can be of significance, because differences in diets induced by parasitism have the potential to cascade to affect plant community composition and structure, which is vital to ecosystem primary production. Thus, similar fitness-associated behavioral changes resulting from parasitism should be studied further to understand parasite evolution and adaption more deeply.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (grant numbers 32071605 and 31530007). The authors are grateful to Wenlong Zhou and Tan Li for their field assistance and the Qinghai–Tibetan Research Base of Southwest University for Nationalities for providing research facilities.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.




