Toxicity of Phenyl Isothiocyanate and Its Sublethal Effects on Growth, Development and Oviposition in Bt-Susceptible and Bt-Resistant Pink Bollworms
Funding: This work was supported by National Natural Science Foundation of China, 32472550.
ABSTRACT
Pest development of resistance to Bacillus thuringiensis (Bt) insecticidal proteins threatens the sustainability of Bt crops, and it is therefore necessary to explore ecofriendly alternative insecticides for controlling Bt-resistant populations. Isothiocyanates are plant secondary metabolites that exhibit a diverse array of types and resistance against a broad spectrum of insect pests, but their effects on pink bollworms remain unexplored. Here, we evaluated the effects of phenyl isothiocyanate on Bt-susceptible and Bt-resistant pink bollworms (Pectinophora gossypiella). The LC50 values of phenyl isothiocyanate for Bt-susceptible and Bt-resistant larvae were 26.4 and 28.6 μg/mL, respectively. The LC30 sublethal dose of phenyl isothiocyanate significantly impaired the 7-day larval survival, the pupation rate, neonate-to-adult survival, the pupal weight, eggs per female and the egg hatching rate and prolonged the larval developmental period but not pupal duration for both strains. The response to phenyl isothiocyanate ingestion did not differ significantly between the two strains, implying that the Bt-resistant strain did not possess cross-resistance to phenyl isothiocyanate. These results provide scientific evidence for enriching green control technologies, resistance management tactics and comprehensive management measures for pink bollworm.
1 Introduction
Genetically modified crops that produce insecticidal proteins derived from Bacillus thuringiensis (Bt) have been utilized globally for pest control for nearly 30 years, conferring specific resistance to target pests (ISAAA 2021; Tilgam et al. 2021). Bt-transgenic crops not only are harmless to nontarget organisms but also allow reduced use of chemical insecticides and promote natural ecological regulation in the field, yielding significant economic, social, and environmental benefits (Dively et al. 2018; Romeis et al. 2019; Li et al. 2020). However, in 11 pest species, some field populations have been verified to exhibit practical resistance that reduces the efficacy of Bt crops, which is detrimental to their sustainable application (Jurat-Fuentes et al. 2021; Tabashnik et al. 2023a; Afzal et al. 2024). Thus, it is essential to explore other environmentally friendly insecticides with distinct mechanisms of action to effectively control Bt-resistant populations of these pests.
The pink bollworm (Pectinophora gossypiella) is a globally destructive cotton pest and one of the target pests of Bt-transgenic cotton. Commercialization of Bt-transgenic cotton effectively suppressed the occurrence of pink bollworms and consequent damage in fields in China, Mexico, and the USA (Wan et al. 2017; Tabashnik et al. 2023b).
However, multiple resistance alleles at the cadherin locus have been detected in some populations of pink bollworms in the Yangtze River Valley of China (Wang et al. 2020; Wang et al. 2022). Furthermore, some populations of pink bollworms in Pakistan and India have been confirmed to exhibit practical resistance to Bt-transgenic cotton generating Cry1Ac, and populations in India have even been confirmed as resistant to Bt cotton expressing two insecticidal proteins, Cry1Ac and Cry2Ab (Naik et al. 2018; Tabashnik et al. 2023b), posing a severe threat to the sustainable application of Bt-transgenic cotton. Therefore, novel alternative methods for effectively suppressing pink bollworms, especially Bt-resistant individuals or populations, are urgently needed.
Plants have developed defense mechanisms against herbivorous insect pressures, such as the production of secondary metabolites, which exhibit toxicity, antifeedant, repellence, and growth inhibitory effects on herbivorous pests, through long-term coevolution (Wang et al. 2019; Gajger and Dar 2021; Zhang et al. 2021). As essential sources of environmentally benign insecticides, plant secondary metabolites play crucial roles in preventing the invasion of herbivorous pests, including direct lethality to pests and negative impacts on insect feeding behavior, growth, development, reproduction, etc. (Benelli and Maggi 2022; Lai et al. 2022). The secondary metabolites naturally present in plants are important sources for the development of environmentally friendly insecticides. Isothiocyanates (ITCs) are bioactive products of glucosinolates, the most abundant secondary metabolites naturally occurring in cruciferous plants; these compounds have toxic and repellent effects on insect pests, antimicrobial effects on pathogens, and beneficial effects for human health (Khokon et al. 2011; Nowicki et al. 2016; Gonzalez et al. 2018; Sontowski et al. 2022; Flor-Weiler et al. 2023).
ITCs exhibit a broad spectrum of resistance against insect pests and encompass a diverse array of types (Romeo et al. 2018; Chen et al. 2020). For example, allyl ITC exhibited insecticidal activity against all four developmental stages (egg, larva, pupa, and adult) of the chive maggot Bradysia odoriphaga (Diptera: Sciaridae), with the highest activity observed against adult-stage insects (Shi et al. 2017), and also caused high larval mortality, an altered larval period, and a prolonged pupal period in the melon fruit fly Zeugodacus cucurbitae (Diptera: Tephritidae) (Singh et al. 2022). 4-Methylsulfinylbutyl ITC has significant detrimental effects on caterpillar mortality, caterpillar weight, and the development period of the cabbage looper Trichoplusia ni (Lepidoptera: Noctuidae) but has no influence on pupal weight (Ghosh et al. 2023). Benzyl ITC, phenethyl ITC, and phenyl ITC repelled Haemaphysalis longicornis nymphs to varying degrees (Kim et al. 2021). However, the effects of ITCs on pink bollworms and Bt-resistant pests remain unexplored.
Here, for the first time, we tested the effects of phenyl ITC on both Bt (Cry1Ac)-susceptible and Bt (Cry1Ac)-resistant strains of pink bollworms, with the aim of evaluating the insecticidal activity of phenyl ITC against larvae; its effects on the growth, development, and reproduction of pink bollworms at sublethal doses (LC15 and LC30); and the differences in its effects on the above two strains. Our findings provide scientific evidence for enriching green control technologies, resistance management tactics, and comprehensive management measures for this pest.
2 Materials and Methods
2.1 Insects and Phenyl ITC
We used two strains of pink bollworm, the Bt (Cry1Ac)-susceptible strain XZ-S and the known Cry1Ac-resistant strain AQ47. The XZ-S strain originated from Xinzhou (Wuhan City, China) and was reared in the laboratory for more than 3 years without any exposure to insecticides or Bt toxins. The AQ47 strain, derived from a field-collected individual obtained from the Yangtze River Valley in China in 2013, was composed of homozygotes carrying the resistance allele r13r13 at the cadherin locus (Wang et al. 2018). The AQ47 strain underwent selection with 10 μg/mL Cry1Ac protoxin every four to five generations, and surviving individuals were reared to maintain the strain and its resistance. The larvae were reared under the following conditions: temperature of 28°C ± 1°C, photoperiod of 16 h light:8 h dark, and relative humidity (RH) of 50 ± 10%. The rearing conditions for the adults were the same as those for the larvae, except that the relative humidity was increased to 70 ± 10%. The phenyl ITC used in this study was purchased from Qingdao Bangtai Chemical Co. Ltd.
2.2 Preparation of an Artificial Diet With Phenyl ITC
The effects of phenyl ITC on insecticidal activity and the growth, development and reproduction of pink bollworms were determined by mixing phenyl ITC with a wheat germ artificial diet (Wu et al. 2008). When the prepared artificial diet had cooled to approximately 50°C, an appropriate amount of phenyl ITC liquid to create diets with final phenyl ITC concentrations of 0, 14, 18, 22, 26, and 30 μg/mL was added, and the mixtures were blended thoroughly with an electric mixer. Once the diets had completely solidified, they were divided into small cubes of approximately 1 cm3 and then placed into 24-well cell culture plates for later use.
2.3 Larval Bioassay
To generate offspring, 50 pairs of newly emerged virgin adults from each strain (XZ-S and AQ47) were placed into separate plastic boxes (10 cm in diameter and 15 cm in height) and were supplied with 8% honey water. Eggs of the Cry1Ac-susceptible (XZ-S) and Cry1Ac-resistant (AQ47) strains were collected, and neonates were transferred singly into each well after hatching. For each strain, each concentration of phenyl ITC was tested three to four times, with each replicate including 24 larvae (one cell culture plate). All plates were placed in the insect culture room with the environmental conditions described above. The number of larvae that survived until the seventh day after treatment was recorded for each replicate under each treatment and used to generate a concentration–mortality curve. The number of newly pupated individuals and dates of pupation for each individual were recorded daily from the 10th day until all surviving larvae pupated. All pupae were identified individually under a microscope to distinguish females and males, and their weights were recorded. A longitudinal crack-like structure displays on the central ventral surface of the 8th–9th abdominal segments in female pupae, while a distinct circular or elliptical depression exhibits on the central ventral surface of the 9th abdominal segment in male pupae. Daily observations and records of the number of emerged adults and dates of emergence for each individual were performed after pupation. The 7-day larval survival, pupation rate, eclosion rate, survival rate from neonate to adult, and larval developmental period were calculated as previously described (Wang et al. 2024). The 7-day larval survival = (number of larvae that survived until the seventh day after treatment/number of neonates tested) × 100%. The pupation rate = (number of pupae/number of neonates tested) × 100%. The eclosion rate = (number of adults/number of pupae) × 100%. The survival rate from neonate-to-adult = (number of adults/number of neonates tested) × 100%. The larval developmental period was calculated as the date of pupation minus the date of the tested neonates. The pupal duration was calculated as the date of emergence minus the date of pupation.
2.4 Female Fecundity Bioassay
Four pairs of newly emerged virgin adults from the 0 (control), 18, and 22 μg/mL phenyl ITC treatment groups of XZ-S and AQ47 were dispensed into a plastic box with a diameter of 10 cm and a height of 15 cm. A small cap (diameter 2 cm, height 1 cm) containing absorbent cotton was placed into each box, which was filled with 8% honey water and replaced with clean absorbent cotton once daily. The top of each box was covered with a sheet of rough white art paper produced by Wenzhou Snow Mountain Paper Co. (Wenzhou, Zhejiang, China), which served to collect eggs and prevent the adults from escaping. Female fecundity was tested three to five times for each concentration for each strain. Each day (until the female died), the egg collection paper on the top of the box was replaced with a new sheet of paper, and the number of eggs on the collected piece of paper was recorded. We selected 14–34 pieces of egg collection paper containing at least 50 newly laid eggs each from each treatment, transferred each paper into a small plastic box with a diameter of 5 cm and a height of 3 cm and placed these boxes into the insect culture room for 7 d. The total number of eggs on each selected paper and the number of unhatched eggs remaining on the seventh day were observed and recorded, and the hatching rate was calculated as the difference between the total number of eggs and the number of remaining eggs that failed to hatch divided by the total number of eggs, multiplied by 100%.
2.5 Data Analysis
Statistical analysis was conducted using IBM SPSS Statistics 22.0 software. For each strain, we conducted a probit regression analysis of the number of larvae that died within 7 days to calculate the LC15/LC30/LC50 values along with their 95% fiducial limits (FLs), as well as the slope of the concentration–mortality line and its standard error (SE). General linear models were used to perform two-factor ANOVA to analyze the effects of two fixed factors (strain and concentration) and their interaction on the 7-day larval survival, pupation rate, eclosion rate, neonate-to-adult survival, eggs per female, and egg hatching rate. Three-factor ANOVA (significance level α = 0.05) were conducted using general linear models to examine the effects of three fixed factors (strain, concentration, and sex) and their main interactions on pupal weight. Tukey's B multiple comparison test (significance level α = 0.05) was used for post hoc analysis to analyze the differences in the response of the same strain to different concentrations of phenyl ITC. Independent sample t tests (α = 0.05) were carried out to evaluate the difference in response between the two strains under the same concentration of phenyl ITC. The Scheirer–Ray–Hare tests and Dunn's tests were conducted to analyze the differences in larval developmental period and pupal duration.
3 Results
3.1 Insecticidal Bioassay of Phenyl ITC on Bt-Susceptible and Bt-Resistant Pink Bollworm Larvae
Phenyl ITC bioassays were performed on pink bollworms of the Bt (Cry1Ac)-susceptible strain XZ-S and the Bt (Cry1Ac)-resistant strain AQ47. The concentration of phenyl ITC needed to kill 50% of the larvae (LC50) was 28.6 μg/mL for AQ47 and 26.4 μg/mL for XZ-S, whereas the concentration of phenyl ITC needed to kill 90% of the larvae (LC90) was 52.1 μg/mL for AQ47 and 43.2 μg/mL for XZ-S (Table 1). Although the LC50 and LC90 values of phenyl ITC were slightly higher for the AQ47 strain than for the XZ-S strain, the differences were not significant at the conservative criterion of overlap of their 95% FLs (Table 1). The LC15 and LC30 values of phenyl ITC were also not different between the strains. These results indicated that the insecticidal activity of phenyl ITC was similar for Cry1Ac-susceptible and Cry1Ac-resistant pink bollworm larvae.
| Strain | Slope (SE)a | LC15 (95% FL)b | LC30 (95% FL) | LC50 (95% FL) | LC90 (95% FL) |
|---|---|---|---|---|---|
| XZ-S | 6.02 (0.851) | 17.8 (15.6–19.3) | 21.6 (20.1–22.9) | 26.4 (25.0–28.4) | 43.2 (37.7–54.5) |
| AQ47 | 4.93 (0.916) | 17.6 (14.4–19.6) | 22.4 (20.3–24.1) | 28.6 (26.4–32.6) | 52.1 (42.1–81.6) |
- a Slope of the concentration-mortality line with its standard error in parentheses.
- b Concentration killing 15% with 95% fiducial limits in parentheses, in μg phenyl isothiocyanate per ml diet.
3.2 Effects of Phenyl ITC on Survival, Pupation Rate, and Eclosion Rate in Pink Bollworms
Phenyl ITC had a significant negative effect on neonate-to-adult survival, 7-day larval survival, pupation rate, and eclosion rate in both the XZ-S and AQ47 strains (Table 2). The 7-day larval survival of the XZ-S strain at the LC15 and LC30 were significantly reduced by 14.6% and 28.2%, respectively, whereas those of the AQ47 strain were reduced by 14.5% and 27.0%, respectively, compared with the control groups (Table 2). Similarly, the pupation rates at the LC15 and LC30 were significantly lower (by 16.7% and 29.2%, respectively) for the XZ-S strain and by 15.6% and 30.2%, respectively, for the AQ47 strain, compared with the control groups (Table 2). Both the eclosion rate and neonate-to-adult survival also decreased with increasing phenyl ITC content. Compared with the control group, the eclosion rates of AQ47 strain in the LC30 treatment group significantly decreased by 19.3%, whereas that of the XZ-S strain showed a non-significant reduction of 12.5%. The neonate-to-adult survival rates of the two strains in the LC30 treatment group decreased significantly by 35.1% and 40.7%, respectively, compared to the control group (Table 2). However, no significant differences in 7-day larval survival, pupation rate, eclosion rate, or neonate-to-adult survival were detected between the two strains (Tables 2 and S1).
| Treatment (concn. in μg/mL) | The 7-day larval survival (%) | Pupation rate (%) | Eclosion rate (%) | Neonate-to-adult survival (%) | ||||
|---|---|---|---|---|---|---|---|---|
| XZ-S | AQ47 | XZ-S | AQ47 | XZ-S | AQ47 | XZ-S | AQ47 | |
| 0 (Control) | 91.7 ± 2.4 aA | 88.5 ± 2.6 aA | 91.7 ± 2.4 aA | 88.5 ± 2.6 aA | 93.9 ± 3.0 aA | 97.7 ± 1.3 aA | 86.1 ± 3.7 aA | 86.5 ± 2.0 aA |
| 18 (LC15) | 77.1 ± 5.5 bA | 74.0 ± 3.6 bA | 75.0 ± 4.5 bA | 72.9 ± 3.6 bA | 84.7 ± 2.6 aA | 81.4 ± 2.7 bA | 63.5 ± 4.6 bA | 59.4 ± 3.6 bA |
| 22 (LC30) | 63.5 ± 2.0 bA | 61.5 ± 3.6 cA | 62.5 ± 2.9 bA | 58.3 ± 3.8 cA | 81.4 ± 4.2 aA | 78.4 ± 2.8 bA | 51.0 ± 4.3 bA | 45.8 ± 3.8 cA |
- Note: Values are means ± SE. Different lowercase letters represent significant differences in the same strain under different treatment concentrations (SPSS 22.0, Tukey's B, α = 0.05). Different capital letters represent significant differences between two strains under the same treatment concentration (SPSS 22.0, t-test, α = 0.05).
3.3 Effects of Phenyl ITC on Larval Developmental Periods and Pupal Duration in Pink Bollworms
The larval developmental periods of both the XZ-S and the AQ47 strains increased significantly with increasing concentrations of phenyl ITC. The prolonged larval development was caused by the difference in phenyl ITC concentration (p < 0.001) rather than the difference in strain (p = 0.522), and there was no interaction effect between strain and phenyl ITC concentration (p = 0.739) (Table S2). For the XZ-S strain, the larval developmental period in the control group (12.83 ± 0.21 d) was significantly shorter than those in the LC15 (14.75 ± 0.21 d) and LC30 (15.37 ± 0.23 d) treatment groups (p < 0.001), but there was no significant difference between LC15 and LC30 groups (p = 0.172) (Figure 1A and Table S3). For the AQ47 strain, the larval developmental period in the control group (12.93 ± 0.12 d) was significantly shorter than those in the LC15 (14.79 ± 0.171 d) and LC30 (15.29 ± 0.23 d) treatment groups (p < 0.001), but no significant difference was observed between LC15 and LC30 groups (p = 0.915) (Figure 1A and Table S3).
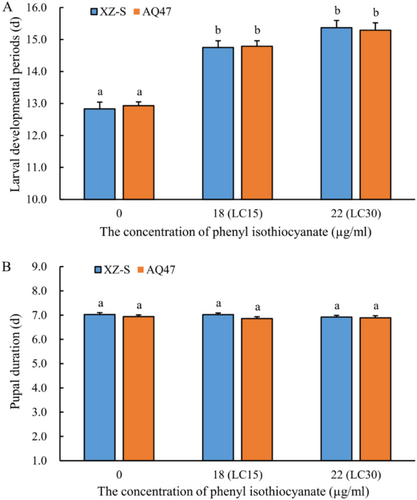
The pupal durations for the XZ-S strain in the control, LC15 and LC30 groups were 7.03 ± 0.073 d, 7.02 ± 0.068 d, and 6.92 ± 0.070 d, respectively, with no significant differences among the three groups (p > 0.05) (Figure 1B). The pupal durations for the AQ47 strain in the control, LC15 and LC30 groups were 6.94 ± 0.067 d, 6.86 ± 0.073 d, and 6.89 ± 0.087 d, respectively, which were not significantly different among the three groups (p > 0.05) (Figure 1B). The strains (p = 0.082) and the LC15/LC30 concentrations of phenyl ITC (p = 0.678) both showed no significant effect on pupal duration (Table S4).
3.4 Effects of Phenyl ITC on the Pupal Weight of Pink Bollworms
ANOVA revealed that pupal weight was significantly affected by the phenyl ITC concentration (LC15 vs. LC30) and sex but not by strain (Table S5). The pupal weights of females and males for XZ-S strain decreased significantly by 12.5% and 14.3%, respectively, at the LC30 concentration of phenyl ITC compared to the control group; similarly, those of AQ47 strain were also reduced significantly, by 17.7% and 22.1%, respectively (Table 3). For both the XZ-S and AQ47 strains, the pupal weight of females was significantly greater than that of males at each concentration (Table 3). In addition, there was no interaction effect between sex and the concentration of phenyl ITC, indicating that the inhibitory effect of phenyl ITC on pupal weight was affected only by its concentration and not by the sex of the insect (p = 0.294, Table S5), that is, that phenyl ITC had the same inhibitory effect on pupal weight in both females and males.
| Treatment (concn. in μg/mL) | Female | Male | ||
|---|---|---|---|---|
| XZ-S | AQ47 | XZ-S | AQ47 | |
| 0 (Control) | 19.2 ± 0.84 aA | 20.3 ± 0.35 aA | 14.7 ± 0.48 aB | 14.5 ± 0.42 aB |
| 18 (LC15) | 16.6 ± 0.53 bA | 17.3 ± 0.75 bA | 12.8 ± 0.46 bB | 13.1 ± 0.41 aB |
| 22 (LC30) | 16.8 ± 0.67 bA | 16.7 ± 0.75 bA | 12.6 ± 0.48 bB | 11.3 ± 1.04 bB |
- Note: Values are means ± SE. Different lowercase letters represent significant differences in the same strain under different treatment concentrations (SPSS 22.0, Tukey's B, α = 0.05). Different capital letters represent significant differences between two strains under the same treatment concentration (SPSS 22.0, Tukey's B, α = 0.05).
3.5 Effects of Phenyl ITC on Reproduction in Pink Bollworms
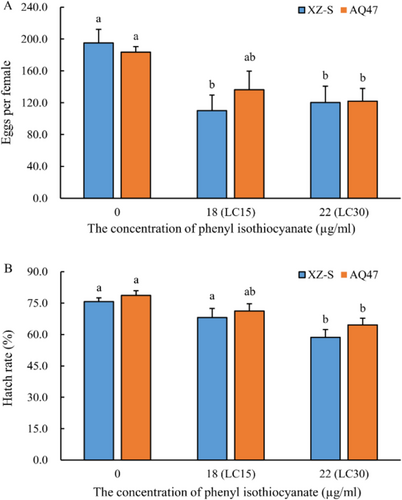
Phenyl ITC had a significant negative effect on the number of eggs per female and the egg hatching rate of both the XZ-S and AQ47 strains, but there was no significant difference between the two strains (Tables S6 and S7). In the XZ-S strain, the number of eggs per female decreased from 195.1 ± 17.0 in the control group to 110.1 ± 19.5 at the LC15 concentration (p = 0.017) and 120.3 ± 20.4 at the LC30 concentration (p = 0.015) (Figure 2). In the AQ47 strain, the number of eggs per female was 183.6 ± 6.8 in the control group, 136.3 ± 23.3 in the LC15 group (nonsignificant difference, p = 0.060), and 121.9 ± 15.9 in the LC30 group (p = 0.022) (Figure 2A). At the LC30, the egg hatching rate of the XZ-S strain decreased significantly, from 75.7 ± 1.8% to 58.7 ± 3.6% (p < 0.001); similarly, that of the AQ47 strain decreased significantly, from 78.7 ± 2.3% to 64.6 ± 3.2% (p = 0.002) (Figure 2B). Based on the life history traits summarized above, the net reproductive rates of XZ-S and AQ47 were 3.4 times and 2.3 times greater, respectively, when reared on the diet without phenyl ITC than on the diet containing the LC30 concentration of phenyl ITC (Table 4).
| Trait | Treatment groupa | Control groupb | Treatment group/control group | |||
|---|---|---|---|---|---|---|
| XZ-S | AQ47 | XZ-S | AQ47 | XZ-S | AQ47 | |
| Neonate-to-adult survival | 0.51 ± 0.04 | 0.46 ± 0.04 | 0.86 ± 0.04 | 0.86 ± 0.02 | 0.59 | 0.53 |
| Proportion of females | 0.49 | 0.68 | 0.48 | 0.46 | 1.02 | 1.48 |
| Eggs per female | 120 ± 20 | 122 ± 16 | 195 ± 17 | 184 ± 7 | 0.62 | 0.66 |
| Hatch rate | 0.59 ± 0.04 | 0.65 ± 0.03 | 0.76 ± 0.02 | 0.79 ± 0.02 | 0.78 | 0.82 |
| Net reproductive ratea | 17.7 | 24.8 | 61.2 | 57.5 | 0.29 | 0.43 |
- a Diet with phenyl isothiocyanate at the LC30 concentration.
- b Diet without phenyl isothiocyanate.
4 Discussion
This study revealed that the secondary metabolite phenyl ITC not only had direct toxic effects on the larvae of both Bt-susceptible and Bt-resistant pink bollworms but also significantly inhibited growth, development, and fecundity in both strains. For the Bt-susceptible strain XZ-S, larval ingestion of phenyl ITC significantly increased both larval and neonate-to-adult mortality and decreased the pupation rate, adult eclosion rate, pupal weight, egg number per female, and egg hatching rate, along with extending the duration of the larval stage, and its efficacy increased in a concentration-dependent manner (Tables 1–3, Figure 1 and Figure 2). Our results are similar to those of several earlier reports, which revealed a significant increase in larval mortality, prolonged development period and decreased larval weight in Z. cucurbitae and T. ni with increasing allyl ITC or 4-methylsulfinylbutyl ITC concentrations (Singh et al. 2022; Ghosh et al. 2023). Similarly, the accumulation of 4-methylsulfinylbutyl ITC in Plutella xylostella larvae impaired larval weight gain, larval pupation, and adult reproduction (Sun et al. 2019). Moreover, the inhibition of emergence and oviposition upon exposure to sublethal concentrations of allyl ITC was also observed in Callosobruchus maculatus and Sitophilus zeamais (de Souza et al. 2018; Vilela et al. 2020). These results suggest that some negative effects of ITCs on pests are conservative and widespread.
Interestingly, allyl ITC significantly reduced the total development period and pupal period in Z. cucurbitae, with its effect on the larval period varying by concentration (Singh et al. 2022). This contrasts with observations in pink bollworms, where phenyl ITC significantly shortened the larval developmental period but did not significantly affect the pupal duration. Moreover, 4-methylsulfinylbutyl ITC had no significant effect on pupal weight in T. ni (Ghosh et al. 2023), whereas phenyl ITC significantly reduced pupal weight in pink bollworms. These results indicate that there may be some differences in the effects of different ITCs on insects or that different species of insects may have slightly different responses to ITCs.
Notably, the response to phenyl ITC ingestion was essentially consistent between the Bt-resistant strain AQ47 and the XZ-S strain. The concentration of phenyl ITC, rather than the strain, was the key factor in significant impairments in 7-day larval survival, pupation rate, adult eclosion rate, neonate-to-adult survival, pupal weight, and larval duration in pink bollworms (Tables S1–S5). These results demonstrate that phenyl ITC is equally efficacious against the Bt-susceptible strain XZ-S and the Bt-resistant strain AQ47 and therefore that the AQ47 strain did not display cross-resistance to phenyl ITC. The main pathways used to detoxify ITCs in lepidopteran and Diptera pests are conjugation with L-glutathione (GSH), the mercapturic acid pathway and the ITC-specific hydrolytic pathway, whose activation involves the upregulated expression of genes encoding detoxifying enzymes such as specific glutathione-S-transferases or P450s (Schramm et al. 2012; Halon et al. 2015; Jeschke et al. 2016; Sontowski et al. 2022). The main mechanism of resistance to Bt (Cry1Ac) in the AQ47 strain is a mutation in the cadherin gene (PgCad1), named the r13 allele (Wang et al. 2018). Thus, the lack of resistance to phenyl ITC in the AQ47 strain implies that the r13 allele does not affect detoxification metabolic pathways.
Planting pyramided Bt crops containing two or more Bt proteins is currently a key strategy for delaying the development of pest resistance. However, cross-resistance among Bt proteins threatens the sustainable use of pyramided Bt crops, which can ultimately promote the evolution of pest resistance to Bt crops (Wei et al. 2019; Kennedy et al. 2023). Thus, the development of novel alternative control agents without cross-resistance to Bt proteins is necessary. Here, we observed that the Cry1Ac resistance conferred by the r13 mutant allele at the cadherin gene locus in pink bollworms did not exhibit cross-resistance to phenyl ITC. This finding suggests that phenyl ITC could serve as a promising potential alternative for controlling Cry1Ac-resistant pink bollworm populations, given that mutations in PgCad1 are the primary mechanism underlying resistance to Bt cotton expressing Cry1Ac in this pest (Fabrick et al. 2014; Wang et al. 2022).
Acknowledgments
This work was funded by research grants from National Natural Science Foundation of China (32472550).
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




