Odorant binding protein TcOBPC02 contributes to phytochemical defense in the red flour beetle, Tribolium castaneum
Abstract
The red flour beetle, Tribolium castaneum, is an agricultural and storage pest with a global distribution. Studies have shown that eucalyptol has strong contact toxicity against larvae of this beetle, whereas odorant binding proteins (OBPs) are known to contribute to larval defenses against this phytochemical toxin. However, the mechanisms underlying the protective effect of insect OBPs against eucalyptol remain unclear. Here, TcOBPC02 from T. castaneum was cloned and characterized. Gene expression profile analysis showed that TcOBPC02 is highly expressed at early larval and early pupal stages. Additionally, tissue expression profiling revealed that, in the adult, TcOBPC02 was most highly expressed in the head, followed by the epidermis, whereas in larvae, TcOBPC02 was mainly expressed in hemolymph and the epidermis. These developmental stages and tissues that exhibit high TcOBPC02 expression are closely related to the detoxification of heterologous substances. Furthermore, the mRNA level of TcOBPC02 was significantly increased after exposure to eucalyptol, whereas TcOBPC02-targeted RNA interference increased the susceptibility of T. castaneum to eucalyptol, indicating that TcOBPC02 participates in the tolerance of this beetle to eucalyptol. Additionally, recombinant TcOBPC02 was expressed in Escherichia coli and isolated, enabling a straightforward fluorescence competition binding assay. In combination, these results have demonstrated that TcOBPC02 is required for defenses against phytochemicals in T. castaneum. This study provides a theoretical basis for understanding the mechanisms underlying the degradation of exogenous toxicants in insects and adds to the repertoire of potential target genes for pest control.
Introduction
Insects comprise the most species-rich animal group on earth and are closely associated with humans. Insects mainly rely on their olfactory system to perceive the external environment, and their olfactory system is crucial for a variety of biological functions, including foraging, mating, reproduction and the avoidance of natural enemies (Bruce et al. 2005; Takken 2011; Vet & Dicke 1992). The olfactory system of insects consists primarily of odorant-binding proteins (OBPs), chemosensory proteins (CSPs), sensory neuron membrane proteins (SNMPs) and olfactory receptors (ORs) (Wan & Du 2015). Odor molecules in the environment enter sensory organs through micropores present on their surface, are recognized by OBPs in the lymph fluid and are transported to the dendrites of sensory neurons. Here, they activate ORs on the dendrite membrane, leading to the transformation of chemical signals into electrical signals, which triggers action potentials in olfactory neurons, consequently resulting in a series of behavioral reactions (Leal & Walter 2013). Odorant-binding proteins bind specifically to fat-soluble odorant molecules and play a preliminary filtering role, which is a key step in insect olfactory recognition (Li et al., 2016b).
Insect OBPs are highly soluble globular proteins of approximately 120–150 amino acids in length that are abundantly secreted in the recipient lymph (Pelosi et al. 2018). Pheromone binding protein was the first member of the OBP family to be identified and was discovered in the antennae of the moth Antheraea polyphemus (Sehring et al. 2014). Employing protein–ligand binding assays and nucleotide sequencing, many OBPs have since been found in a wide variety of insects, including 52 in Drosophila melanogaster (Menuz et al. 2014), 21 in Apis mellifera (Foret & Maleszka 2006) and 32 in the antennae of Micromelalopha troglodyte (Zhang et al. 2022). The progress made in molecular biology techniques has gradually led to the identification of the physiological function of insect OBPs (Zhu et al. 2017). Recent studies have shown that OBPs are specifically expressed in antennae in most insects. For instance, Wang et al. (Wang et al. 2021) found that CchiOBP1, CchiOBP2, CchiOBP3, CchiOBP4 and CchiOBP6 are expressed in the antennae of Callosobruchus chinensis, both male and female, and at significantly higher levels than in other tissues. Similarly, CpunPBPl was reported to also be highly and specifically expressed in the antennae of Conogethes punctiferalis (Jia et al. 2015). However, not all OBPs are exclusively expressed and distributed in the antennae. For example, TcOBPC12 is highly expressed in the epidermis of Tribolium castaneum, indicating that OBPs may participate in physiological functions other than olfactory perception. TcOBPC12 is also highly expressed in both the early and late stages of larval development, suggesting that it may be involved in the growth and development of insects (Gao et al., 2021b).
Tribolium castaneum, a worldwide stored grain pest belonging to the order Coleoptera, is extensively distributed in tropical and warmer regions and is found in at least 23 provinces (regions) in China (Zhang 2017). The adult can secrete an odorous liquid containing benzoquinone and other carcinogens promotes flour lumping, resulting in considerable economic losses (Islam 2017). Following the sequencing of the whole genome of T. castaneum in 2008 this beetle has become another type insect, like Drosophila, and is representative of Coleoptera, the order of animals with the highest species richness (Richards et al. 2008). Fumigation with chemical agents such as bromoethane and phosphine is an effective method for controlling T. castaneum; however, the excessive or inappropriate use of chemical fumigants can harm non-target organisms as well as the natural environment. Thus, the identification and development of environmentally friendly insecticides that can control this pest has become a key focus of research. Several plant essential oils have been found to be effective in insect control. For example, the essential oil of Artemisia vulgaris has certain repellent, fumigation and contact effects on both larval and adult T. castaneum (Hamdy et al. 2015; Hu et al. 2018), and was reported to significantly induce the expression of the genes TcOBPC11 and TcOBPC17 in T. castaneum (Zhang et al. 2020; Gao et al., 2021a). In addition, RNAi-mediated knock-down of TcOBPC11 and TcOBPC17 further increased the mortality rate resulting from exposure to the essential oil, suggesting that these two genes are related to the defense of T. castaneum against this potential insecticide. Moreover, compared with other moxa plant essential oils, the essential oil of A. vulgaris does not contain thujone, which is harmful to humans (Jiang et al. 2019). In this study, eucalyptol, a monoterpenoid compound and the main active component of the essential oil of A. vulgaris (Gao et al. 2020), was selected as the test irritant. Eucalyptol and its main constituents have the advantages of low toxicity, ease of degradation, environmental friendliness and anti-insect effects, rendering it a potential new pesticide.
In our previous study, we found that TcOBPC02 (Tc010067), encoding an OBP, was significantly upregulated after exposure to eucalyptol (Gao et al. 2023). Therefore, in this study, we obtained the sequence of TcOBPC02 by TA cloning. Subsequently, we generated the expression profile of TcOBPC02 using quantitative real-time PCR (qPCR), investigated its function and associated regulatory mechanisms through RNA interference (RNAi), gene expression analysis and eucalyptol sensitivity testing, and produced large quantities of recombinant TcOBPC02 in a prokaryotic expression system. The results contribute to understanding the functional characteristics of TcOBPC02 and provide a novel idea for the prevention and control of T. castaneum.
Materials and methods
Insect rearing
The eggs of T. castaneum Georgia-1 were collected at Nanjing Normal University and were brought back to the laboratory. Culture bottles were sterilized, moistened, dried and marked to facilitate passage. The eggs were cultured in whole-wheat flour containing 5% yeast particles in an artificial climate incubator at 30°C and 40% humidity to obtain T. castaneum at every stage of development (Gao et al. 2020).
RNA extraction and cDNA library preparation
Samples were collected at different stages of development, 50 mg from each group, as follows: early eggs (1 day old); late eggs (3 days old); early larvae (1 day old); late larvae (20 days old); early pupae (1 day old); late pupae (5 days old); early adults (1 day old); and late adults (10 days old). RNA was extracted from adult tissue (whole adult, head, fat body, epidermis, gut, ovary, antennae, testis and accessory gland) and larval tissue (whole larva, head, fat body, epidermis, gut and hemolymph) using Trizol reagent, with 50 mg required for different tissues. RNA concentration and quality were determined using a NanoDrop 2000 (Thermo Fisher Scientific, Waltham, MA, USA) and the integrity of the RNA was assessed by 1% agarose gel electrophoresis. The extracted RNA was treated with 4× gDNA wiper Mix and 5× HiScript qRT SuperMix II, reverse-transcribed into cDNA. The samples were diluted 10-fold and stored at −20°C, whereas the extracted RNA was stored at −80°C for later use.
Cloning of the open reading frame of the TcOBPC02 gene
The cDNA obtained from early adults was used as a template to amplify the full-length target fragment (20 μL reaction mixture) using primers designed by Premier 5.0 (Table 1). The PCR products were purified using the FastPure Gel DNA Extraction Mini Kit (Vazyme, Nanjing, China). Then, the target fragment was linked to a T-carrier and transformed into competent Escherichia coli DH5α cells. A strain containing the target gene was obtained through blue–white screening and colony PCR verification and sequencing performed by Shanghai Kehua Bio-engineering Co. Ltd. (Shanghai, China). The sequencing data were compared with sequences in the GenBank database.
| Primer name | Primer sequence (5′→3′) | Product length (bp) | Purpose |
|---|---|---|---|
| OBPC02-F | GTCTGCGTAATCTTCATTTTAGTGG | 212 | qPCR |
| OBPC02-R | TTCATTATTCCCACTTTTTTTCCAA | ||
| OBPCO2-FF | ATGAACTTCGTCTGCGTAATCTTCA | 405 | Cloning |
| OBPC02-FR |
TCATTCAACTGGTGAAAACTTTGG |
||
| OBPC02-RF |
TAATACGACTCAATATAGGGGTGCCTTGACCGGAGTGTCTC |
222 | RNAi |
| OBPC02-RR | TAATACGACTCAATATAGGGTTTTTGACCGCACATTTGTTGTA | RNAi | |
| OBPC01-F | CAATAAGCAAAGAATGCCGAGA | 209 | qPCR |
| OBPC01-R | CACTTCGTCGTCGTTCTCACTC | ||
| OBPC07-F | GCAAAAATCAGTCGGGAGTGT | 291 | qPCR |
| OBPC07-R | AGAAGTTGGGTTTGTTGGCG | – | – |
- F, forward; R, reverse; FF, forward full-length; FR, reverse full-length; RF, forward interference; RR, reverse interference. The underlined letters indicate the T7 promoter sequence for dsRNA synthesis.
Expression profiling of the TcOBPC02 gene
The specific primers used to quantify TcOBPC02 expression are shown in Table 1. cDNA derived from eight key periods and different adult and larval tissues was used as a template for qPCR, with the housekeeping gene TcRPS3 (ribosomal protein S3) serving as an internal reference. Three biological repetition experiments were performed for different developmental stages and different tissues.
The expression pattern of TcOBPC02 following eucalyptol stimulation
Eucalyptol (99%; CAS no. 470-82-6) was obtained from the Aladdin Company (Shanghai, China). The median lethal concentration (LC50) of eucalyptol for T. castaneum derived from our previous study was 43.294 mg/mL (diluted using acetone) (Gao et al. 2023).
For treatment, 30 late larvae were soaked in eucalyptol (LC50) or acetone (50 μL) for 1 min, air-dried on filter paper and then allowed to grow in a Petri dish under standard conditions (Lu et al. 2012). Each treatment was carried out in three independent biological replicates. Three larvae were collected at different time points (12, 24, 36, 48, 60 and 72 h) for RNA extraction, reverse-transcribed into cDNA and then underwent qPCR to assess the effect of eucalyptol on the expression of TcOBPC02.
dsRNA synthesis and injection
Primers were designed for the synthesis of double-stranded RNA (dsRNA) from the gene TcOBPC02 (Table 1). The reaction system includes 0.5 μL of forward and reverse primers (10 μmol/L), 12.5 μL of 2× nPrimer STAR Mix, 9.5 μL of DEPC H2O and 2 μL of plasmid DNA (20 ng/μL) in which the TcOBPC02 open reading frame (ORF) was inserted. PCR amplification was performed using the following conditions: 94°C for 5 min; 35 cycles of 94°C for 30 s, 55°C for 30 s and 72°C for 2 min; and 72°C for 8 min. PCR products were purified as templates for dsRNA synthesis, and dsRNA was synthesized using the TranscriptAid T7 High Yield Transcription Kit (Fermentas, Vilnius, Lithuania), in accordance with the manufacturer’s instructions. After purification and chloroform extraction, the upper liquid layer was collected and the dsRNA was precipitated with 2× the volume of ethanol, washed in 1 mL of 70% ethanol (−20°C), centrifuged and dissolved in 20 μL of DEPC-treated water. The dsRNA was diluted to a concentration of 2 μg/μL, aliquoted (3 μL) in 0.5 mL centrifuge tubes and stored at −80°C.
For the injection, the dsRNA was dissolved, centrifuged for a few seconds and then mixed with 3 μL of 2× injection buffer. Two control groups (IB and ds-ver) and one experimental group (ds-TcOBPC02) were set up for microinjection, and 30 late larvae were selected from each group. In the ds-TcOBPC02 or ds-ver group, each larva was injected with 200 nL of solution containing 200 ng of dsRNA; in the IB group, each larva was injected with 200 nL of 1× injection buffer. RNAi of ver (T. castaneum vermillion, GenBank AY052390), encoding tryptophan oxygenase, causes the color of the eyes in T. castaneum to change from black to white, without any effect on the normal growth and development of the insect (Lorenzen et al. 2002). The ds-ver group was used as a positive control to test the effectiveness of RNAi by monitoring the eye pigmentation of T. castaneum (Arakane et al. 2009). Three biological replicates were set up for each group. After 30 min of injection, the larvae were transferred to Drosophila tubes and cultured normally. Biological characterization was performed at the same time every day for five consecutive days; on day 4, three larvae from each group were selected for the assessment of interference efficiency and off-target effects using qPCR.
Insecticidal efficacy assay for eucalyptol
After TcOBPC02-targeted RNAi, the larvae were assessed for morphological defects. In the absence of defects, the 30 larvae of the ds-TcOBPC02 group and the control group were subjected to contact experiments with the LC50 of eucalyptol. The larvae were first exposed to eucalyptol for 1 min, then dried on filter paper for 2 min and then finally transferred to clean Drosophila tubes for normal feeding. Larval mortality was recorded 12, 24, 36, 48, 60 and 72 h after treatment.
Construction of the TcOBPC02 gene expression vector
The recovered target fragment was ligated to the pET-22b (+) vector after double digestion with the restriction enzymes NdeI and XhoI, yielding the expression vector. Receptor cells containing the cloned vector were transferred to coated plates for culture and a single colony was selected for PCR- and sequenced-based verification. The bacteria harboring the correct clone were expanded and cultured and the pET-22b (+)-TcOBPCO2 recombinant plasmid was extracted and stored at −80°C for subsequent expression in E. coli.
Prokaryotic expression and purification of TcOBPC02
The recombinant plasmid was transformed into competent E. coli cells using heat shock at 42°C and the transformed cells were coated on a plate containing 50 μg/mL ampicillin and cultured at 37°C. Monoclonal colonies were selected and cultured at 37°C in liquid medium containing ampicillin. At an OD600 (optical density measured at 600 nm) of 0.6, isopropyl β- d-thiogalactopyranoside (IPTG) (0.5 mmol/L) was added and the cells were cultured overnight at 16°C. Bacteria were suspended in binding buffer and fully fragmented using an ultrasonic crusher; then, after centrifugation, the supernatant was taken for purification.
For affinity purification, 2 mL of nickel nitrilotriacetic acid (Ni-NTA) was placed in the purification column, the column was cleaned with binding buffer at 5× the column bed volume, after which the crude protein was added dropwise into the column and the outflow was collected. Subsequently, the column was cleaned with binding buffer, then washed with washing buffer and the effluent was collected. The protein was eluted via the sequential addition of Elution-20 buffer, Elution-100 buffer and Elution-500 buffer, followed by effluent collection. The crude protein and effluent components were treated separately. Finally, the purified components were concentrated in preservation buffer (PBS pH 7.4), filtered through a 0.22 μm membrane, aliquoted into 1 mL samples and stored at −80°C.
Data analysis
Relative gene expression levels were calculated using the 2−ΔΔCt method (Livak & Schmittgen 2001). Data regarding the effect of eucalyptol on gene expression and the mean values relating to RNAi treatment versus the control conditions were compared using the Student’s t-test and one-way analysis of variance (ANOVA), followed by Fisher’s least significant difference (LSD) multiple comparison test, respectively, using SPSS software (IBM, Armonk, NY, USA). All data are presented as mean ± standard deviation, with P < 0.05 considered significant.
Results
Identification of the target gene TcOBPC02
The ORF of TcOBPC02 was cloned from the total cDNA obtained from late pupae (Fig. 1). The ORF of the TcOBPC02 gene was 405 bp in length, encoding a protein of 134 amino acids with a relative molecular weight of 14 933.

Spatiotemporal expression profile
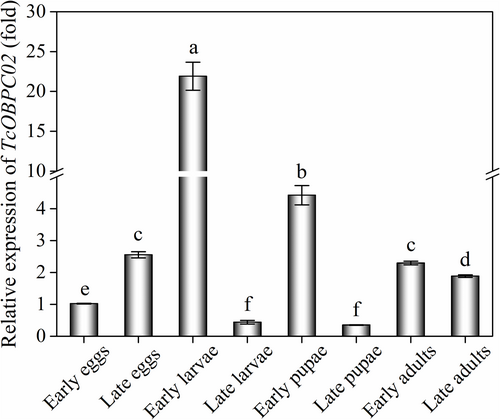
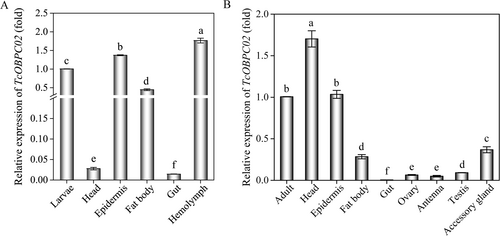
The abundance of the TcOBPC02 transcript at different developmental stages and tissues of T. castaneum was assessed by qPCR. The results showed that TcOBPC02 is expressed at varying levels in eight key periods, suggesting that it might be involved in several physiological processes. TcOBPC02 was highly expressed in early larvae, followed by late eggs, early adults and early pupae, and its expression was relatively low in the other developmental stages assessed (Fig. 2). The expression pattern of TcOBPC02 was also evaluated in adult and larval tissues. TcOBPC02 was found to be expressed in all tissues in both adults and larvae. In the larval stage, Tc0BPC02 was highly expressed in the epidermis, fat body and hemolymph, but its expression was low in other tissues (Fig. 3A). In the adult stage, the expression level of Tc0BPC02 was highest in the head and the epidermis, followed by the fat body and the accessory gland; the expression of Tc0BPC02 was low in the other tissues evaluated (Fig. 3B).
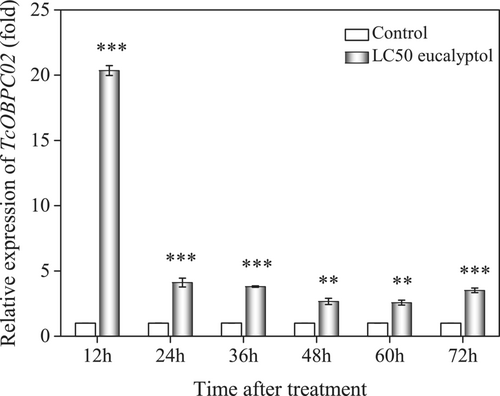
The expression of TcOBPC02 was induced after exposure to eucalyptol
To investigate whether eucalyptol treatment affects the expression of TcOBPC02, T. castaneum larvae were exposed to LC50 eucalyptol and then cultured under normal conditions (acetone served as the control). Larvae were collected at different treatment time points and the transcript levels of TcOBPC02 were assessed by qPCR. As shown in Figure 4, compared with the control treatment, the expression of the TcOBPC02 gene was upregulated to varying degrees after 12–72 h of exposure to eucalyptol. The expression level of TcOBPC02 peaked at 12 h, and then gradually declined. This suggested that treatment with eucalyptol induced the expression of the TcOBPC02 gene.
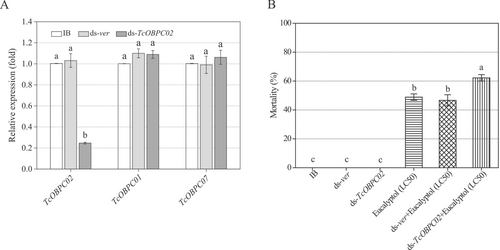
The effect of TcOBPC02 RNAi on the response of T. castaneum to eucalyptol
To further evaluate the susceptibility of TcOBPC02 expression to eucalyptol, the expression of TcOBPC02 was knocked down using RNAi. Compared with the control treatment, RNAi significantly reduced the expression level of TcOBPC02 without concomitantly affecting the expression levels of the OBP family genes TcOBPC01 and TcOBPC07 (Fig. 5A). This indicated that interference with the target gene TcOBPC02 was successful and did not cause off-target effects. On day 5 after injection, the larval mortality rate of the larva was similar among the three groups, indicating that the injection of ds-TcOBPC02 did not affect the health of the larvae at this time point. Consequently, the larval drug sensitivity test was carried out with the LC50 of eucalyptol on day 5 after ds-TcOBPC02 injection. The results showed that the mortality rate was increased in all groups, but was higher in the ds-TcOBPC02 group than in the control group (Fig. 5B).
Expression in E. coli and protein purification
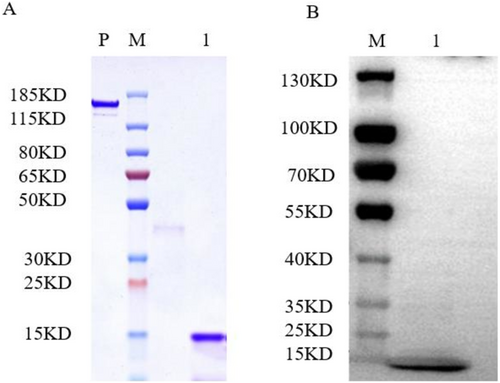
To obtain a large quantity of soluble TcOBPC02 protein and further explore its function in T. castaneum, TcOBPC02 was expressed in E. coli strain BL21. The target protein was induced by IPTG at the optimal induction temperature of 16°C. After purification, sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) of the fusion protein resulted in clear bands near its theoretical molecular weight (Fig. 6A), indicating that it was indeed the fusion protein. To further confirm that it was the target protein, we performed Western blotting with tetramethyl benzidine (TMB). As shown in Figure 6(B), clear and uniform bands were detected in the corresponding positions, again indicating that it was indeed the target protein that had been purified.
Discussion
Insects have evolved an extremely sensitive olfactory system. OBPs are key components of this system, serving as carriers that bind and transport sex pheromones and odor molecules to the dendrite endings of olfactory neurons, leading to olfactory responses (Pelosi et al. 2018). In this study, we cloned the cDNA of TcOBPC02 and found that it encoded a protein of 134 amino acids with a relative molecular weight of 14 933. TcOBPC02 was found to contain a signal peptide (aa 1–18) but no transmembrane region. TcOBPC02 had a predicted isoelectric point of 8.00, rendering it an alkaline water-soluble protein, similar to EoblOBP11, the encoded amino acid sequence of which contains 38.7% alkaline residues and a predicted isoelectric point of 8.1 (Yan et al. 2021). The temporal and spatial expression patterns of OBPs in insects are related to their specific physiological functions (Song et al. 2014; Sun et al. 2016; Zhang et al. 2017). For example, at the mating peak stage, the expression levels of five Carposina sasakii OBP genes (CsasOBP7, CsasOBP12, CsasOBP15, CsasOBP19 and CsasOBP21) were significantly higher in male antennae than in other tissues (Li et al. 2022), suggesting that these genes may play an important role in how males seek out females. In the present study, qPCR analysis showed that TcOBPC02 is highly expressed in the head of adult T. castaneum. The head of adult T. castaneum has a variety of mouth parts, suggesting that TcOBPC02 participates in olfactory recognition. Consistent with previous reports (Liu et al. 2014), we observed that TcOBPC02 is also highly expressed in hemolymph, suggesting that it may be involved in the transport of foreign bodies in this fluid (Armbruster et al. 2009; Kim et al. 2017; Paskewitz & Shi 2005). Although TcOBPC02 is also highly expressed in the epidermis and fat bodies of adult T. castaneum, the biological functions of OBPs in these tissues are not clear. Nevertheless, the fact that in insects these tissues are involved in metabolism and detoxification (Beyenbach et al. 2010; Chen et al. 2019; Li et al. 2019) suggests that TcOBPC02 may play an indispensable role in the degradation of toxic substances. OBPs are expressed in both larval and adult stages in insects (Gao et al. 2020; Qu et al. 2021), and Cheng et al. (2021) found that HaxyOBP5 and HaxyOBP12 were abundantly expressed in Harmonia axyridis during the larval stage, suggesting that they are related to the larval biology of this beetle; however, two other OBPs, HaxyOBP3 and HaxyOBP15, were highly expressed in the adult stage, suggesting that they may participate in adult-specific behavior. In Sitophilus zeamais, SzeaOBP1 and SzeaOBP28 are expressed in both larval and adult stages, albeit at different levels (Tang et al. 2019). Here, we found that the expression of TcOBPC02 was highest in the larval stage, indicating that T. castaneum is more sensitive to odor at this stage of development. Consistent with this, TcOBPC02 is expressed to varying degrees in all developmental stages (from egg to adult), suggesting that it may be involved in several physiological activities, including growth, development and courtship.
We found that the TcOBPC02 gene is responsive to eucalyptol stimulation. Studies have shown that TcOBPC11 may contribute to the defense against the essential oil of A. vulgaris (Zhang et al. 2020). The close homology of these two genes implies that TcOBPC02 may have similar functions to TcOBPC11. In this study, we observed that the expression level of TcOBPC02 was significantly increased in larvae exposed to the LC50 of eucalyptol, as determined by qPCR (Fig. 4). The expression pattern of TcOBPC02 under stimulation with this odor insecticide is similar to that of OBPs in other insects following exposure to different insecticides. In Plutella xylostella, OBP13 was significantly upregulated after treatment with permethrin (Bautista et al. 2015), whereas the expression of OBPjj7a and OBP28 in late Culex pipiens larvae was positively correlated with deltamethrin resistance (Li et al., 2016a; Liu et al. 2017). This suggests that the expression of OBPs may be affected to similar extents under stimulation with different insecticidal substances. RNAi is a powerful tool for studying gene function in a wide range of both single- and multicellular organisms (Baum et al. 2007; Bautista et al. 2009; Guo et al. 2015; Kim et al. 2015). Here, we injected dsRNA to knock down the expression of the TcOBPC02 gene and performed an off-target efficiency assessment to ensure that the expression of homologous genes was not affected. We found that ds-TcOBPC02 injection led to an increase in larval mortality, indicating that TcOBPC02 may be involved in the resistance of T. castaneum to odor-based insecticidal substances. Exposure to eucalyptol leads to increased mortality; however, RNAi-mediated TcOBPC02 knock-down before exposure resulted in higher mortality relative to that observed with the control treatment, indicating that TcOBPC02 may have a protective, buffering effect against eucalyptol. In addition, our findings suggest that the expression pattern of TcOBPC02 may be time dependent. We noted that the mRNA level of TcOBPC02 peaked after 12 h of eucalyptol treatment, and then gradually declined. In response to Beauveria bassiana infection in whitefly (Bemisia tabaci), 654 and 1681 genes were observed to be differentially expressed after 48 and 72 h of infection, respectively (Xia et al. 2013). In T. castaneum, the expression level of TcOBPC02 had declined after 72 h of eucalyptol treatment, possibly because many defense mechanisms and resistance genes had been activated to cope with the effects of eucalyptol. This suggested that the strength of expression of different OBPs at different time points may represent mechanisms for protecting different tissues in the insect. In summary, these results strongly support that TcOBPC02 is responsive to eucalyptol stimulation. Besides eucalyptol, the essential oil of A. vulgaris also contains other complex compounds. Some of these compounds, such as α-terpineol (Pandey & Singh 2017; Hu et al., 2018) and β-pinene (Benelli et al. 2018; Chu et al. 2011), can also be lethal to insects. Furthermore, there may be a synergistic relationship between these complex compounds, which improves the insecticidal effect of essential oils. More studies are needed to better characterize the mechanisms involved in the insecticidal effects of these environmentally friendly insecticides.
Fluorescence competitive binding assays are commonly used to test the binding of insect OBPs to target ligands. For instance, Zhan et al. (2021) examined the binding properties of recombinant OBP69a and OBP76a of Drosophila suzuki to 26 potential ligands, including cVA, employing a fluorescence-based ligand binding assay. Using a similar methodology, Qu et al. (2021) showed that HparOBP14 had an affinity for 6-methyl-5-heptene-2-one (a plant volatile), 3-methylindole, p-cymene, methanol, formaldehyde, α-pinene and geraniol (organic fertilizer volatiles). Owing to experimental limitations, we could not perform a fluorescence competitive binding experiment in the current study. However, after continuous optimization of the experimental conditions, the optimal conditions for TcOBPC02-induced protein expression was successfully explored, and has been expressed and purified, which provides an important guarantee required for subsequent functional studies of this protein.
Conclusion
In this study, we cloned TcOBPC02 and determined its spatiotemporal expression profile. Our findings suggest that it may be involved in multiple physiological activities. The RNAi and drug sensitivity experiments revealed that TcOBPC02 plays a significant role in defending against the exogenous poison eucalyptol in T. castaneum. We also optimized the prokaryotic expression conditions and generated a large quantity of soluble TcOBPC02 protein. In summary, our results provide insight into the mechanism of olfactory communication in T. castaneum, as well as a theoretical basis for the study of green methods for the prevention and control of T. castaneum.
Acknowledgments
This research was supported by the Natural Science Foundation of Henan Province (242300421554) and the Crossing Research Project (KYHT2024007).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.




