Solitary Bees as Vital Bioindicators: A Comprehensive Review of the Diversity, Decline, and Conservation Imperatives of the Halictidae Family
ABSTRACT
Pollination, a keystone ecological process sustaining most flowering plant communities, is indispensable to human survival, with over 500 cultivated plant species relying on insect pollinators. Solitary bees (Hymenoptera: Apoidea) are critical contributors to this service, requiring specialized foraging, nesting, and habitat resources. Plant diversity strongly correlates with pollinator community composition, underscoring the ecological interdependence of these groups. Within solitary bees, the family Halictidae (~4500 species) plays a disproportionately significant role in global pollination networks. Halictids exhibit remarkable diversity in social organization—ranging from solitary to communal, semi-social, and primitively eusocial behaviors—shaped by floral resource availability, geographic distribution, and climatic factors. The subfamily Halictinae represents the group's greatest diversity, with the tribe Halictini comprising 53.3% of described species. Key pollinator genera such as Lasioglossum (e.g., Lasioglossum marginatum, Lasioglossum leucozonium) dominate temperate ecosystems. However, population declines in solitary bees have severely disrupted pollination services across wild and cultivated plant systems, exacerbating global concerns over insect biodiversity loss and biomass reduction. These declines threaten foundational ecosystem services, necessitating urgent research to refine species diversity estimates, identify habitat conservation priorities, and implement evidence-based protective policies. This review highlights the need for standardized methodologies to accurately assess global bee diversity and proposes targeted strategies to mitigate conservation challenges for Halictidae and other solitary bee taxa.
1 Introduction
Pollination is a critical ecological process for sustaining the majority of angiosperms on Earth (Asar et al. 2022). Insect pollination is essential for the reproduction of nearly 75% of cultivated and wild plant species (Kaur and Kaleka 2022) making pollinators crucial for human survival. While honey bees are well-known pollinators, nearly 85% of bee pollinators are solitary and live in burrows under diverse ecological conditions (Zattara and Aizen 2021). Though their pollination mechanism is not as developed as that of honey bees, their ecological importance is undeniable (Vázquez et al. 2023). Female solitary bees mate independently and build nests comprised of 10–15 brood cells in soil, wood, litter, rocks, and other substrates (Danforth et al. 2019; Loukola et al. 2020).
They forage on various crops for pollen and nectar, contributing to pollination in the process. The gathered food is stored in a ball-like structure inside each cell, where they later lay eggs, a process known as mass provisioning (Pizza et al. 2023). Many solitary bees are losing their natural habitat due to anthropogenic activities, leading to a rapid decline in pollinator populations (Dar et al. 2017). The availability of nesting sites is a key factor in bee restoration. Farmers worldwide have begun providing suitable habitats and provisions for solitary bees. The decline of honey bee populations due to colony collapse disorder and environmental pollution has heightened concerns about wild bee conservation (Panziera et al. 2022).
Solitary bees do not live in colonies; instead, they raise their young independently by mass provisioning in newly constructed cells within diverse substrates (Danforth et al. 2019). They seek holes or tunnels in soil, sand, clay, wood, or other materials for shelter (Sexton et al. 2021). They may also use old mud walls, houses, cavities, and naturally formed spaces such as fissures in bricks. Habitat loss due to modern infrastructure development, synthetic chemicals, and pollution leads to biodiversity loss and increases the risk of disease outbreaks (Barbier 2021; Wilkinson et al. 2018; Yuan et al. 2024). To counter this, specially designed structures and solitary bee houses can encourage nesting (El Abdouni et al. 2022). Nearly 90% of the total bee population comprises solitary bees (El Abdouni et al. 2022; Lemanski et al. 2022).
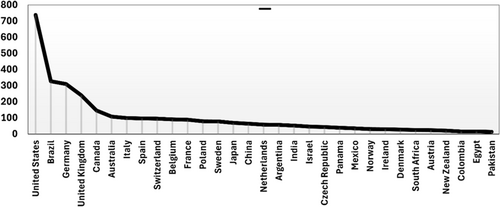
Globally, there are approximately two trillion bees, including 81 million honeybee colonies and 100 million managed beehives. The seven recognized bee families—Apidae, Megachilidae, Halictidae, Colletidae, Melittidae, Andrenidae, and Stenotritidae—encompass over 20,000 species. The Apidae subfamily contributes around 5700 species, including bumblebees and honey bees (Orr et al. 2021; Seltmann and Poelen 2024). The Andrenidae family consists exclusively of solitary bees that nest in the ground (Pisanty et al. 2022). Solitary bee species, including mason, leafcutter, mining, carder, and digger bees, number between 20,000 and 30,000. Over the past 10 years decade, 623 studies on solitary bees have been conducted across 67 countries. The United States, Brazil, Canada, Russia, Germany, China, the United Kingdom, India, Argentina, and Australia account for 58% of total publications (Figure 1).
Solitary bees are crucial for biodiversity and agriculture. Among them, the Halictidae family plays a significant role in maintaining ecosystems, yet their populations are declining. This decline threatens plant pollination and ecosystem stability. Plant diversity and pollinator variety are closely linked, emphasizing the importance of habitat preservation. Despite advances in research, knowledge gaps remain regarding solitary bee population dynamics and responses to environmental changes. These gaps hinder conservation efforts against climate change, habitat fragmentation, and pesticide use. By analyzing the diverse social and nesting behaviors of Halictidae and their adaptability to stressors, this review aims to enhance ecological understanding and conservation strategies. Protecting these vital pollinators is essential for ecosystem stability and agricultural productivity.
This review examines the ecological significance of solitary bees, particularly Halictidae, and highlights threats to their populations. By synthesizing research on their behaviors, nesting habits, and links to plant diversity, it addresses critical knowledge gaps and explores conservation strategies. Emphasis is placed on habitat conservation, plant-pollinator networks, and mitigating threats such as pesticide use and climate change. The goal is to provide insights that inform future research and effective conservation efforts.
The novelty of this review lies in its focus on solitary bees, especially Halictidae, as essential yet understudied pollinators. While research often centers on social bees like honeybees and bumblebees, solitary bees play a crucial role in both wild and agricultural ecosystems. This review explores Halictidae diversity, from solitary to semi-social behaviors, nesting requirements, and floral preferences influenced by environmental conditions. By analyzing key genera, such as Lasioglossum, and recognizing Halictidae as bioindicators of ecosystem health, it underscores their ecological importance. Conservation strategies tailored to solitary bees, such as plant-pollinator networks and predictive models for environmental impacts, are urgently needed. Addressing knowledge gaps, this review contributes to a more comprehensive understanding of pollinator conservation.
1.1 Solitary Bees as a Crop Pollinator
Broadly, bees exhibit various forms of social behavior, including sociality (as seen in honey bees), eusociality, semi-social, solitary, and communal behaviors (Smith et al. 2022). Globally, there are hundreds of genera of bees, comprising both domesticated and solitary species. Most solitary bees belong to nine families within the order Hymenoptera. Among the bee families currently studied, the most researched include Colletidae (membrane bees), Andrenidae (digger bees), Halictidae (sweat bees), Megachilidae (leafcutter bees and mason bees), Anthophoridae (carpenter and minor bees), and other families such as Melittidae, Oxaeidae, Fideliidae, Apidae (honey bees), stingless bees, orchid bees, and bumblebees. The majority of bees are solitary and exhibit fossorial behavior, which involves excavating and building nests in various structures such as soil, sand, leaf litter, twigs, hollow reeds, wood, and other materials (Odanaka and Rehan 2020).
In each nest, mated females (gynes) create specific structures of varying dimensions, called cells, for egg laying. After laying the eggs, they provision the cells and seal them until the adults emerge. Generally, each female builds one or two nests during her lifetime. Males typically emerge first, preparing to mate with the early-emerging females. The nest tunnel contains multiple cells, with those closer to the entrance allowing males to emerge earlier than those in the deeper cells of the nest (Buckley et al. 2016).
Bees are efficient pollinators of entomophilous crops, with over 500 cultivated plant species relying directly on insect pollination, particularly from members of the superfamily Apoidea and solitary bees, for their yield or seed production. Entomophilous plants cover more than half of all cultivable land and account for roughly one third of all agricultural products. While honey bees pollinate the greatest number of plants, wild bee species also make significant contributions to pollination but are often overlooked (Weekers et al. 2022). Because different plants have varying flower morphologies in terms of structure and function, wild bee activity is crucial for the cultivation and pollination of a wide variety of plants. Many flowers are small and are not effectively pollinated by honey bees. The Fabaceae family of field crops, which includes red clover and alfalfa, is cultivated for hay and green mass to feed cattle. These crops are entirely dependent on solitary bee species for pollination, especially when used as mulch in vegetable gardens (Badawy et al. 2022). It was explored by Riggi et al. (2021) that the relationship between red clover yield and bumblebee activity, focusing on the flower morphology that facilitates pollinator visits, nectar harvesting, and subsequent pollen transfer. The length of the flower corolla varies from 7.5 to 12.4 mm (with an average of 10 mm), but the nectar, which is mostly released from the base of the flower, rarely rises higher than 1.35–1.47 mm.
All solitary bees are effective pollinators of Medicago sativa, one of the most important forage crops grown worldwide. The floral architecture of alfalfa delivers pollen explosively onto the bee's head (a process known as tripping) when the bee attempts to sip nectar from the flower's center. However, honey bees tend to avoid the tripping mechanism and instead attempt to steal nectar from the side of the flower to avoid being directly hit by the pollen (Zhang et al. 2022).
Alkali bees (Nomia melanderi) and alfalfa leafcutting bees (Megachile rotundata) are effective pollinators because they do not mind being hit directly by pollen while collecting nectar for nourishment (Vijayakumar et al. 2022). Solitary bees effectively pollinate approximately 8% of blooming plants through a process known as buzz pollination or sonication. Numerous carpenter bees of the genus Xylocopa can vibrate tomatoes and other plants due to the rapid contraction of their indirect flight muscles (Holley et al. 2022). The contraction of muscles in Xylocopa bee species during foraging forces hidden anthers to eject pollen onto their heads, resulting in effective pollination of various crops (El Abdouni et al. 2022). However, honey bees lack this mechanism and are therefore less efficient pollinators for many angiosperms. Honey bees are effective pollinators for various plant species in the Rosaceae family and are commonly used as commercial pollinators for apple orchards, alongside mason bees (Osmia cornifrons), Japanese horn-faced bees, and blue orchard bees (Osmia lignaria). Since the mid-1990s, the Japanese have been using horn-faced bees to pollinate apples. Additionally, according to Wurz et al. (2021), fruit trees respond strongly to the pollination efforts of solitary bees. Carpenter bees, due to their high tolerance for extreme weather conditions, can forage in hot environments where honey bee activity is minimal. They also perform pollination in low sunlight, rainy conditions, and on windy days (Chapman et al. 2022). By employing this trait, carpenter bees can serve as excellent pollinators in hot, cold, arid environments, as well as in hot and microclimatic polyhouses (Dar et al. 2023). Over time, many plants and solitary bees have coevolved to form mutualistic relationships. For example, sweat bees often pollinate crops that bloom at night or in moonlight, a behavior that is uncommon among honey bees. In general, for some solitary bee species, wide eyes and ocelli have evolved for crepuscular foraging on crops that bloom at night, when honeybee activity is low. Carpenter bees are more polylectic than other oligolectic solitary bees and also engage in extensive foraging flights even under dim light. In regions where honeybee pollination is inefficient, carpenter bees serve as significant pollinators (Anandhan et al. 2020). Solitary bees are also important in areas where honeybee species are absent (Shaw et al. 2021). This is managed by keeping brood cells in deep refrigeration to maintain a dormant state for an extended period. These dormant bees can then be used for pollination in agricultural and horticultural crops during the blooming season. Bringing the brood cells to optimal temperatures under controlled climatic conditions allows males to emerge first, followed by females the next day. However, in many cases, females may emerge from the brood cells a week or more later, depending on climatic conditions (De Manincor et al. 2023).
1.2 Major Solitary Bee Families
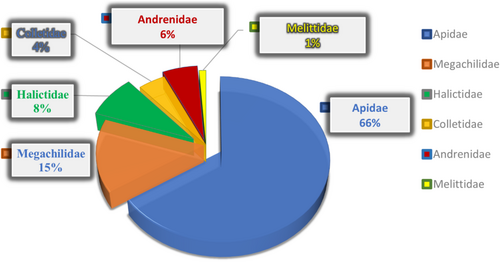
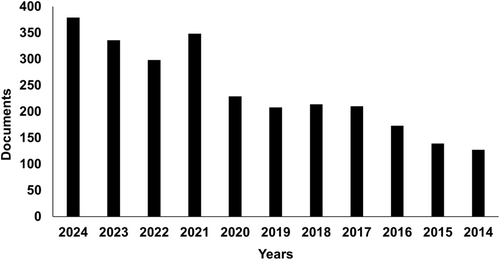
Most solitary bees belong to nine families within the order Hymenoptera. Among all currently existing bee families, the most researched include the Membrane bees (Colletidae), Digger bees (Andrenidae), Sweat bees (Halictidae), Leaf cutter bees (Megachilidae), Mason bees (Anthophoridae), Carpenter bees, and Minor bees, particularly from the families Melittidae, Oxaeidae, Fidellidae, Apidae (honey bees), Stingless bees, Orchid bees, and Bumblebees (Batra 1984). Table 1 provides an overview of solitary bee families and their common names. In Figure 2, a pie chart illustrates the top six families of solitary bees ranked by the percentage of publications over the last 10 years. The family Apidae leads with 66% of the total articles, followed by Megachilidae with 15%. During this period, the family Stenotritidae was the least studied. Based on data from the Scopus database, the number of documents related to six Hymenopteran families namely Apidae, Megachilidae, Halictidae, Colletidae, Melittidae, and Andrenidae (Figure 3). Table 1 summarizes the primary families of solitary bees in the order Hymenoptera, along with their common names. These bees, unlike social species, nest and forage alone, contributing uniquely to pollination across diverse habitats.
| Bee family | Description | Geographic distribution | Conservation status | Research attention (% of publications) | Notes |
|---|---|---|---|---|---|
| Apidae | Includes honey bees, bumblebees, carpenter bees, stingless bees, and orchid bees. Both solitary and social species. | Cosmopolitan | Varies: Managed honey bees stable; some bumblebees Vulnerable (e.g., Bombus spp.). | 66% (leading family) | Dominates research due to ecological/economic importance. |
| Megachilidae (Leafcutter bees) | Solitary; construct nests using leaves or resin. | Global (except Antarctica) | Mixed: Some species declining due to habitat loss. | 15% | Key pollinators for crops and wild plants. |
| Halictidae (Sweat bees) | Mostly solitary; includes polylectic species attracted to human sweat. | Worldwide (except polar) | Least Concern overall; some species at risk from pesticides. | ~7% (estimated from remaining 19%) | Adaptable to diverse environments. |
| Andrenidae (Digger bees) | Solitary ground nesters; diverse in temperate zones. | Northern Hemisphere and South America | Local declines noted (pesticides/habitat loss). | ~5% | Critical for early spring pollination. |
| Colletidae (Membrane bees) | Solitary; line nests with cellophane-like secretions. | Australia and South America | Least Concern; some Australian species declining. | ~4% | Primarily studied in Southern Hemisphere. |
| Melittidae | Specialist pollinators (e.g., oil-collecting bees). | Africa, Europe, and North America | Declining due to habitat fragmentation. | ~3% | Rare; reliant on specific host plants. |
| Anthophoridae (Mason bees) | Solitary; nest in pre-existing cavities (e.g., wood and stems). | Widespread in temperate/arid zones | Stable in agricultural areas; habitat-dependent declines. | Not specified (grouped under Apidae in some taxonomies) | Often confused with Apidae; key for orchard pollination. |
| Oxaeidae | Lesser-known solitary bees; neotropical specialists. | Americas (neotropical) | Data deficient; habitat loss suspected. | <1% | Rarely studied; limited data. |
| Fidellidae | Small family of solitary bees. | South America, southern Africa | Not evaluated; likely threatened by land-use changes. | <1% | Minor research focus. |
| Stenotritidae | Least studied family; endemic to Australia. | Australia | Data deficient; climate change risks. | 0% (no publications in last decade) | Urgent need for research. |
- Note: The bold emphasis on certain entries in this table is used to highlight key results or categories that are of particular importance in the context of our study.
1.3 Family Halictidae
The family Halictidae (true social bees) is the second-largest bee family after Apidae, with approximately 4500 species. Apidae, the largest family, includes around 6000 species today and originated nearly 115 to 95 million years ago during the Eocene Epoch. This family is represented by some of the oldest known fossil bees from Burma (Poinar and Danforth 2006). Similarly, Halictidae also dates back to the Early Eocene, with fossil records from the Okanagan Highlands of British Columbia, Canada (Wappler and Engel 2003). Species within Halictidae are found in diverse habitats and exhibit significant variation in appearance and behavior. They are cosmopolitan in distribution, occurring on every continent except Antarctica, and show a range of species-specific adaptations (Allahverdi et al. 2022; Buckley et al. 2016). Color differentiation in Halictidae is highly distinct and conspicuous, ranging from dark brown, black, metallic, and non-metallic hues to green, red, purple, yellow, and even blue (Buckley et al. 2016; Howard et al. 2021; Miyanaga et al. 1999). Bees in this family exhibit a variety of body dimensions, patterns, and behavioral features. Some species have characteristic yellow markings, especially on the facial region, short tongues, and arcuate (curved) basal wing veins. Halictidae are among the most abundant bee families, surpassing all but Apis species in population. The diverse evolutionary adaptations, categorizations, and eco-adapted genetic modifications in Halictidae are notable, encompassing solitary, communal, semi-social, and eusocial behaviors (Kocher et al. 2018).
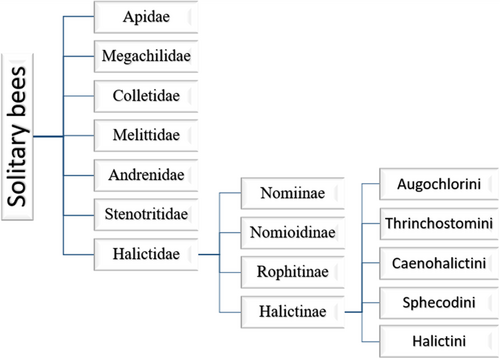
Solitary and eusocial behaviors in bees depend on factors such as time, location, altitude, forage availability, pests, diseases, and anthropogenic pressures. Generally, female bees across all families are larger and better foragers than males (Brant and Camilo 2021; Larson et al. 2022). Behavior Halictidae, commonly known as sweat bees, are small and often attracted to perspiration, which can create a nuisance (Reed and Landolt 2019). Taxonomically, the most significant subfamilies of Halictidae include Halictinae, Nomiinae, Nomioidinae, and Rophitinae. Generally, Rophitinae overwinter as pre-pupae, while Nomioidinae can have both sexes overwinter. In contrast, Halictinae and Nomiinae typically have only mated adult females overwinter at the end of summer.
In spring or mid-summer, female bees emerge and mate if they have not done so previously. After mating, females (gynes) begin excavating nests and mass provisioning cells with spherical pollen to be used by larvae until they pupate. The larvae then undergo species-specific developmental stages (Smith et al. 2022). Some species may remain in the prepupal stage for up to a year during drought conditions, while others overwinter in cells or emerge immediately as adults. Most Halictidae species are polylectic, but a few are oligolectic in their foraging behavior. Adult Halictids consume nectar and collect both pollen and nectar for their larvae.
1.4 General Characteristics of Halictidae
1.4.1 Ecology
Most Halictidae nest in the ground, particularly in clay soil at moist riverbanks. Some also nest in rotten wooden logs, beetle galleries, and deep wooden crevices (Dar et al. 2017). They predominantly use mass provisioning, where an egg is laid on top of a pollen mass, and the cell is sealed. In this method, the larva receives all its food at once, unlike “progressive provisioning,” as seen in honeybees, where the larva is fed repeatedly as it grows. To provide stiffness to the cell walls, Halictids use lignin, tannin, and other plant exudates, which are applied in a specific pattern to the inner walls and sides of the cells, creating a waterproof structure (Potts et al. 2005; Zhou et al. 2022). Additionally, some Halictid species use lactone and associated secretions to line their tunnels, which helps workers navigate back to the nest (Allasino et al. 2019). Each bee is believed to have a unique chemical signature. Except for kleptoparasites, all Halictidae species are pollen feeders and can be significant pollinators (Tsiolis et al. 2022).
1.4.2 Eusocial Species
Some eusocial bee species are crucial for crop pollination worldwide (Lemanski et al. 2022). Notable examples include the alkali bee, Lasioglossum vierecki, and Lasioglossum leucozonium (Dar and Sofi 2018). Many species in the subfamily Halictinae exhibit partial eusocial behavior, such as Lasioglossum malachurum and Halictus rubicundus, which have relatively well-defined queen and worker castes (though not as rigid as the caste system in honey bees). Social behavior in these species can vary facultatively across different lineages. Primitive eusocial species, like Lasioglossum zephyrus, lack a permanent, rigid division of labor (Crone et al. 2022; Kocher et al. 2018).
Another example of a primitive eusocial bee species is Halictus ligatus (Brant and Camilo 2021), where aggression plays a significant role in establishing hierarchy and social organization within the colony (Crone et al. 2022). Primitively eusocial species provide insights into the early evolution of eusociality. Halictus sexcinctus, which exhibits social, communal, and eusocial organization, offers clues about the evolutionary reversal of eusociality. Phylogenetic data from H. sexcinctus suggest that a communal strategy may serve as a transitional step between eusociality and a reversion to solitary nesting (Danforth et al. 1999; Richards 2001; Xu et al. 2022).
Eusociality is not a dominant feature in the family Halictidae. Most species, such as L. malachurum, exhibit eusocial behavior only in part of their life cycle, with relatively well-defined queen and worker castes, though these differ from the castes found in honey bees (Price and Field 2022).
The various behavioral traits of Halictids are influenced by a range of ancestral characteristics. In their nests, the first brood or offspring often continue to build and protect the nest, as well as gather food for new brood larvae. Halictids exhibit a wide range of social and nesting behaviors, including solitary, communal, semi-social, and primitively eusocial forms (Steitz et al. 2021), which are influenced by factors such as floral resources, location, altitude, season, and climate. Among the most common and primitive eusocial species are L. zephyrus and H. rubicundus, which lack a permanent and rigid division of labor within the colony (Gardner and Gibbs 2020). The hierarchy and social organization are more clearly established in H. ligatus, another primitive eusocial bee species from Halictidae. This species provides valuable insights into the evolution of eusociality and colony organization among solitary bees.
1.4.3 Parasitic Behavior
Sphecodes is a significant genus within Halictidae, known for its high kleptoparasitic behavior. Despite having similar size, mating, and foraging behaviors to other Halictid species (Gonçalves 2021), kleptoparasitic behavior has evolved independently at least nine times within the family, making Sphecodes one of the most effective parasitic genera targeting Lasioglossum (Dar et al. 2017). The most well-known kleptoparasitic species belong to Sphecodes, which resemble wasps with their blood-red abdomens and body lengths ranging from 4.5 to 10 mm. Female Sphecodes enter the nesting cavities of Lasioglossum, attack the provisioned mass, prey on host eggs, and lay their own eggs in the host cells (Astafurova et al. 2020; Cross 2017).
Halictidae is one of only four bee families that include crepuscular species, which are active only at dusk or early evening and are considered “vespertine” (e.g., the subgenus Sphecodogastra of Lasioglossum), or occasionally truly nocturnal (e.g., in the genus Megalopta, such as Megalopta genalis). These bees have significantly enlarged ocelli. The other three families with crepuscular species are Andrenidae, Colletidae, and Apidae (Jones et al. 2020).
1.4.4 Economic Importance
Insect pollinators are economically significant (Buxton et al. 2022). Many species from the family Halictidae are crucial pollinators, visiting a wide variety of flowering plants (Funayama et al. 2022). Among them, Lasioglossum marginatum, L. vierecki, and L. leucozonium are particularly important. Some Halictid species are oligoleges, such as Rophites algirus, which specialize in visiting hedgenettle flowers. However, most species within the genus Lasioglossum are generalists, making them valuable potential pollinators While some Halictid species, such as the oligolege, R. algirus, exhibit specialized pollination behaviors—exclusively visiting certain plants like hedgenettle—most species within the genus Lasioglossum are generalists. These generalist pollinators are of value due to their ability to pollinate a wide variety of plants, enhancing their role in sustaining ecosystems and providing essential services for agriculture and natural habitats. Their flexibility and widespread presence underscore their critical contribution to both ecological balance and economic activities reliant on pollination. Bees represent a vast and diverse group of pollinators, with over 20,000 species exhibiting a wide array of behaviors, forms, and ecological roles. From solitary bees like mason and leafcutter bees to highly social species such as honeybees and bumblebees, their diversity spans different nesting habits, feeding specializations, and interactions with plants. This incredible variation allows bees to thrive in a multitude of environments, from forests and grasslands to urban areas, making them indispensable to both natural ecosystems and agricultural systems worldwide (Layek et al. 2022).
1.5 Phylogeny and Biology of Halictidae
Bees are highly diverse in species and behavior, with major behavioral categories including solitary, eusocial, and parasitic. The Halictidae family contains an exceptionally high proportion of kleptoparasitic species. The presence of eusociality and kleptoparasitism in bees complicates the resolution of their phylogenetic relationships, particularly with the Apidae family and solitary species. The strong convergence on parasitic traits among kleptoparasitic species makes it challenging to differentiate between them based on morphological features. These distinct kleptoparasitic traits have added complexity to discussions on phylogenetic relationships and the evolutionary connections between Halictidae and Apidae. The tendency for parasitic traits has made it difficult to distinguish similarities caused by shared hereditary convergence. Fossil records relevant to Halictidae are typically found in amber from the Baltic region and the Dominican Republic (Engel 2009). These records suggest that Halictidae have existed from 96 to 75 million years ago (De Meulemeester et al. 2012). According to another study, the earliest fossil record of Halictidae dates back to the Early Eocene (56–33.9 million years ago), with many species recovered from amber deposits, such as Neocorynura electra and Augochlora leptoloba (Lepeco and Gonçalves 2022).
Since the family Halictidae is broadly divided into four subfamilies, morphological analysis and molecular data suggest that the subfamily Rophitinae is closely related to the sister groups Nomiinae, Nomioidinae, and Halictinae (Figure 4). Generally, most species overwinter as pupae. In some species of the subfamily Rophitinae, only females overwinter, while in the subfamily Nomioidinae, both sexes overwinter. In Halictinae and Nomiinae, mated females overwinter (Smith et al. 2003). Most mated females emerge in the spring, though some emerge in the summer. They begin by digging nests and later provision the cells with a mixture of pollen and nectar. Each cell contains a single egg, and after the larva hatches, it begins to consume the pollen provision until it is depleted. Larval defecation depends on factors such as food availability, moisture content, species, season, and other 32 pupae may or may not enter a prepupal period the following year, which is dependent on the species. In some species, the pupae are thought to remain dormant for the current year or longer due to adverse conditions. Some prepupae emerge as adults in the same year, while in other cases, adults may remain in their cells over the winter (Michener 2007a; Wcislo and Engel 1996).
Phylogenetic studies conducted worldwide reveal that Rophitinae make larval cells in a slanting to horizontal fashion along the sides of their tunnels, while Nomiinae construct cells in a vertical, clustered pattern. Nomioidinae, on the other hand, build sub-horizontal cells off the vertical burrow. Halictinae create a series of cells that can be either scattered or clustered. In the clustered arrangement, most cells are typically lined with a waxy material released from the Dufour's glands located in the abdomen. Nests built in rotten wood are irregular in terms of external entrance, diameter, and cell structure and arrangement. Due to the nature of the wood, fine cuts are not made, resulting in rough-walled cells (Patiny et al. 2008).
Parasitic bee species neither construct their own nest cavities nor gather floral resources for provisioning; instead, they rely on other soil-dwelling non-parasitic bee species for shelter and food. The genus Sphecodes is of Palearctic origin, with species like Microsphecodes and several small-sized Lasioglossum being among the most prevalent parasitic bees in the family Halictidae. Sweat bee populations are dominant pollinators for many crops, and their numbers can be increased by planting a variety of flowering plants, especially vegetables, field crops, and fruit crops. Generally, sunny locations are preferred for nest building and foraging by all species. However, excessive tillage, pesticide application, construction, overgrazing, and soil burning can lead to total population loss through habitat degradation and a deficit of forage crops (Sless et al. 2022).
1.6 Differences in Subfamilies
Mature larvae and pupae of the multivoltine Nomiodes patruelis Cockerell and its kleptoparasite Chiasmognathus pashupati Engel have been reported from Pakistan. The mature larvae of the Halictidae subfamilies Rophitinae, Nomiinae, and Halictinae share most diagnostic traits, differing mainly in mandibular morphology and body size. However, Pesenko (2000) found that mature larvae are identical in all aspects. The kleptoparasite C. pashupati is multivoltine, and its pupae differ morphologically from those of Ammobatini. Mature larvae of C. pashupati exhibit traits similar to those of Nomadinae in Ammobatini. The mature oocytes of Chiasmognathus orientanus (Warncke) are also similar to those of C. pashupati (Rozen and Özbek 2003).
1.6.1 Nomiinae
The Nomiinae are a subfamily of the Halictidae family, comprising 11 genera and 550 described species (Seltmann and Poelen 2024; Zhang et al. 2022, 2020). Recently, two new species from the genus Lipotriches were discovered in China (Zhang et al. 2022). A distinctive feature of the Nomiinae is the presence of three submarginal cells on the forewings, with the first and third cells being nearly equal in length, while the second cell is comparatively shorter. Additionally, their antennae are located near the middle of the eyes, with an episternal groove near the scrobe, and a small depression below the scrobal groove. Male legs are highly modified to aid in mating (Pesenko 2000).
Like other ground-nesting hymenopteran insects, Nomiinae build subterranean nests that feature turrets and a main shaft reaching depths of 60.5–69.33 cm. These nests contain oblique to horizontal cells, numbering 13–21 per nest, arranged in cluster patterns at various depths (Cosarinsky and Roces 2012; Vijayakumar et al. 2022). The main tunnel diameter ranges from 0.35–0.46 cm. Generally, the diameter ranges from 0.45–0.55 cm at depths greater than 12.7 cm (5 in.) and 0.33–0.36 cm at depths less than 12.7 cm (5 in.). The diameter of primary, secondary, and tertiary cells decreases further after a depth of 29.21–36.84 cm (11.5–14.5 in.) from the surface, similar to what is observed in termites (Astafurova 2008; Chiu et al. 2015; Johansen et al. 1978). However, the depth at which branching and cell clusters originate varies by species and can extend up to 70 cm (27.55 in.) from the surface (Njoya et al. 2019; Udayakumar and Shivalingaswamy 2018). Bees lay their eggs in the terminal portion of cells, seal them, and then move on to the next empty cell. On average, species complete their life cycles in 36–41 days. It has been observed that species within the subfamily Nomiinae complete their egg, larval, and pupal stages in approximately 5–7, 20–21, and 11–13 days, respectively (Derstine et al. 2023, 2021; Udayakumar and Shivalingaswamy 2018). The larvae are curved (in the pre-defecating stage, when feeding on pollen provisions) and range in color from whitish to creamy. Fully grown, defecating larvae (without a pollen mass) are also curved, yellowish to whitish, and defecate within the cells. Larvae have eyes that vary in color from whitish to brown to dark brown. No pupal diapause has been found in any Nomiinae species (Du et al. 2022; Karp 2021; Zhang et al. 2022). Female bees (typically two to five individuals per nest entrance) exhibit the behavior of blocking the nest entrance after laying eggs, which may help prevent pest and disease attacks on the brood mass. There is little to no brood parasitism or predation observed (Danforth et al. 2008; Pauly 2014). Mature females typically collect pollen, making 10–12 foraging trips per nest per day. Some species of Nomiinae, such as Nomia tetrazonata and Hoplonomia westwoodi, exhibit communal nesting behavior, with three to five bees sharing the same nest tunnel (Derstine et al. 2023, 2021). The mean nest entrance diameter and turret height were recorded as 0.45 cm and 1.09 cm, respectively. Generally, nest tunnels branch within the soil, with females accessing the main tunnel from multiple entry points. This suggests that a network of exit entrances originates from the same nest tunnel. A greater number of exit entrances and increased branching are indirect indicators of higher bee and nest density, as they provide more cell clusters for oviposition. The deeper placement of cells and brood within the soil is an adaptation in solitary bees to protect eggs, larvae, and pupae (the next generation) from pests (kleptoparasites) and abiotic hazards such as rainfall, floods, fire, and erosion (Derstine et al. 2023, 2021). It has been observed that the cells are oval in shape, with side walls coated by glandular secretions to protect the eggs from moisture and kleptoparasites. Cell length and width vary depending on several soil parameters and the bee species, generally ranging from 9.45–12.58 mm in length and 4.35–4.40 mm in width (Udayakumar and Shivalingaswamy 2018).
Once oviposition is completed, the cells are covered by a thin layer of soil. Provisioning is a common behavior in Halictidae bees, and the provision mass of the pollen ball varies among species (Field 1996).
In the subfamily Nomiinae, the pollen ball's mass, diameter, length, and height (width) vary from 3.5–5.9 mg, 4.4–4.6 mm, 2.25–2.30 mm, and 2.31–2.45 mm, respectively. A single egg is deposited in each cell, placed on the pollen mass either at the side or on top. The pollen mass is not completely dry, containing a moisture content of 5%–10%, which is attributed to the bee's secretion. The pollen mass supports various life stages concealed within the cells, including the egg, non-defecating and defecating larvae, prepupa, and pupa (Bernauer et al. 2022; Brown et al. 2020). Nomiinae bees have a diverse foraging range and visit plant families such as Acanthaceae, Asteraceae, Convolvulaceae, Fabaceae, Lamiaceae, Malpighiaceae, Polygonaceae, Rubiaceae, and Solanaceae (Salatnaya et al. 2021). The Nomiinae population peaks from June to November. While foraging on solanaceous crops like brinjal and tomato, particularly Hoplonomia westwoodi, the species demonstrated high levels of buzz pollination. Foraging characteristics varied between species (Murao and Gibbs 2019).
1.6.2 Nomioidinae
Members of the subfamily Nomioidinae have a cosmopolitan distribution and include both ground- and wood-nesting bees. They exhibit a range of behaviors from solitary to eusocial (Pesenko and Pauly 2005). These bees engage in mass provisioning with pollen and nectar. Oviposition is done singly in cells, with most eggs laid in terminal cells, which are later sealed with a thin film of soil. The subfamily has a diverse foraging range and comprises two subgenera: Ceylalictus and Nomioides (Rust et al. 2004). Nomioidinae consists of small-bodied bees, ranging from 2.9 to 3.51 mm in length, with bright coloration, particularly metallic green to blue. These bees exhibit pale integument markings. The classification of Nomioidinae is often misunderstood, and they are sometimes placed within the tribe Halictidae. However, based on their distinct morphological characteristics, they warrant a clear subfamily status (Pesenko and Pauly 2005; Rozen and Özbek 2003). This subfamily shows communal associations among nest-mates, with long vertical burrows that have side-clustered horizontal cells. The nests are typically grouped in clusters of 4–6, spaced at least 14–18 cm apart, with circular openings averaging 2.12–2.33 mm in diameter, lacking conspicuous tumuli and turrets (Pauly 2014). Normally, the nest mound is eroded by rainfall and wind. Each female constructs her own separate nest cavity and occasionally uses older galleries. The nest cavity is vertical in shape, sealed with a thin film of soil, and constructed at various sites with different soil textures and slopes. In many cases, the nest tunnel is obstructed by pebbles, rocks, roots, and sandy soils, causing the tunnel to deviate from being perfectly vertical. The diameter of the tunnel varies from the exposed external opening to deeper inside, ranging from 3.11 to 2.08 mm. This suggests that the diameter near the surface is larger than the diameter at greater depths (Pesenko 1977).
The main tunnel descends 15–20 cm and ends in a blind, short passage, a classic trait of Halictidae bees. Unlike most Halictidae bees, the main passage, branches, and terminal branches of Nomioidinae have rough, uneven, irregular, and unlined side walls (Eickwort 1978; Radchenko 1980). Horizontal branches (85%) and oblique branches (15%), each approximately 6 to 10 cm in length, arise 10–18 cm below the main tunnel's depth. The side passages and lateral branches further bifurcate into horizontal and oblique cells, which are mostly arranged in clusters. Side passages range from 2.25 to 6.7 cm in length and 1.7 to 1.98 mm in diameter, featuring rough walls and irregular diameters (Sakagami 1962). It was found that side passages are created in a sequential sliding and descending pattern from the original tunnel. However, in many cases, the passages are not made directly downwards but are excavated randomly, leading to the conclusion that side branches are mostly oblique (Antoine and Forrest 2021; Dos Santos et al. 2020; Riaño-Jiménez et al. 2023; Udayakumar and Shivalingaswamy 2018). Secondary and tertiary tunnels arise from the main tunnel, and these are shorter and narrower, leading to specific cells off lateral passages. Brood cells are typically ellipsoidal, polished by the abdomen, and coated with oral secretions. These cells have an average length of 4.01–4.73 mm. Clustered cells can be either horizontal or vertical, with diameters ranging from 2.22–2.56 mm in horizontal cells and 1.90–2.10 mm in vertical cells. The diameter of the entrance at the soil surface varies from 1.90–2.09 mm (Antoine and Forrest 2021; Dos Santos et al. 2020; Houston 2020; Riaño-Jiménez et al. 2023; Udayakumar and Shivalingaswamy 2018).
In soil-dwelling bees, each cell is filled with food once constructed, and a single female typically constructs an average of 15–24 cells over her lifetime. We observed that side passages are often filled with soil, making it difficult to determine which main tunnel a particular cell belongs to. Additionally, the distance between nest entrances on the soil surface is often less than the combined length of the primary and secondary passages. For unknown reasons, larval mortality in cells from the previous year was notably higher, especially if the cell depths were greater than the surface. During the first 7–10 days of nest construction, the number and dimensions of cells were observed to decrease as larval stages progressed (Antoine and Forrest 2021; Dos Santos et al. 2020; Houston 2020; Riaño-Jiménez et al. 2023; Udayakumar and Shivalingaswamy 2018) underside of the abdomen. Due to their comparatively short life cycle and busy schedules, bees often do not manage to construct many cells for oviposition. In such cases, they choose the lowest cells from abandoned nests of the previous year and lay their eggs there. Food storage is a crucial adaptation and essential for the survival of the brood during periods of scarcity. Bees collect pollen and nectar from a variety of plants and store them in pellet form in the cells, starting from the upper cells and moving to the lower ones (Burkle and Irwin 2009; Cane and Love 2021).
The moistened pollen pellet is typically spherical or round but often flattened at the ends. It is positioned at the floor of the cell, usually at one end, with a single egg laid on top. The dimensions of the pellet vary, generally ranging from 2.09 to 2.30 mm in horizontal diameter and 1.87 to 1.96 mm in vertical diameter. The emerging larvae are initially white to milky in color and feed on the nectar-moistened pollen pellets. As they complete feeding, they turn black due to the dark excrements they produce (Burkle and Irwin 2009; Cane and Love 2021; Filipiak 2019). The larvae initially appear milky in color, but this changes after defecation. Fecal pellets are deposited at the end of the cells. The pupae position their heads near the entrance of the cells and later transform into adults, with the entire process taking 35–39 days. In temperate climates, the species produces only one generation per year. Overwintering stages are imagoes, which hibernate in earthen cells and are often subjected to fungal infections that contribute to high larval mortality through mummification. Additionally, larvae tend to die when soil humidity exceeds 25% and moisture content surpasses 13%. Nests are typically constructed in soils with a first layer of vegetation and sandy loam. Higher humidity and excess moisture in the cells—particularly in the lower cells—result in increased larval death (Burkle and Irwin 2009).
1.6.3 Rophitinae
Rophitinae is a subfamily of sweat bees comprising two tribes, 13 genera, and 260 described species with a cosmopolitan distribution. The Rophitinae are found in the Holarctic and Afrotropical regions, with five species in these areas. Additionally, a few species are limited to the South American region, and nine species are found in Russia (Patiny et al. 2008).
Nests are typically excavated in sloping landscapes, with larval cells positioned either at an angle or horizontally off from the main and lateral tunnels. Generally, the main tunnel is occupied by a single female. The dimensions of the tumuli vary, with lengths ranging from 2.89 to 3.12 cm, widths from 1.98 to 2.10 cm, and diameters from 1.98 to 2.40 mm. Excavated soil is heaped on the downhill side of the main entrance. The main tunnel has a diameter of 1.98 to 2.24 mm, is horizontal to oblique, and descends 32 to 39 cm below the soil surface. Typically, the lateral tunnels have a smaller diameter compared to the main tunnel, ranging from 1.97 to 2.21 mm. However, in some species, both the main tunnel and the lateral tunnels have the same diameter. The laterals, which are 12 to 27 mm in length, end with a linear series of cells starting at a depth of 18 to 25 cm from the entrance. Each nest contains 17 to 25 cells, with new cells constructed at even greater depths. The cells are generally round, with lengths ranging from 3.76 to 4.41 mm and diameters from 2.79 to 3.20 mm. They are usually arranged end-to-end, with minimal variation in size and spacing among individual cells (Dar et al. 2023; Genise 2017; Yang et al. 2022).
Cells may occur either in clusters or singly. They are hard, non-reflective, and have poorly defined walls, coated with an invisible lining of glued-together sand grains, making them semi-waterproof compared to the surrounding substrate. Female solitary bees have modifications on the tibia, the fourth segment of the leg located between the femur and tarsus, known as the scopa (Abdelmegeed 2016). The scopa consists of various modifications on the bodies of non-parasitic bees, forming an apparatus for carrying pollen. It often appears as a dense mass of elongated, branched, hair-like structures originating from the hind leg. Occasionally, it is present on the hind legs as modified hairs on the tibia (Proctor et al. 1996).
In bees, the tibia is covered with spines and hairs that help pollen grains adhere. In honey bees and a few other species, these spines and hairs form a structure known as the corbicula. The pollen mass collected from various flowers and transported to the cells is sticky and forms small spherical pollen-nectar masses with diameters ranging from 1.35 to 1.56 mm. In species such as Calliopsinio of the genus Panurgus, additional pollen mass is added to an already prepared pollen ball, resulting in larger mealy-moist masses with diameters ranging from 2.24 to 2.53 mm. The pollen ball is coated with a thin, non-reflective, partially transparent, waterproof layer similar to the shiny food coatings of Calliopsini. It rests in the posterior area of the cell floor, free from any mechanical attachment except for a loose connection to the interior cell wall through glandular secretions (Khurelchuluun et al. 2021; Klinger et al. 2021; Kueneman et al. 2023).
In Raphitinae, the egg is occasionally positioned at the top of the nectar-pollen spherical ball, with both sides attached to the pollen mass (resulting in two attachments) (Burgett et al. 2005). The egg is creamy to white in color, curved, clear to semi-transparent, and smooth with a shiny chorion. Its length ranges from 1.25 to 1.34 mm, and its diameter ranges from 0.29 to 0.35 mm. Late embryonic reorientations are observed in Raphitinae, a feature common to most bees. The first instar larvae attach to the pollen mass from the inside near the hatching period (Kocher et al. 2018).
1.6.4 Halictinae Nesting, Provisioning, and Development
The Halictinae subfamily is the most diverse, largest, and most recently diverged group within the family Halictidae Table 2 (Allahverdi et al. 2022). It encompasses around 2400 bee species across five tribes: Augochlorini, Thrinchostommini, Caenohalictini, Sphecodini, and Halictini. However, some entomologists have classified them into just two tribes: Augochlorini and Halictini. Halictinae belong to the monophyletic clade Aculeata (Brothers 2021; Buckley et al. 2016), which is characterized by a needle-like ovipositor that injects venom into predators and prey as a defense mechanism. The Halictinae include both eusocial and kleptoparasitic taxa, feed on pollen, and exhibit mass provisioning of their young. Bees within this subfamily display a wide range of behavioral social polymorphism, from solitary nesting to obligate eusociality. Fossil records indicate that eusociality in Halictinae originated relatively recently, about 20 to 22 million years ago, during the early Eocene period (Bernadou et al. 2021). Notable species such as Neocorynura electra (Engel 2009) and Augochlora leptoloba (Lepeco and Gonçalves 2022) have been found in amber deposits.
| Topic | Details | References |
|---|---|---|
| Diversity and classification of Halictinae | Halictinae is the most diverse and largest subfamily within Halictidae, comprising around 2400 species across five tribes (Augochlorini, Thrinchostommini, Caenohalictini, Sphecodini, and Halictini). Some entomologists classify them into only two tribes: Augochlorini and Halictini. | (Allahverdi et al. 2022) |
| Monophyletic clade | Halictinae belong to the monophyletic clade Aculeata, characterized by a needle-like ovipositor that injects venom as a defense mechanism. | (Buckley et al. 2016; Brothers 2021) |
| Social behavior | Halictinae includes eusocial and kleptoparasitic taxa, with a wide range of social behaviors from solitary nesting to obligate eusociality. | (Bernadou et al. 2021) |
| Fossil records | Eusociality in Halictinae originated 20–22 million years ago during the early Eocene period, with species like Neocorynura electra and Augochlora leptoloba found in amber deposits. | (Lepeco and Gonçalves 2022) |
| Nest structures behavior | Halictinae nests typically consist of a series of cells, either scattered or clustered, with a waxy lining secreted by the Dufour's gland. | (Derstine et al. 2021, 2023; Kingwell et al. 2021; Orlova et al. 2022) |
| Wood-nesting behavior | Some Halictids nest in wood, where irregular cell arrangements are due to the wood's nature and lignin content, hindering excavation. | (Morato and Martins 2006) |
| Pollen feeding and provisioning | All species feed on pollen, and larvae are fed through mass provisioning. Halictidae larvae receive food all at once, unlike honey bees, which exhibit progressive provisioning. | (Cane 2004; Michener 2007a; Cane 2016; Doyle et al. 2020) |
| Kleptoparasitism | Some Halictid species, like those in the subgenus Sphecodogastra of Lasioglossum, are kleptoparasitic and nocturnal, with large ocelli. | (Dorey et al. 2020) |
| Soil-dwelling behavior | Halictinae typically create soil nests with circular to semicircular entrances. The main burrow descends at an angle and bifurcates into side branches. | (Dar et al. 2023) |
| Nest depth and structure | Nests were found 16–30 cm below the surface, ending in short, blind burrows. Cells were radially distributed along the main and branch burrows, with no lateral extensions. | (Udayakumar and Shivalingaswamy 2018; Dos Santos et al. 2020; Antoine and Forrest 2021; Riaño-Jiménez et al. 2023) |
| Cell construction | Cells were horizontal, bilaterally symmetrical, and sealed with loose soil after oviposition. Pollen balls were firm, slightly hemispherical, and moistened with nectar. | (Cane 2004, 2016; Michener 2007b; Doyle et al. 2020) |
| Larval distribution | The number of larvae per nest varied, with no fixed proportionality between the number of nests, cells, and larvae. Larvae curl onto pollen balls moistened with nectar during feeding. | (Udayakumar and Shivalingaswamy 2018; Dos Santos et al. 2020; Antoine and Forrest 2021; Dharampal et al. 2022; Kueneman et al. 2023; Riaño-Jiménez et al. 2023) |
| Brood development | The brood developmental gradient was generally progressive, with younger stages deeper in the nest. Each nest contained 1–6 females, with no direct correlation between the number of females and cells. | (Vogt 2013) |
| Caste differentiation | Castes in Halictus scabiosae were ill-defined, with pollen collectors having varying ages and similar ovarian group sizes. | (Aamidor et al. 2022; Wendzonka et al. 2022) |
| Ovary size and age | Bees with narrower ovaries were generally younger. Differences in social organization suggest the presence of sibling species. The older nest-founding female guards the nest while daughter's forage. | (Farinosus 2012) |
| Associated organisms | Mites, gregarine protozoa, nematodes, and pupal conopids were associated with Halictus scabiosae. | (Eini et al. 2022) |
| Kleptoparasitic avoidance | H. scabiosae occasionally visited nesting areas, but kleptoparasitic Sphecodes species rarely entered their nests. | (Dar et al. 2017) |
This evidence suggests that Halictinae are model primitive insects within Hymenoptera with advanced eusocial behavior, making them valuable models for studying the social evolution of arthropods. Halictinae exhibit diverse nest structures, typically consisting of a series of cells that may be either scattered or clustered. Regardless of cell pattern or arrangement, a common feature is the waxy lining on the walls of the nest tunnel, secreted from the Dufour's gland, located on the underside of the abdomen (Derstine et al. 2023, 2021; Kingwell et al. 2021; Orlova et al. 2022).
Some Halictids nest in wood, where the cell arrangement is usually irregular due to the nature of the wood and its lignin content, which hinders excavation. All species are pollen feeders, and their immatures are fed through mass provisioning, where pollen and nectar are mixed and sealed inside a waterproof cell. The bees lay an egg on the pollen ball and then seal it off. Unlike honey bees, which exhibit advanced progressive provisioning, Halictidae larvae are provided with food all at once. A few Halictid species are kleptoparasites, robbing food and eggs from nests and laying their own eggs on the stolen provisions. For example, species from the subgenus Sphecodogastra of the genus Lasioglossum are considered vespertine or nocturnal, such as Megaloptagenalis, which possesses large ocelli. Halictinae are typically soil-dwelling bees, with circular to semicircular nest entrances located centrally within a loose tumulus. However, in most cases, the tumulus is washed away by rain or wind. The average diameter of the nest entrance (4.6–11.23 mm) is smaller than that of the main burrow (10.23–12.34 mm) (Dar et al. 2023). The main burrow descends at an angle slightly less than 90°, often appearing tortuous and bifurcating into three to seven inclined side branches. The first branches arise at a depth of 7.45–7.89 cm from the surface (Reed and Landolt 2019).
Frequently, the diameters of the side branch burrows were the same as that of the main burrow; however, in most cases, the diameter of the side branches was smaller than that of the main burrow. All the burrows were lined with smooth soil. Nests were found at depths ranging from 16 to 30 cm below the surface, with a mean depth of 21.5 cm across six nests. The nests typically ended in short, blind burrows beneath the lowest cells (Antoine and Forrest 2021; Dos Santos et al. 2020; Riaño-Jiménez et al. 2023; Udayakumar and Shivalingaswamy 2018).
The cells were radially distributed along the main burrow and its branches, with no lateral extensions. The cells were located 7 to 25 cm below the soil surface and contained between 6 and 45 cells. Each cell was roughly horizontal, bilaterally symmetrical, and measured 17–23 mm in length, 8–11 mm in width, and 5–7 mm in diameter (including plugs). After oviposition, the cells were sealed with approximately 5 mm of loose soil (Buckley et al. 2016; Michener 2007a).
Each nest contained between 1 and 39 cells from which bees had emerged. These cells were either empty or partially or entirely filled with soil (Antoine and Forrest 2021; Dos Santos et al. 2020; Riaño-Jiménez et al. 2023; Udayakumar and Shivalingaswamy 2018). The upper distal walls of the cells were plastered with fecal material. Pollen balls, slightly hemispherical in shape, measured 8 mm in length, 6.8 mm in width, and 4.2 mm in height, with a wide groove on top where the arched egg (3.2 × 0.75 mm) was located. These orange pollen balls were sealed in circular, semicircular, oblique, ovoid, or rectangular cells. The pollen balls were firm yet crumbly, with a glazed and shiny surface moistened with nectar (Cane 2016, 2004; Doyle et al. 2020; Michener 2007a). On average, in Halictidae, the number of larvae per nest varies. For instance, one nest contained two small larvae, three nests contained a total of seven medium-sized larvae, and two nests contained a total of four large larvae, indicating that there is no fixed proportionality between the number of nests, cells, and larvae (Antoine and Forrest 2021; Dos Santos et al. 2020; Riaño-Jiménez et al. 2023; Udayakumar and Shivalingaswamy 2018). The newly hatched larvae curl into a semicircular shape on the pollen ball, which is moistened with a drop of nectar during feeding. The larvae cling tightly to the pollen ball when they sense any physical disturbance (Dharampal et al. 2022; Kueneman et al. 2023). Although there were several anomalies, the brood developmental gradient generally appeared progressive, with the youngest stages located deeper in the nest. For example, each of the seven nests contained one to six females. Since the number of adult females varies as offspring are born and die, it is likely that there is no direct correlation between the number of females per nest (sex ratio) and the total number of cells.
Castes in the Geneva population of Halictus scabiosae were ill-defined, particularly in terms of workers, eggs, ovaries, and mated individuals. For instance, two of the eight pollen gatherers had fully developed ovaries, and seven of the eight were inseminated (likely laying eggs as well as foraging). The pollen collectors appeared to range in age; however, the size of various ovarian groups was identical (Aamidor et al. 2022; Wendzonka et al. 2022). Generally, bees with narrower ovaries tend to be younger (less worn) than those with larger ovaries, some of which are almost egg layers. These may have been young bees created earlier in the summer. Females that were the least worn (youngest) had not yet been sexed. It is likely that in the population, differences in social organization may suggest the presence of sibling species. The older nest-founding female typically remains in the nest, guarding its entrance while the daughters forage. This is consistent with findings from a study of a Halictidae population in France. Among the organisms associated with H. scabiosae were mites attached to female bees on pollen balls, similar to gregarine protozoa found in the mid-gut of honey bee females, as well as nematodes and pupal conopids (wasps) (Eini et al. 2022).
H. scabiosae occasionally visited the nesting area; however, the kleptoparasitic species from the genus Sphecodes rarely, if ever, entered their nests. The adult females were radially distributed along the “branching burrows,” where cells containing brood of varying ages were present. Each nest contained one to six females, with no clear differentiation into worker and queen castes. The majority of Geneva's pollinators were inseminated, and some may have even laid eggs (Dar et al. 2017).
1.7 Halictidae Tribes
The genus Lasioglossum (formerly Dialictus) within the family Halictidae is one of the world's largest bee genera, comprising around 1700 species across various subgenera. These species exhibit an incredibly diverse range of behaviors (Gibbs et al. 2012; Zhang et al. 2022). They vary widely in size, color, and markings. Some species are kleptoparasites, others are nocturnal, and some are oligolectic (Lim et al. 2022; Yu et al. 2022). Except for parasitic species such as Sphecodes, female halictids carry pollen on the tibia and femur of their hind legs. Males are often slenderer than females of the same species, lack scopa, and sometimes have a yellow clypeus (the sclerite located below the antennae on the bee's face). Most species lay their eggs in the ground, though some lay eggs in rotting wood (Gibbs 2011; Gibbs et al. 2013).
Lasioglossum species exhibit a wide range of social behaviors, including solitary nesting, primitive eusociality, and social parasitism. Colony sizes vary greatly across populations, ranging from those with only one worker to colonies with a single queen and up to 16 workers. Some species have as many as 400 workers, with permanent life cycles (Danforth et al. 2008).
The distal veins of the forewings serve as a distinguishing characteristic and are used to differentiate the genus Lasioglossum. Although some species, such as Lasioglossum aegyptiellum, are eusocial and exhibit clear evidence of division of labor, the genus Lasioglossum (often referred to as “strong-veined Lasioglossum”) is primarily composed of solitary and communal species. In contrast, species from the genus Hemi-halictus (also known as “weak-veined Lasioglossum”) exhibit a variety of social structures, including solitary, communal, kleptoparasitic, eusocial, and social parasitic behaviors (Zhang et al. 2022).
Eusocial species can form enormous colonies with hundreds of workers or smaller colonies with just one or a few. Large colony sizes are common in L. marginatum, the only Halictine bee species with perennial colonies that survive for 5 or 6 years and can have hundreds of workers. Mature larvae have a grub-like appearance, lack setae, and are usually less than 15 mm long. Pupae are extremely delicate and due to their brief developmental period, are rarely encountered. A strongly curved basal vein in the wing distinguishes this bee family from others; further distinctions are made based on weak or reduced wing veins (Dar et al. 2023).
1.7.1 Augochlorini
The tribe Augochlorini comprises roughly 646 species, primarily found in the New World Neotropics and some regions of North America. Although not well understood, sociality in Augochlorini is significantly polymorphic across its range, varying between and within species and genera. Kleptoparasitism has independently developed in three augochlorine genera and subgenera, including Temnosoma, Megalopta (Noctoraptor), and Megommation (Cleptommation) (Lepeco and Gonçalves 2022). The evolutionary complexity of the Augochlorini tribe underscores its critical ecological role within pollinator networks. The polymorphism in sociality, ranging from solitary to highly eusocial structures, reflects the tribe's adaptability to diverse environmental pressures and habitats. Moreover, the independent development of kleptoparasitism across multiple genera points to a sophisticated level of ecological interaction, where species not only compete for resources but also exploit the nesting efforts of others. This behavioral diversity suggests that Augochlorini bees have evolved intricate survival strategies that enhance their ability to occupy varied ecological niches, making them essential contributors to both natural ecosystems and the stability of plant-pollinator relationships. As our understanding of their biology and sociality deepens, Augochlorini could provide valuable insights into the evolution of social behavior and parasitism in bees, offering potential implications for the conservation of pollinators globally.
Another fascinating adaptation within the Augochlorini tribe is the independent evolution of kleptoparasitism in at least three genera or subgenera: Temnosoma, Megalopta (Noctoraptor), and Megommation (Cleptommation). Kleptoparasitic species do not construct their own nests but instead invade the nests of other bees, laying their eggs within the host's provisions. This form of parasitism suggests a high degree of ecological specialization, as these bees must closely synchronize their life cycles with their hosts to successfully exploit their resources. The evolution of kleptoparasitism within multiple lineages of Augochlorini highlights the complex survival strategies that have emerged within this tribe, reinforcing their ecological importance and adaptability.
Augochlorini bees play a crucial role in pollination networks, contributing significantly to the reproduction of both wild plants and agricultural crops. Their frequent floral visits make them efficient pollinators, essential for maintaining ecosystem biodiversity and stability. However, despite their ecological significance, these bees face several threats, including habitat destruction, climate change, pesticide exposure, and declining genetic diversity. Understanding their social evolution, ecological interactions, and survival strategies can provide valuable insights into the broader field of pollinator conservation, ensuring that their vital role in ecosystem sustainability is preserved. Further research into Augochlorini could shed light on the mechanisms driving eusociality, kleptoparasitism, and pollinator resilience, offering crucial implications for biodiversity conservation and the future of pollination services.
1.7.2 Thrinchostomini
The tribe Thrinchostomini is a small but ecologically significant group of Halictid bees, comprising only two genera: Thrinchostoma and Parathrinchostoma. These bees are distinct from many other halictids due to their large, non-metallic bodies, which contrast with the often iridescent appearance of their relatives. They are primarily distributed across Madagascar, Africa, and Asia, with Madagascar being home to 12 endemic species of Thrinchostoma, some of which exhibit host-plant specificity (Danforth et al. 2008). This suggests that they may have evolved specialized relationships with certain plant species, playing a crucial role in the pollination of local flora. Despite their potential ecological importance, the biology of these bees remains poorly understood, particularly their social behavior, nesting preferences, and foraging strategies (De Meulemeester et al. 2012).
Both Thrinchostoma and Parathrinchostoma are believed to be solitary bees, meaning they do not form colonies like some of their halictid relatives. However, nesting behavior has only been documented for a few species. Some Thrinchostoma species construct nests in hollow plant stems, while others are thought to excavate burrows in soil or rotting wood. Their foraging behavior suggests a preference for certain floral resources, further reinforcing the idea of specialized plant-pollinator interactions in some species. The genus Parathrinchostoma is particularly intriguing, as it includes two known kleptoparasitic species, identified based on female morphology (De Meulemeester et al. 2012). These kleptoparasites do not build their own nests but instead lay their eggs in the nests of other bees, relying on the host's food provisions to sustain their offspring. This parasitic behavior highlights a fascinating evolutionary adaptation within the tribe and suggests that competition for nesting sites and floral resources may have driven the emergence of alternative reproductive strategies.
Despite their low species diversity, Thrinchostomini bees may play an important role in maintaining regional pollination networks, particularly in tropical and subtropical ecosystems where they are found. Their niche specialization and potential vulnerability to habitat changes make them a valuable subject for further research in bee conservation and pollination ecology. Given the ongoing threats of habitat loss, deforestation, and climate change, understanding the behavior, reproductive biology, and ecological interactions of these bees is crucial to ensuring their preservation and the continued stability of the ecosystems they support. Expanding research on Thrinchostomini could also shed light on broader evolutionary trends within Halictidae, particularly regarding the development of sociality, nesting behavior, and kleptoparasitism in bees.
1.7.3 Caenohalictini
Caenohalictini is a tribe of Halictid bees found exclusively in the New World, primarily distributed across North, Central, and South America. These bees closely resemble members of the Augochlorini tribe in both morphology and behavior. They exhibit a diverse range of nesting strategies, including both solitary and communal lifestyles, with some species forming small social groups within shared nests.(Gonzalez-Vaquero and Roig-Alsina 2019) Caenohalictini species construct their nests in a variety of substrates, typically choosing soil or rotting wood as nesting sites. Their nesting habits range from strictly solitary, where each female independently provisions and maintains her own brood cells, to communal living, where multiple females share a nest entrance but care for their offspring separately. Some species exhibit primitive eusocial behavior, with division of labor among nestmates, although this is less developed compared to highly eusocial bees like honeybees (Apis spp.) A notable feature of some Caenohalictini species is their adaptation to nocturnal activity, a relatively uncommon trait among bees. These nocturnal bees are often active during twilight or at night, possibly as an adaptation to avoid daytime predators, reduce competition, or exploit floral resources that are more abundant or accessible during cooler nighttime temperatures. Their visual systems are thought to be adapted to low-light conditions, enabling them to navigate and forage efficiently in dim environments (Gonzalez-Vaquero and Roig-Alsina 2019). As important pollinators, Caenohalictini species contribute significantly to the reproduction of various flowering plants, including both wild flora and cultivated crops. Their presence in diverse ecosystems, from forests to arid regions, suggests their ecological plasticity and ability to adapt to different environmental conditions. Their interaction with native plant species is crucial for maintaining pollination networks, making them key players in ecosystem stability and biodiversity conservation. Like many other bee groups, Caenohalictini faces threats from habitat loss, pesticide exposure, and climate change. The destruction of nesting habitats due to agricultural expansion and deforestation poses a major risk to their populations. Additionally, the increasing use of chemical pesticides can impact their foraging success and reproductive viability. Conservation efforts aimed at preserving native plant diversity and reducing chemical exposure in agroecosystems could help sustain their populations.
1.7.4 Sphecodini
The species from this genus are the most abundant and diverse, parasitizing many species from the genus Lasioglossum (Pereira et al. 2021). The tribe contains four kleptoparasitic bee genera that lay their eggs on or near their hosts' pollen stores in the cells (Gonçalves 2021). These bees are host generalists and belong to an ancient parasitic lineage, sharing no specificity with any non-parasitic Halictine taxa. As aggressive parasites, they often attack solitary bees and nests of social bees before ovipositing eggs in the cells. These parasites are distributed worldwide, except in Australia, and are often considered members of the Halictini tribe (Gonçalves and Pereira 2022). Sphecodini species are widely distributed across temperate and tropical regions but are absent from Australia. Their adaptability to different environments allows them to thrive in diverse ecosystems, from forests to grasslands and even urbanized areas (Portman et al. 2018). Although they are sometimes considered members of the Halictini tribe, molecular studies suggest they represent a distinct evolutionary lineage, with their parasitic behavior emerging independently from other kleptoparasitic (Danforth et al. 2019).
1.7.5 Halictini
Halictini is the largest tribe of Halictid bees, comprising over 2400 described species (Schwarz et al. 2007), which represents nearly 53.3% of the approximately 4500 described species in the family Halictidae. Halictini are widely distributed across various landscapes and exhibit significant behavioral diversity. The most important genera include Lasioglossum, Mexalictus, and Patellapis. The genus Lasioglossum is particularly diverse, with species exhibiting a range of behaviors, including nocturnal, diurnal, socially parasitic, solitary, eusocial, and communal activities. Lasioglossum species are distinguished by their weakened outer wing venation, in contrast to Mexalictus species, which have strong wing venation. The genus Mexalictus consists of 20 described species found in humid, high-elevation areas ranging from southeast Arizona to northern Guatemala. Although the detailed social behavior of Mexalictus species is not well understood, some communal nesting has been observed, with up to eight females sharing a nest. Patellapis species are distributed in Africa, Madagascar, tropical Asia, and Australia (Eickwort 1978; Seltmann and Poelen 2024). Halictini bees are known for their adaptability to various climatic conditions, which may contribute to their evolutionary success. Some species have evolved tolerance to extreme temperatures, allowing them to thrive in both temperate and tropical regions. Their nesting habits also vary widely, with some species constructing underground nests, while others use pre-existing cavities or even exhibit kleptoparasitic behavior, laying their eggs in the nests of other bees.
Despite their ecological importance, Halictini bees face threats from habitat loss, climate change, pesticide exposure, and competition from invasive species. Conservation efforts, including habitat restoration and pollinator-friendly agricultural practices, are essential to maintain their populations and the ecosystem services they provide. Further research is needed to understand their social structures, foraging behaviors, and responses to environmental changes, which will aid in developing effective.
1.8 Global Bee Declines and Population Estimation
Over the past three decades, numerous high-quality studies based on local and regional data have sounded the alarm about the widespread decline in insect diversity and biomass, emphasizing the urgent need for bee conservation and restoration (Manley et al. 2019).
One of the most critical ecological services impacted by this decline is pollination, which supports both wild and cultivated flowering plant species (e Silva et al. 2020; Erickson et al. 2022). Insects are the primary agents of pollen transfer, with the family Apidae (Hymenoptera: Anthophila) being the most significant, comprising approximately 20,000 recognized bee species (Boustani et al. 2021). In addition to Apidae, solitary bees play an essential role in the reproduction of thousands of wild plant species and significantly contribute to crop productivity (Klaus et al. 2021).
Growing evidence suggests that the decline in wild bee populations aligns with broader insect population trends. Bees depend on floral resources for food and various substrates for nesting, both of which have been severely impacted by habitat loss due to large-scale agriculture, urban expansion, and other intensive land uses (Panziera et al. 2022). These environmental changes threaten bee populations and, consequently, the pollination services essential for maintaining ecosystem stability and agricultural production.
Although research on bee decline is predominantly focused on the Northern Hemisphere, particularly North America and Europe, much of the data centers on Bombus species using local, regional, and country-level datasets (Briggs et al. 2022). It is widely recognized that bee populations have been declining globally over the past several decades in both size and range, with a growing gap in overall abundance (Zattara and Aizen 2021).
However, most evidence comes from localized studies, making it difficult to determine a comprehensive global status. A review of literature published in standard journals found that between 2006 and 2015, only 25% of the total bee diversity was collected and reported compared to pre-1990 records (Zattara and Aizen 2021) highlighting gaps in data collection and necessitating caution when interpreting trends. Nevertheless, immediate conservation measures are needed to prevent further losses (Koh et al. 2016).
Human activities are significantly impacting pollinators, leading to declines in species richness and abundance. Almost every form of resource use is linked to habitat destruction (Table 3). In particular, pollution—especially from pesticides and heavy metals—plays a major role in pollinator decline (Askri et al. 2023).
| Bee common name | Bee spices/family | Region/country | Factors | Impacts | References |
|---|---|---|---|---|---|
| Solitary red mason bees |
Osmia bicornis (Fam.: Megachilidae) |
Dublin, Ireland |
Insecticides: - Pyrethroid (lambda-cyhalothrin) - Neonicotinoid (acetamiprid) |
- Behavior - Pollination services |
(O'Reilly and Stanley 2006) |
| Stingless bees honeybee |
Partamona helleri Apis mellifera (Fam.: Apidae) |
Viçosa, Brazil |
Agrochemical pollutants: - Neonicotinoid (imidacloprid) - Fungicide mixture (thiophanate-methyl and chlorothalonil) |
- Color preference - Respiration rates - Group locomotory activities |
(Almeida et al. 2021) |
| Honeybee |
A. mellifera (Fam.: Apidae) |
Beheira, Egypt | - Organophosphorus pollutants |
- Oxidative stress - Ultrastructural biomarkers in the midgut |
(El-Saad et al. 2017) |
| Honeybee |
A. mellifera (Fam.: Apidae) |
Brazil | - Neonicotinoid (imidacloprid) | - Risk assessment procedures | (de Assis et al. 2022) |
| Sweat bees |
Lasioglossum sp. (Fam.: Halictidae) |
Chicago, USA | - Urbanization |
- Bee community's composition - The richness of native and non-native bees - Bee functional traits composition |
(Gruver and CaraDonna 2021) |
|
Bumble bee Sweat bees |
- Bombus sp. (Fam.: Apidae) - Lasioglossum (Dialictus) (Fam.: Halictidae) |
Southeastern Michigan, USA | - Urbanization, floral resources, bee functional traits, temperature, farm size, and the spatial scale of analysis | - Bee community composition | (Wilson and Jamieson 2019) |
|
Bumble bee Sweat bees |
- Bombus sp. (Fam.: Apidae) - Lasioglossum (Dialictus) (Fam.: Halictidae) |
Southeastern Michigan, USA | - Urbanization, floral resources, bee functional traits, temperature, farm size, and the spatial scale of analysis | - Bee community composition | (Wilson and Jamieson 2019) |
| Bumble bee species | (Fam.: Apidae) | Michigan, USA |
- Roads pose substantial barriers to bee movement. - Loss of nesting habitat from landscape weed fabrics |
- Movement of insect pollinators, and consequent pollination, bee health & bee diversity | (Fitch and Vaidya 2021) |
|
Honey bee Bumble bee |
- A. mellifera, - Bombus pascuorum—B. terrestris—Bombus lucorum (Fam.: Apidae) |
England and France |
- Varroa destructor—Deformed wing virus (DWV) - Honey bee pathogen |
- Colony health and longevity | (Manley et al. 2019) |
|
Mason bees Mining bees |
Osmia spp. (Fam.: Megachilidae) Andrena spp. (Fam.: Andrenidae) |
Northeast USA | - Warm winters | - Decreased abundances | (Kammerer et al. 2021) |
|
Bumble bee |
- Bombus spp. (Fam.: Apidae) |
North America and Europe | - Increased number of unusually hot days | - The severe decline | (Soroye et al. 2020) |
| Bumble bee |
- Bombus terrestris audax (Fam.: Apidae) |
Biobest, Westerlo, Belgium |
- Gut parasite Nosema ceranae, - Neonicotinoid (Thiamethoxam) - Pyrethroid insecticide cypermethrin - EBI fungicide tebuconazole |
- Colony health, expression levels of immunity and detoxification related genes and colony performance | (Botías et al. 2021) |
| Honey bee |
A. mellifera (Fam.: Apidae) |
Central-northern Europe |
- Nutritional deficiencies - Parasites and pathogens - Pesticides - Changing climate |
- Mortality rates in managed honey bee colonies | (Wood et al. 2020) |
| Solitary bees | (Fams.: Andrenidae, Apidae, Colletidae, Halictidae, Megachilidae, Melittidae) | Mediterranean region | - Climatic warming | - Body shrinking effect | (Herrera et al. 2023) |
|
Ground-nesting bee Solitary hole-nesting bee Social paper wasp |
Halictus scabiosae (Fam.: Halictidae) Osmia cornuta (Fam.: Megachilidae) Polistes dominula (Fam.: Vespidae) |
Milan (Italy) |
- Temperature - Green areas fragmentation - Vegetation productivity |
- Body size, wing loading, wing aspect ratio and wing fluctuating asymmetry | (Ferrari and Polidori 2023) |
| Stingless bees |
Tetragonula elegante (Fam.: Apidae) |
Brazil | - Herbicide (Glyphosate) |
- Viruses: (DWV, ABPV, BQCV, KBV, IAPV, and CBPV) - Microsporidia (N. apis and N. ceranae) - Pesticide residues |
(Guimarães-Cestaro et al. 2020) |
| Stingless bee |
Melipona colimana (Fam.: Apidae) |
Center in Zapotlan, Jalisco, Mexico |
- Insecticide (Thiamethoxam) - Nosema ceranae |
- Survivorship - Cellular immunity (hemocyte concentration) |
(Macías-Macías et al. 2020; Siviter et al. 2021) |
| Bumble bee |
B. terrestris (Fam.: Apidae) |
Egham, UK |
- Insecticide (Sulfoxaflor) - Nosema bombi |
- Mortality - Larval growth |
(Askri et al. 2023) |
| Bumble bee |
B. impatiens (Fam.: Apidae) |
Michigan, USA |
- Fungicide (Chlorothalonil) - Nosema bombi |
- Worker produced - Microcolonies |
(Calhoun et al. 2021) |
| Bumble bee |
B. terrestris (Fam.: Apidae) |
Agralan, Wiltshire, UK |
- Herbicide (Glyphosate) - Crithidia bombi |
- Mortality - C. bombi concentration - Worker reproduction |
(Straw and Brown 2021) |
Climate change disrupts pollination by altering flowering seasons, displacing species, and shifting plant distributions, which can introduce new competitors and modify plant-pollinator interactions (Inouye 2020; Vanbergen and Initiative 2013). Additionally, increased pesticide use—particularly neonicotinoids—has had severe consequences for native bees, impairing immune function, reducing foraging efficiency, and leading to cognitive impairments (Bakker et al. 2020; Wood and Goulson 2017). Pesticide exposure has also been linked to higher disease susceptibility due to weakened immune systems (Czerwinski and Sadd 2017; Gill and Raine 2014; Moffat et al. 2015; Stanley and Raine 2016). Multiple factors contribute to bee population declines, including habitat loss, pesticide exposure, climate change, diseases, competition from invasive species, and inadequate nutrition (Figure 5). Addressing these challenges requires an integrated approach to conservation.
1.9 Estimating of Bee Populations and Abundance
Currently, evidence of population declines in bees and other pollinators has generated significant interest in monitoring bee abundance, diversity, and distribution across various habitats. In the United States, nine bee species have been protected under the Endangered Species Act of 1973. In response to repeated calls for action, large-scale monitoring efforts led to the formation of the US National Native Bee Monitoring Research Coordination Network in 2020. This research network comprises scientists dedicated to the conservation and protection of bees in the United States (Aldercotte et al. 2022; Turley et al. 2022).
Bee monitoring provides ecological benefits by supporting pollination and maintaining ecological balance. Common sampling methods in monitoring studies include pan traps, vane traps, trap nests, netting from flowers, and timed observations (Packer and Darla-West 2021; Prendergast et al. 2021). Each method provides a population index, typically representing bee count per sampling effort rather than absolute population density (Kulhanek et al. 2021). Since bees are highly mobile, their response to floral and nesting resources may appear geographically random. However, body size, resource availability, and spatial dispersal influence bee density, which is often interpreted based on relative species abundance. The accuracy of this interpretation depends on a consistent relationship between population indices and true density over time and space within a landscape (Moylett et al. 2020). While statistical indices are useful for estimating population trends, a single species' index over a large area may be insufficient, as capture probabilities vary across habitats, potentially leading to biased representations of species composition. Studies suggest that, like small mammals and terrestrial arthropods, population indices from trap catches cannot fully represent the relative abundance of species due to differential capture probabilities and environmental influences (Hennessy et al. 2020; Shi et al. 2022; Briggs et al. 2022). The relationship between sampling indices and community composition remains underexplored, leaving gaps in understanding community-scale variations in bee populations (Orr et al. 2021; Clair et al. 2022; Fairo et al. 2022; Gerner and Sargent 2022; Su et al. 2022). Different indexing methods yield varying depictions of bee communities, especially when using pan traps, sweep netting, and molasses traps (Prendergast et al. 2021), particularly when employing techniques such as pan traps, sweep netting, and molasses traps. Sampling and quantification methods exhibit substantial differences across habitats, taxa, topography, plant stands, elevation, slope gradients, and overall forage resources (Packer and Darla-West 2021). Mark-recapture methods are often effective for estimating bee abundance with higher precision (Cecala and Rankin 2020; Briggs et al. 2022) and have been applied to other taxa such as mammals, birds, and amphibians (Larson et al. 2022). For example, an experiment in Sweden on 10 populations of the solitary red-listed Andrena hattorfiana used survey transits and mark-recapture methods, revealing significant differences in population estimates (Larsson and Franzén 2008). Globally, research is increasingly focused on estimating bee diversity, implementing conservation measures, and protecting habitats from further degradation. This underscores the urgent need for accurate strategies in pollinator conservation, ensuring the stability of ecosystems that depend on them.
1.10 Methods for Future Research and Conservation Efforts
To effectively protect solitary bees in the Halictidae family, we can take several practical steps for future research and conservation. First, establishing long-term monitoring programs will help us gather important data on bee populations and their health. Developing predictive models that consider factors like climate change and habitat loss can enhance our understanding of their dynamics. Additionally, studying the genetic diversity of these bees will reveal how they adapt to changing environments. We should also promote Halictidae as bioindicators of ecosystem health by monitoring their responses to environmental stressors. Creating and restoring habitats that connect fragmented areas is crucial for their survival, as is optimizing plant-pollinator networks to ensure these bees have access to their preferred flowers. Advocating for reduced pesticide use and promoting alternative pest management practices will further protect them. Lastly, engaging the community through citizen science initiatives can raise awareness about the importance of solitary bees and involve local efforts in their conservation. By implementing these strategies, we can support the health of Halictidae populations and the ecosystems that rely on their vital pollination services. To effectively safeguard solitary bees in the Halictidae family, targeted research and conservation efforts are essential to mitigate habitat loss, climate change, and pesticide exposure. Key strategies include establishing long-term monitoring programs, such as the UK Pollinator Monitoring Scheme (PoMS) and the US National Native Bee Monitoring Research Coordination Network, to track population trends (Potts 2024). Predictive modeling, like species distribution models and network-based assessments, can help anticipate environmental impacts on Halictidae. Genetic studies on population connectivity and adaptation inform conservation priorities, while habitat restoration with native plants ensures year-round foraging and nesting resources. Strengthening plant-pollinator networks through intercropping, organic farming, and urban pollinator gardens further supports Halictidae diversity. Reducing pesticide exposure via Integrated Pest Management (IPM) and pesticide-free buffer zones is crucial, as seen in the EU's Farm to Fork Strategy (Carayannis et al. 2020). Urban conservation efforts, including bee-friendly green spaces and artificial nesting sites, provide vital refuges, exemplified by the BeePathNet Project. Additionally, citizen science initiatives, such as the Great Sunflower Project and the Australian Wild Pollinator Count, engage communities in pollinator conservation, fostering awareness and data collection for ongoing research (Drossart and Gérard 2020). The Pampean region, one of the world's most productive grasslands, has undergone significant agricultural expansion, leading to habitat fragmentation and biodiversity loss, including solitary bees. Research in four Argentine Pampean agroecosystems analyzed 3500 individuals from 82 wild bee species, 20 cleptoparasitic bees, and various predatory insects. Findings indicate that low floral diversity dominated by exotic species and agrochemical use are key threats to bee populations. Crop homogenization and habitat loss further challenge their survival, emphasizing the urgent need for sustainable agricultural practices that support biodiversity and ecosystem health (Torretta et al. 2025). Socioeconomic changes significantly impact ecosystem services (ES), yet their effects on crop pollination remain less understood at larger scales. A decade-long study in Brazil (2006–2017) examined how socioeconomic drivers influence the demand, diversity, and provision of pollination services. Findings revealed that the shift from small, diverse farms to large monocultures led to a 3.3% increase in pollination demand but a 16.1% and 22.5% decline in diversity and provision, respectively. These deficits were linked to wealth concentration and social inequality, as regions with concentrated land ownership and restricted credit access experienced reduced pollination services. The study highlights the need for a multidimensional approach to address socioeconomic drivers in conservation strategies (Bergamo et al. 2025). Expanding conservation efforts requires a multi-faceted approach that integrates ecological, economic, and social dimensions. Sustainable land-use strategies, such as agroforestry and diversified cropping systems, can enhance pollinator habitats while maintaining agricultural productivity. Strengthening policy frameworks through conservation incentives and stricter pesticide regulations will further protect pollinators from environmental stressors. Selecting seed sources for restoration based on local adaptation and plant traits can enhance pollinator interactions, improving restoration success. Taller plants and those from climates similar to the restoration site showed greater pollinator visitation and network centrality (Aslan et al. 2025). Additionally, fostering habitat connectivity by restoring semi-natural areas and planting native floral resources can mitigate the negative effects of habitat fragmentation. Public engagement through citizen science initiatives and educational campaigns is also essential to raise awareness and promote community-driven conservation actions. Moreover, economic incentives for farmers who implement pollinator-friendly practices can help bridge the gap between conservation and agricultural needs. By combining scientific research with proactive conservation policies and community involvement, we can create a resilient and sustainable environment for solitary bees and other pollinators (Drossart and Gérard 2020; Potts 2024; Storkey et al. 2020; Torretta et al. 2025).
2 Conclusion
Given the urgent threats facing pollinators, particularly solitary bees within the Halictidae family, it is essential to explore innovative conservation strategies. Despite progress in understanding their biology and ecological roles, knowledge gaps remain, especially regarding population dynamics and responses to environmental changes. Future research should focus on developing predictive models that incorporate climate change, habitat fragmentation, and floral resource availability to forecast the effects on Halictidae. Additionally, studying the genetic and phenotypic adaptability of these bees may reveal resilience mechanisms useful for conservation. Halictidae could also serve as bioindicators of ecosystem health due to their sensitivity to environmental stressors like pesticides and habitat loss. To protect these vital pollinators, conservation efforts should prioritize creating plant-pollinator networks that cater to their floral preferences and implementing landscape-scale strategies to connect fragmented habitats. Addressing broader environmental threats, such as pesticide use and climate change, is also crucial. By integrating ecological, genetic, and conservation research, we can better protect Halictidae and ensure the stability of ecosystems and agricultural systems dependent on them.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available upon reasonable request from the corresponding author.




