Epistatic interactions between pterin and carotenoid genes modulate intra-morph color variation in a lizard
Abstract
Color polymorphisms have become a major topic in evolutionary biology and substantial efforts have been devoted to the understanding of the mechanisms responsible for originating such colorful systems. Within-morph continuous variation, on the other hand, has been neglected in most of the studies. Here, we combine spectrophotometric/visual modeling and genetic data to study the mechanisms promoting continuous variation within categorical color morphs of Podarcis muralis. Our results suggest that intra-morph variability in the pterin-based orange morph is greater compared to white and yellow morphs. We also show that continuous variation within the orange morph is partially discriminable by conspecifics. Genotyping results indicate that allelic variants at the BCO2 locus (responsible for deposition of yellow carotenoids) contribute to generate continuous variation in orange individuals. However, other intrinsic and/or extrinsic mechanisms, such as body size, might be involved, opening a new avenue for future research on the drivers of continuous variation within-morphs.
INTRODUCTION
Animal coloration can serve a number of biological purposes such as camouflage, aposematism, thermoregulation, or communication (e.g. Roulin 2004; Endler 2006; Stuart-Fox & Moussalli 2009; Schaefer 2010; Bradbury & Vehrencamp 2011; Allen et al. 2013). Color polymorphisms represent a special case where 2 or more discrete and genetically-determined color morphotypes (i.e. morphs) are present in an interbreeding population (Ford 1945; Huxley 1955). Color polymorphisms occur in all major animal groups (Wellenreuther et al. 2014) providing exceptional systems to test hypothesis in functional and evolutionary biology. Among them, lizards stand out by displaying a vast diversity of polymorphic color patterns (Stuart-Fox & Ord 2004; Nicholson et al. 2007) that have repeatedly emerged and been lost (Corl et al. 2010; Feldman et al. 2011; Stuart-Fox et al. 2021).
The striking coloration of lizards results from the interplay of 3 different layers of chromatophores (i.e. xanthophores, iridophores, and melanophores) combining light-absorbing pigments and reflective/scattering structures (Olsson et al. 2013). Long wavelength-based colors (i.e. those spanning from yellow to red) are commonly achieved through the deposition of carotenoids and/or pterins in the xanthophores (Olsson et al. 2013), and recent studies have provided evidence on the link between genes involved in carotenoid and pterin metabolism and color polymorphisms in lizards (e.g. McLean et al. 2017, 2019; Andrade et al. 2019). However, animal coloration usually arises from rather complex interactions between multiple pigmentary and/or structural components (Saenko et al. 2013; San-Jose et al. 2013; San-Jose & Roulin 2017) potentially involving pleiotropic/epistatic interactions or a tight linkage between the genes involved in coloration (Sinervo & Svensson 2002; Jamie & Meier 2020).
In spite of recent advances in the understanding of cellular and molecular mechanisms underlying discrete color morphs, intra-morph variation has only been explored in a handful of studies (e.g. Teasdale et al. 2013; Paterson & Blouin-Demers 2017). One of the few studies characterizing continuous variation within-morphs in lizards showed that orange and yellow coloration in Ctenophorus decresii vary continuously, and its expression behaves as a quantitative trait, likely regulated by multiple genes and environmental effects (Rankin et al. 2016). Furthermore, differences in the methods to classify color morphs have led to inconsistent or controversial classifications (e.g. Vercken et al. 2007, 2008; Cote et al. 2008), hampering the drawing of firm conclusions. Thus, exploring within-morph variation would not only inform about the reliability of genotypic inferences from color morphs but would also provide insights into the mechanisms responsible for generating color and its functionality.
The common wall lizard, Podarcis muralis (Laurenti, 1768), is a widespread lacertid species displaying up to 5 discrete color morphs in syntopy: white, yellow, and orange pure morphs, as well as white-orange and yellow-orange intermediate mosaic morphs (Pérez i de Lanuza et al. 2013). This complex polymorphism is likely under the influence of both natural and sexual selection (e.g. Calsbeek et al. 2010; Galeotti et al. 2013; Pérez i de Lanuza et al. 2017, 2018a; Sacchi et al. 2017; Pérez i de Lanuza & Carretero 2018; but see Abalos et al. 2020, 2021). This variation in color expression is associated with 2 regulatory regions near genes responsible for pterin (sepiapterin reductase [SPR]) and carotenoid (beta-carotene oxygenase 2 [BCO2]) metabolism (Andrade et al. 2019). Adult P. muralis display orange colorations when homozygous for a recessive regulatory allele (o) at SPR, whereas individuals exhibiting yellow colorations are homozygous for a regulatory recessive allele (y) at BCO2 (Andrade et al. 2019). Genotyping results from this study suggested that the orange locus is epistatic over the yellow locus, since the expression of yellow will not occur if a lizard is homozygous for the orange allele. The orange morph is explained by the same haplotype across the whole distribution of the species, but despite this, a recent study identified a significant degree of intra-morph variation in the achromatic profile of the orange morph comparing 2 phylogenetically distant lineages (Pérez i de Lanuza et al. 2019). In addition, field observations (e.g. Pérez i de Lanuza et al. 2018a) indicate that even within single localities there is considerable variation in orange-morph lizards.
Even though much attention has been focused on between-morph differences, the magnitude of within-morph differences in polymorphic systems, as well as how they are generated or perceived, has seldom been assessed. In this study, we seek to shed light on intra-morph color variation and discriminability within the orange morph of P. muralis. Available evidence suggests that diurnal lizards recognize color-based cues along 300–700 nm (e.g. Loew et al. 2002; Bowmaker et al. 2005; Fleishman et al. 2011; Pérez i de Lanuza et al. 2014; Martin et al. 2015). Yet, only recently have studies begun to test lizard's ability to discriminate color variation in conspecifics (e.g. Teasdale et al. 2013), and to our knowledge the only available experimental evidence confirming that lizard's color variation represents actual categorical color morphs was obtained for P. muralis (Pérez i de Lanuza et al. 2018b). Therefore, here we address intra-morph variation in the orange morph of P. muralis by using visual modeling. Given that orange lizards can possess all 3 possible genotypes at the ‘yellow’ locus (i.e. YY, Yy, and yy; Andrade et al. 2019), and thus putatively vary in their degree of accumulation of yellow carotenoids in ventral skin, we hypothesize that interactions between pterin and carotenoid pigmentation lead to intra-morph variability in the orange morph of P. muralis. Thus, we examine genetic variation at BCO2 in orange individuals on several populations from the Pyrenees which have been subject to an extensive study (e.g. Pérez i de Lanuza et al. 2013, 2016, 2019).
MATERIALS AND METHODS
Sample collection
We collected 2001 adult P. muralis (915 females and 1086 males) from the eastern Pyrenees during spring-summer season in 2018, 2019, and 2020. Samples were distributed along 28 different localities covering an area of ≈800 km2 (see sampling details in Table S1, Supporting Information). All lizards were captured by noose, measured, and images from their ventral surface were taken with a scanner (LiDE 700F, Canon). Although a conventional scanner does not allow to consider the way in which lizards perceive coloration (which is analyzed with spectral data, see below), it ensures a standardized illumination that allows us to obtain comparable pictures in RGB color space. As in previous studies, lizard's morphs were directly determined by eye and also checked with a reflectance spectrophotometer (e.g. Pérez i de Lanuza et al. 2013; see below). Tissue samples (i.e. tail tips) were also collected and stored in 96% ethanol. Lizards showing intermediate morphs (i.e. a mosaic of scales of 2 different colors: white and orange or yellow and orange) were excluded. After measurements, all lizards were released to the exact same point of capture previously geo-referenced. Intra-morph variation within the orange morph, hereinafter referred to as “redness”, was preliminarily determined by eye on a subsample of 72 males (from 8 localities; see details in Table S1, Supporting Information). For this first “redness” classification we arbitrarily determined 3 different categories: “orange”, “red”, and “deep red” (where “orange” and “deep red” represent the extremes of variation; see representative examples in Fig. 1). We chose these artificial categories because, while color variation is continuous (see results below), their use allows for a practical grouping to perform comparisons.

Color measurement and visual modeling
To check the intra-morph classification and obtain an objective and quantitative measurement of “redness”, we performed spectrophotometric measurements from both the throat and the belly of orange lizards using a USB-2000 portable spectrometer and a PX-2 xenon light source (Ocean Optics Inc., Dunedin, USA), calibrated with a Spectralon white diffuse reflectance standard (Labsphere; see Badiane et al. 2017 for technical details). From spectra, we extracted luminance (i.e. the sum of the relative reflectance over the 300–700 nm range), hue (wavelength at which reflectance is halfway between its minimum and its maximum), and intermediate chroma (the sum of the reflectance between 400 and 600 nm divided by the sum between 300 and 700 nm; that is, the waveband in which the ventral morphs are more variable). As in some adult lizards, the orange coloration of throats is not completely developed (at least in some localities); subsequent analyses were focused on belly coloration. Then, using Vorobyev and Osorio's (1998) receptor noise limited model, we performed visual models of ventral coloration to quantify chromatic and achromatic distances (i.e. discriminability) within the 3 “redness” categories. We used cone absorbance spectra of P. muralis (Martin et al. 2015) and assumed a cone ratio of 1:1:1:4 (corresponding to UV-, short-, middle-, and long-wavelength sensitive cones; Martin et al. 2015; Pérez i de Lanuza et al. 2018b), and a Weber fraction of 0.05 for the long-wavelength sensitive cone (Siddiqi et al. 2004; Martin et al. 2015; Pérez i de Lanuza et al. 2018b). To test if the different “redness” categories are discriminable by conspecifics, we follow the methodology proposed by Maia and White (2018) combining a PERMANOVA analysis (using 999 permutations to determine if the difference between “redness” categories is statistically supported), and a bootstrap analysis to test if the putative differences are perceptually discriminable assuming a discrimination threshold of 1 JND. Spectra processing, visual modeling and analyses were performed using the packages PAVO 2.1.0 (Maia et al. 2019), vegan (Oksanen et al. 2007) and RVAideMemoire (Hervé 2018) in R 4.0.3 (R Core Team 2020). Finally, using pictures corresponding to the same subsample of 72 adult males employed for the by-eye classification (see above) and genotyping (see below), we also extracted RGB mean values from the entire belly surface (including all the ventral scales, but avoiding black spots) using ImageJ (Schneider et al. 2012). Significance level was set at P < 0.05.
Genotyping
To understand if genetic variation associated with yellow pigmentation interacts with orange, we focused on the subsample of 72 orange males previously used for spectral analyses based on their “redness” category. Genomic DNA was extracted using a membrane-based commercial kit (EasySpin; Citomed). Resulting gDNA was used as template to amplify via polymerase-chain reaction (PCR) a 509-bp region upstream of BCO2 known to be associated with yellow coloration in P. muralis (forward primer: 5′-GAGCTCGAAGGAATCCTGAA; reverse primer: 5′-TAGCCACTTTCTGCCAAACC; Andrade et al. 2019). Since several polymorphic sites within this region provide redundant information, we chose a target mutation (chr15:26,161,682 bp) strongly correlated with the deposition of yellow carotenoids (Andrade et al. 2019). Amplification reaction consisted of 1 μL of gDNA (≈50 ng), 5 μL of QIAGEN PCR Master Mix, 0.4 μL of each primer, and 3.2 μL of H2O for a final volume of 10 μL. Thermocycler profile was set as follows: (1) initial denaturing step of 95°C for 15 min; (2) 5 cycles of 95°C for 30 s, 68–64°C for 30 s with a decrease of 1°C per cycle and 72°C for 45 s; (3) 35 cycles of 95°C for 30 s, 64°C for 30 s, and 72°C for 45 s; and (4) a final extension step of 60°C for 20 min. PCR products were Sanger sequenced in an Applied Biosystems ABI3130xl DNA Analyzer, and genotypes at the target mutation for each sample were obtained by analyzing chromatograms in BioEdit v. 7.2.5 (Hall 1999).
Analyses
In order to compare intra-morph variability between the 3 pure morphs we conducted pairwise Levene tests on the spectral variables (i.e. luminance, hue, and intermediate chroma) of our overall sample using the car package (Fox & Weisberg 2019). Moreover, we explored the correlation between the spectral variables of orange bellies and body size (snout-to-vent length, SVL). To avoid undesired consequences of sexual size dimorphism, for this analysis we only considered males. Then, focusing on the subsample of 72 orange males, we performed PERMANOVA analyses (using 999 permutations) using the vegan package (Oksanen et al. 2007) to inspect possible differences on the spectral variables between the 3 “redness” categories, and between the BCO2 genotypes. Additionally, we included SVL as a predictor for both analyses to account for putative differences in pigment accumulation with age. We also used Mann–Whitney U tests for subsequent pairwise comparisons. Finally, we explored the correlation between orange throats and bellies for the spectral variables, and the correlation between the RGB values and the chromatic variables (i.e. intermediate chroma and hue). All analyses were performed in R 4.0.3 (R Core Team 2020) and corrected by Benjamini & Hochberg (1995) with a significance level of P < 0.05.
RESULTS
The orange morph of P. muralis shows an intra-morph variability on the intermediate chroma higher than the white and yellow morphs; white-yellow paired comparison for the intermediate chroma was not significant, while white-orange and yellow-orange were highly significant (Table 1). Moreover, the orange morph occupies a greater volume in the color space (Fig. 2a): the chromatic volume of orange lizards is 0.00034, whereas chromatic volumes of white and yellow lizards are 0.00016 and 0.00010, respectively. All spectral variables from male orange bellies are correlated with SVL (intermediate chroma, Pearson correlation: t = −12.48, P < 0.001, correlation coefficient = −0.54, n = 377; hue, Spearman correlation: S = 11436980, P < 0.001, rho = −0.28, n = 377; and luminance, Spearman correlation: S = 4138209, P < 0.001, rho = 0.51, n = 369).
| Spectral intra-morph variability between morphs (pairwise Levene tests) | |||
|---|---|---|---|
| F | df | P-value | |
| Luminance | |||
| White-yellow | 2.53 | 1 | 0.171 |
| White-orange | 3.30 | 1 | 0.118 |
| Yellow-orange | 0.24 | 1 | 0.649 |
| Intermediate chroma | |||
| White-yellow | 2.26 | 1 | 0.198 |
| White-orange | 369.34 | 1 | <0.001 |
| Yellow-orange | 82.88 | 1 | <0.001 |
| Hue | |||
| White-yellow | 125.18 | 1 | <0.001 |
| White-orange | 410.65 | 1 | <0.001 |
| Yellow-orange | 0.34 | 1 | 0.612 |
| Spectral variables between “redness” categories (PERMANOVA analysis) | ||||
|---|---|---|---|---|
| F | df | P-value | R2 | |
| Luminance | ||||
| “Redness” | 10.02 | 2 | 0.005 | 0.23 |
| SVL | 2.32 | 1 | 0.198 | 0.03 |
| “Redness”:SVL | 0.43 | 2 | 0.689 | 0.01 |
| Intermediate chroma | ||||
| “Redness” | 40.90 | 2 | 0.003 | 0.53 |
| SVL | 4.00 | 1 | 0.100 | 0.03 |
| “Redness”:SVL | 1.13 | 2 | 0.392 | 0.01 |
| Hue | ||||
| “Redness” | 42.50 | 2 | 0.003 | 0.56 |
| SVL | 1.73 | 1 | 0.250 | 0.01 |
| “Redness”:SVL | 0.50 | 2 | 0.641 | 0.00 |
| Spectral variables between genotypes (PERMANOVA analysis) | ||||
|---|---|---|---|---|
| F | df | P-value | R2 | |
| Luminance | ||||
| Genotype | 4.94 | 2 | 0.012 | 0.12 |
| SVL | 5.90 | 1 | 0.036 | 0.07 |
| Genotype:SVL | 2.22 | 2 | 0.155 | 0.05 |
| Intermediate chroma | ||||
| Genotype | 4.22 | 2 | 0.032 | 0.10 |
| SVL | 11.59 | 1 | 0.005 | 0.13 |
| Genotype:SVL | 1.28 | 2 | 0.353 | 0.03 |
| Hue | ||||
| Genotype | 2.54 | 2 | 0.155 | 0.06 |
| SVL | 8.02 | 1 | 0.011 | 0.10 |
| Genotype:SVL | 0.60 | 2 | 0.584 | 0.02 |
- Tests were considered significant if P < 0.05.
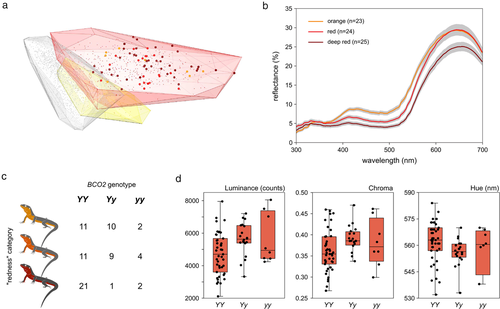
The 3 arbitrary categories of “redness” differ in both the achromatic (i.e. luminance) and chromatic (i.e. intermediate chroma and hue) spectral variables, while SVL and the interaction were not significant (Table 1 and Fig. 2b). “Redness” categories are partially discriminable (PERMANOVA: F2,69 = 5.41, P = 0.003, R2 = 0.14): “orange” and “red” are discriminable (P = 0.007; bootstrap mean = 1.43 JNDs; bootstrap 95% CI = 1.04–1.98), “orange” and “deep red” are also discriminable (P = 0.007; bootstrap mean = 2.06 JNDs; bootstrap 95% CI = 1.60–2.62), but “red” and “deep red” are not discriminable (P = 0.245; bootstrap mean = 0.64 JNDs; bootstrap 95% CI = 0.35–1.23; Fig. S1, Supporting Information). Spectral variables of orange throats and bellies are strongly correlated for intermediate chroma (Pearson correlation: t = 13.69, P < 0.001, correlation coefficient = 0.58, n = 72), hue (Spearman correlation: S = 3896471, P < 0.001, rho = 0.47, n = 72), and luminance (Spearman correlation: S = 4073750, P < 0.001, rho = 0.49, n = 72). Values of RGB obtained from scans are also correlated with both chromatic variables obtained from ventral spectra: intermediate chroma (Spearman correlation: S = 108924, P < 0.001, rho = −0.83, n = 72) and hue (Spearman correlation: S = 16996, P < 0.001, rho = 0.72, n = 72).
Finally, orange morph individuals can present all 3 genotypes at the BCO2 locus (Fig. 2c). Lizards with the 3 possible genotypes at BCO2, however, differ in their luminance and intermediate chroma (Table 1 and Fig. 2d). Moreover, the SVL (but not the interaction) is significant for all spectral properties (Table 1). Differences for intermediate chroma are significant for the YY vs. Yy comparison (P = 0.008, YY lizards have lower values of intermediate chroma). Similar patterns are observed when comparing luminance (YY vs. Yy, P = 0.011, YY lizards have lower values of luminance) and hue (YY vs. Yy, P = 0.014, YY lizards have higher values of hue). Other non-significant comparisons with the yy genotype should be viewed with caution due to the small sample size of this group (n = 8).
DISCUSSION
Continuous variation in color polymorphic systems has been greatly overlooked. Here, we combine spectrophotometric and visual modeling analyses to describe intra-morph variation and genotypic data to infer its molecular source. Overall, our results suggest that the orange morph possess a higher degree of variability than white and yellow morphs. Furthermore, chromatic and achromatic distances within the orange morph are partially discriminable, but not surprisingly, chromatic distances within and between “redness” categories are smaller than those reported between different color morphs (see values in Pérez i de Lanuza et al. 2018b).
Our genotyping results confirmed that orange coloration is epistatic over the yellow, as orange individuals can present all 3 genotypes at BCO2. Additionally, BCO2 genotype seems to be associated with differences on luminance and intermediate chroma, particularly when comparing YY against Yy genotypes. These results suggest that BCO2-mediated carotenoid deposition in xanthophores contributes to intra-morph variation in orange individuals. Previous results on gene expression and pigment quantification suggested that the yellow allele leads to down-regulation of BCO2 in xanthophores, followed by deposition of colorful dietary carotenoids such as zeaxanthin or lutein (Andrade et al. 2019). A possible scenario suggested by our present results is thus that orange lizards with at least one copy of the yellow allele (Yy and yy) are impaired in their capacity to fully metabolize these pigments (deeper red coloration is almost exclusively attained in lizards that are homozygous for the non-yellow Y allele), which are then deposited in xanthophores, leading to subsequent chromatic (intra-morph) variation in the orange morph. Furthermore, and according to our results, differences in size could also contribute to generate variability in color, likely as a consequence of differential deposition of pterin and carotenoid pigments with age. Despite the relatively low frequencies at which yellow and yellow-orange morphs are observed in nature (e.g. Pérez i de Lanuza et al. 2017, 2018a), the yellow allele may be maintained in the genetic pool through orange individuals. Finally, the expression of orange coloration regardless of BCO2 genotype posits a question on why ooyy individuals can exhibit either orange or yellow-orange coloration (i.e. why do many adults express a “mosaic” of scales of different colors?), which should be addressed in future studies.
Although our results suggest a role for carotenoid pigmentation and body size/age in modulating pterin-based signals, it is highly likely that other factors apart from these 2 loci contribute to modulate ventral skin coloration. Differences in tissue nanostructure, shape and size of the chromatophores, the abundance and distribution of pterinosomes within the chromatophores, carotenoid intake or the presence and concentration of other pigments (e.g. melanin) are also likely to promote intra-morph variation (Weiss et al. 2012; Cuervo et al. 2016; Merkling et al. 2018). These mechanisms are also likely to affect the chromatic properties of the other morphs of P. muralis, which, even though are less variable than the orange morph, are also likely to harbor variability that is discriminable to conspecifics.
Variability within the orange morph documented in this study is similar to that observed comparing the Pyrenean and the north-Italian lineages, mainly caused by differences in luminance (Pérez i de Lanuza et al. 2019). Continuous variation between orange individuals from different populations could be explained by historical divergence, as suggested by Pérez i de Lanuza et al. (2019), by the above-mentioned mechanisms, or most likely, by a combination of both. Further evidence will be required to clarify if and to what extent these putative mechanisms interact to generate continuous variation within and between lineages of P. muralis, for example by explicitly quantifying pigment content in xanthophores of each “redness” category. In any case, our results highlight the relevance of studying intra-morph color variation, identifying interactions between alleles involved in the expression of color polymorphisms.
AUTHOR CONTRIBUTIONS
Conceptualization: G.P.L. and P. Andrade; data curation: G.P.L., P. Aguilar, and P. Andrade; formal analysis: P. Aguilar and G.P.L.; investigation: P. Aguilar, P. Andrade, and G.P.L.; resources: G.P.L.; supervision: G.P.L. and P. Andrade; visualization: G.P.L. and P. Andrade; writing—original draft: P. Aguilar; writing—review and editing: P. Aguilar, G.P.L., and P. Andrade.
ACKNOWLEDGMENTS
We wish to thank Catarina Pinho and Miguel Carneiro for useful discussions. Lizards were captured under permits 2016-s-09 from the Direction Régionale de l'Environnement, de l'Aménagement, et du Logement, Occitanie, and SF/0092/2019 from the Departament de Territori i Sostenibilitat, Generalitat de Catalunya. G.P.L. was supported by post-doctoral grants Juan de la Cierva-Incorporación, IJC2018-035319-I (from the Spanish Ministerio de Ciencia, Inovación y Universidades), and SFRH/BPD/94582/2013 by Fundação para a Ciência e a Tecnologia under the Programa Operacional Potencial Humano-Quadro de Referência Estratégico Nacional, funds from the European Social Fund and Portuguese Ministério da Educação e Ciência. P. Andrade was supported by the Fundação para a Ciência e Tecnologia (FCT) through a research contract in the scope of project PTDC/BIA-EVL/28621/2017. P. Aguilar was supported by the Fundação para a Ciência e Tecnologia (FCT) through a technician contract in the scope of project PTDC/BIA-EVL/30288/2017-NORTE-01-0145-FEDER-30288. This work was funded by Fundação para a Ciência e Tecnologia through the FCT project PTDC/BIA-EVL/30288/2017-NORTE-01-0145-FEDER-30288 and by the Spanish Ministerio de Ciencia e Innovación through the project PID2019-104721GB-I00.
COMPETING INTERESTS
The authors declare no competing interests.




