Expression, purification, and function of baculovirus infected insect cells-derived hookworm AIP-1 and AIP-2 proteins
Abstract
Anti-inflammatory protein (AIP)-1 and AIP-2, identified as parasite excretory/secretory (ES) proteins, play a crucial role in promoting the survival of the parasite and evading the host immunological response. Both proteins inhibit inflammatory reactions, induce apoptosis in effector cells, and influence the phenotype of the immune response. Numerous parasite-derived proteins have shown promise as therapeutic targets for inflammatory and allergic diseases. Despite this, the precise biological roles, and molecular characteristics of many such proteins remain unclear.
In this study, AIP-1 and AIP-2 were produced in the baculovirus-insect cell expression system (BEVS). The multiplicity of infection (MOI) and the duration of infection conditions for AIP-1 and AIP-2 protein expression were successfully optimized to induce the highest expression levels of AIP-1 and AIP-2 proteins in insect cells. The insect cell-derived AIP-1 and AIP-2 exhibited inhibitory functions against human matrix metalloproteinases (MMPs). This study demonstrates that insect cell-derived AIP-1 and AIP-2 have the potential as therapeutic proteins for MMP-TIMP (Metalloprotease-Tissue inhibitor of metalloprotease) axis related disease.
Introduction
Parasite excretory/secretory (ES) proteins are a mixture of proteins, lipids and carbohydrates that are secreted from the parasite, contain a wide range of structurally and functionally distinct molecules, mostly proteins (Abuzeid et al., 2020). These molecules react with host proteins and act major functions in parasite survival and development and host–parasite relationships, especially, are crucial for the penetration of the host, tissue migration, nourishment, reproduction, and evasion of host immunity (Pearson et al., 2012). These molecules help the parasite to survive and evade the host immunological response by inhibiting the inflammatory reaction, encouraging effector cells apoptosis, and skewing the immune response phenotype (Loukas & Prociv, 2001). Many parasite-derived proteins have proven as therapeutic targets against inflammatory and allergic diseases (Navarro et al., 2013). However, the biological role and molecular function of many of them are still unclear.
AIP-1 and AIP-2 are one of the identified proteins in Ancylostoma caninum ES products. Proteomic analysis of A. caninum ES proteins reveal the relative abundance of two Tissue Inhibitor of Metalloprotease (TIMP)-like proteins, anti-inflammatory protein (AIP)-1 and AIP-2 (Mulvenna et al., 2009). The open reading frame of AIP-1 of A. caninum adult worm corresponds to a putative hookworm TIMP with 33% identity and 50% similarity to the N-terminal domain of human TIMP-2 (Zhan et al., 2002). The recombinant AIP-2 protein contains an unusual multicopy (ten) repeat of the amino acid sequence (KTVEENDE), and inhibits the human matrix metalloproteinases, MMP-2, MMP-7, and MMP-13 (Zhan et al., 2008). Therefore, it is speculated that AIP-1 and AIP-2 proteins might directly interact against MMP (Zhan et al., 2002; Zhan et al., 2008).
The inhibition mechanism of MMP activity by AIP-1 and AIP-2 has not been fully studied. However, Ferreira et al. (2017) reported that recombinant AIP-1 protects against all hallmark parameters of inducible colitis and promotes a regulatory immune environment in treated mice. Navarro et al. (2016) reported that AIP-2 suppresses the expression of costimulatory markers on human dendritic cells (DCs) and suppresses HDM-specific proliferation of peripheral blood mononuclear cells (PBMCs) from allergic asthma patients. These studies suggest the potential efficacy of parasite ES proteins for treating a range of human inflammatory diseases.
Baculovirus-insect cell expression system (BEVS) has stimulated great interest in various therapeutic recombinant protein production (Jarvis, 2009; Lee et al., 2023). The BEVS with advantages of high safety and cost-effectiveness have been considered as a competitive commercial manufacturing platform for vaccines and gene therapy vectors. Currently, vaccines produced using BEVS have been successfully marketed for the influenza virus, HPV, SARS-CoV-2, and some animal viruses (Hong et al., 2023). To date, eleven BEVS products have been approved as therapeutics, including four human vaccines (Cervarix, Flublok, Flublok Quadrivalent, and Nuvaxovid/Covovax), two human therapeutics (Provenge and Glybera), and five veterinary vaccines (Porcilis Pesti, BAYOVAC CSF E2, Circumvent PCV, Ingelvac CircoFLEX and Porcilis PCV) (Hong et al., 2022).
This study aims to optimize the best conditions of expression of AIP-1 and AIP-2 using BEVS and evaluate their inhibitory function against the human MMPs.
Materials and methods
Generation of AIP-1 and AIP-2 gene expression cassettes
The synthetic DNA sequences encoding AIP-1 (GenBank number: AAK58952.1) and AIP-2 (GenBank number: ACB13195.1) were fused to 6x histidine tag and KDEL ER retention motif to produce the recombinant proteins AIP-1 and AIP-2. The genes encoding AIP-1 and AIP-2 were cloned under the control of the polyhedrin promoter (PPH) in a baculovirus expression vector (pFastBac1 vector; Thermo Fisher Scientific, Waltham, MA). AIP-1 had BamHI and EcoRI restriction enzyme sites and AIP-2 had EcoRI and HindIII restriction enzyme sites at 5′ and 3′ ends. The two recombinant vectors were transformed into DH5α Escherichia coli competent cells, respectively.
DH10Bac E. coli transformation and Bacmid PCR
The recombinant plasmids in DH5α E. coli were isolated with a FavorPrep™ Plasmid DNA Extraction Mini Kit (Favorgen, PingTung, Taiwan). The purified plasmid DNA was transformed into DH10Bac E. coli cells for transposition into the bacmid. LB agar plates for blue/white colony selection were contained to 50 μg/mL kanamycin, 7 μg/mL gentamicin, 10 μg/mL tetracycline, 100 μg/mL Blue-gal and 40 μg/mL isopropyl β-d-1-thiogalactopyranoside (IPTG), these were used to select for transformants. Bacmids containing the AIP-1 and AIP-2 gene expression cassettes were isolated from transformed DH10Bac E. coli cells using FavorPrep™ Plasmid DNA Extraction Midi Kit (Favorgen). To confirm the transposition of AIP-1 and AIP-2 gene expression cassettes, Bacmid PCR was conducted according to the manufacturer's recommendations (Thermo Fisher Scientific).
Bacmid transfection into Sf9 insect cells
Sf9 insect cells (Thermo Fisher Scientific) were used as transfection carriers to produce baculovirus, were cultured in 30 mL of Sf900II SFM (Gibco, Gaithersburg, MD) containing 10% penicillin (Welgene, Gyeongsan, Gyeongsangbuk-do, Korea) in culture flask at 140 rpm at 28°C incubator. The Sf9 cells were seeded with 1 × 106 cells/well in 6-well plates (SPL, Seoul, Korea) and incubated at room temperature for 1 h to permit attachment of the cells. AIP-1 and AIP-2 bacmids (2 μg each) were mixed with 10 μL of ExpiFectamine™ Sf Transfection Reagent (Gibco) in 200 μL of Opti-MEM (Gibco) and incubated for 5 min at room temperature. Three mL of antibiotic-free medium and 200 μL of the bacmid mixture were added to every well, and the plate was incubated at 28°C for 72 h to generate passage P0 recombinant baculoviruses.
Generation of P2 recombinant baculovirus
At 72 h post-transfection, the virus-containing medium from each well (∼3 mL) was collected and transferred to a sterile 50 mL tube. The tubes were centrifuged at 1,200 rpm for 5 min, and the P0 baculovirus supernatant was filtered through a syringe-driven filter unit [0.45 μm, polyvinylidene fluoride (PVDF); GVS, Rome, Italy] into a fresh 50 mL tube and stored as a P0 viral stock at 4°C. Cells were infected with the P0 viral stock to generate high-titer P1 and P2 baculovirus stocks. 2 mL of Sf9 cells (1 × 106 cells/well) to each well of a 6-well plate were attached in SF900II SFM for 1 h at 28°C, and 1 mL of P0 viral stock was then added to each well and incubated for 72 h at 28°C. The baculovirus-containing medium (∼2 mL) was collected from each well by centrifugation and filtered to obtain the P1 baculovirus. Sf9 cells seeded in a 6-well plate (1 × 106 cells/well) were infected with 1 mL of P1 baculovirus for 72 h at 28°C to produce P2 baculovirus by the same process.
Measurement of AIP-1 and AIP-2 P2 baculovirus titration values by plaque assay
Plaque assay was used to determine the baculovirus titration values of the AIP-1 and AIP-2 P2 baculovirus stocks according to previously described methods (Lee et al., 2023). Briefly, plaquing media were made by combining 30 mL of Sf-900 medium (1.3×) (Gibco) with 10 mL of microwave-dissolved 4% agarose gel with gentle mixing before the experiment. The plaquing medium was then placed in a 40°C water bath until needed. 2 mL of Sf9 cells (1 × 106 cells/well) to each well of a 6-well plate were attached in SF900II SFM for 1 h at 28°C. After the medium was removed from each well, 1 mL of diluted virus (Virus-free medium, 10−4, 10−5, 10−6, and 10−7) was added to each well and incubated for 1 h at room temperature. The inoculum was removed, 2 mL of plaquing media was added, and was allowed to harden at room temperature for 1 h. The plate was incubated at 28°C for 4 days. To count the plaques in red monolayer, Neutral Red overlay process was performed by previously described methods. The virus titer was calculated based on the following formula: titer (pfu/mL) = number of plaques × dilution factor ÷ mL of inoculum/well.
Optimization of MOI for the expression of AIP-1 and AIP-2 in Sf9 and High-five insect cells
Given the results of the titer assay, insect cells were infected at different MOI (0.05, 0.1, 0.5, 1 or 3) according to previously described methods (Moussavou et al., 2018). The required volume of P2 viral stock for infecting the Sf9 and High-five cells at different MOI values was estimated using formula: inoculum required (mL) = [MOI (pfu/mL) × number of cells)/(titer of viral stock (pfu/mL)] (Table 1). 10 mL of Sf9 and High-five cells (1 × 107 cells, respectively) was infected with P2 viral stock at different MOI values in an incubator for 24 h or 48 h at 28°C and 140 rpm. The cells were collected by centrifugation (12,000 rpm for 10 min at 4°C), and the pellets were lysed with 1 × PBS by sonication (amplitude 50%, 30 sec). The lysed cells were centrifuged (15,000 rpm for 30 min at 4°C) to obtain the cell lysates.
| Vectors | Virus Titer (pfu/mL) | MOI | Required inoculum (mL) |
|---|---|---|---|
| AIP-1 | 3.9 × 107 | 0.05 | 0.0128 |
| 0.1 | 0.0256 | ||
| 0.5 | 0.128 | ||
| 1 | 0.256 | ||
| 3 | 0.768 | ||
| AIP-2 | 2.5 × 107 | 0.05 | 0.02 |
| 0.1 | 0.04 | ||
| 0.5 | 0.2 | ||
| 1 | 0.4 | ||
| 3 | 1.2 |
- MOI: Multiplicity of infection.
Immunoblot for AIP-1 and AIP-2 proteins in baculovirus-infected insect cell lysate
The insect cells infected with AIP-1 and AIP-2 P2 baculoviruses was lysed according to previously described methods (Lee et al., 2023). Briefly, High-five insect cells (Thermo Fisher Scientific) were inoculated into a sterile flask (1 × 106cells/mL). AIP-1 (3.9 × 107 pfu/mL) and AIP-2 (2.5 × 107 pfu/mL) P2 baculovirus was added into High-five insect cells, it was infected using a MOI of 3 pfu/cell for 48 h. Cells were collected by centrifugation (12,000 rpm for 10 min at 4°C), and dissolved by sonication (amplitude 50%, 30 sec) in lysis buffer (50 mM sodium phosphate buffer, 300 mM NaCl, 10 mM imidazole, pH 8.0). The lysed cells were centrifuged (15,000 rpm for 30 min at 4°C) to obtain the cell lysate. The supernatants were passed through 0.45 μm and 0.22 μm PVDF syringe-based filters (GVS).
Insect cell lysates (Sf9 and High-five) were boiled for 5 min with 5 × sample buffer (Genscript Biotech, Piscataway, NJ) and cooled for 10 min. Proteins were separated by 15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a nitrocellulose (NC) membrane (Cytiva, Marlborough, MA). The membranes were incubated with blocking buffer [5% skim milk in Tris-buffered saline (TBS)] for 2 h at room temperature, and then incubated overnight at 4°C with rabbit anti-polyhistidine tag-specific antibody (Bethyl Laboratories, Montgomery, TX) diluted 1:5000 in blocking buffer. The membranes were incubated for 2 h at room temperature with goat anti-rabbit IgG H + L (Bethyl Laboratories), after three washes, protein bands were detected with EzWestLumi plus chemiluminescence substrate (Atto, Tokyo, Japan). Multi-tag protein included 6x histidine tag (Genscript) was used as a positive control, and insect cell lysate infected with no virus and lysate infected with an empty pFastBac1 vector were used as negative controls.
Purification of AIP-1 and AIP-2 expressed in baculovirus-infected High-five cells
The insect-cell derived AIP-1 and AIP-2 were purified by Immobilized Metal Affinity Chromatography (IMAC) according to previously described methods (Kielkopf et al., 2020; Shestak et al., 2023). After adding WorkBeads™ NiMAC resin (Bio-works, Uppsala, Sweden) to Poly-Prep Chromatography Columns (Bio-Rad, Hercules, CA), the solution was washed with binding buffer (50 mM sodium phosphate buffer, 300 mM NaCl, 10 mM imidazole, pH 8.0). After filtered cell lysates were applied, it was washed five times using a washing buffer (50 mM sodium phosphate buffer, 300 mM NaCl, 20 mM imidazole, pH 8.0). Elution buffer (50 mM sodium phosphate buffer, 300 mM NaCl, 300 mM imidazole, pH 8.0) was passed into a microcentrifuge tube for protein elution. The purified protein was dialyzed overnight at 4°C using 1 × PBS. Aliquots of purified protein were stored at −80°C for further studies.
SDS-PAGE and immunoblot analysis of purified AIP-1 and AIP-2
Purified AIP-1 and AIP-2 proteins were boiled for 5 min in 5 × protein sample buffer and cooled for 10 min. Proteins were separated by 15% SDS-PAGE gel or transferred to a nitrocellulose (NC) membrane (Cytiva). The separated SDS-PAGE gels were stained Coomassie brilliant blue for 1 h. The purity and concentration of the purified protein were compared with the band size of bovine serum albumin (BSA) diluted to various concentrations. The blots were conducted for the purified AIP-1 and AIP-2 proteins in the same process as above.
Measurement of human MMPs inhibition activity for AIP-1 and AIP-2 proteins
Inhibitory activity of the purified insect cell-derived AIP-1 and AIP-2 were measured by MMP inhibitor profiling kit (Enzo, Farmingdale, NY) to examine the specificity of inhibitors against a panel of human MMPs. 1.5 μg of the purified insect cell-derived AIP-1 and AIP-2 were incubated with each MMP for 30 min before adding substrate thiopeptide. Each sample was run in duplicates on a 96 well plate. Plate was continuously read at 412 nm in a microplate reader. Data was recorded at 1 min interval for 20 min. N-Isobutyl-N-(4-methoxyphenyl sulfonyl) glycyl hydroxamic acid (NNGH) as a prototypic control inhibitor was included as a positive control.
Result
Construction of AIP-1 and AIP-2 protein expression cassettes
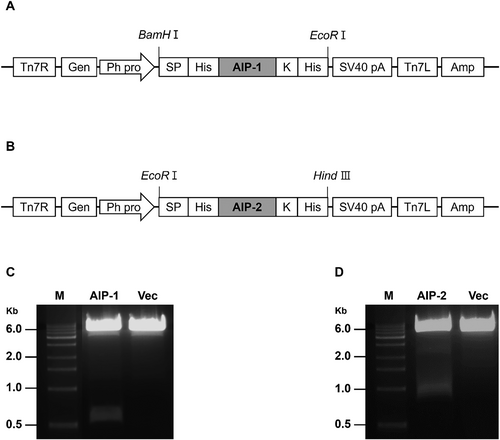
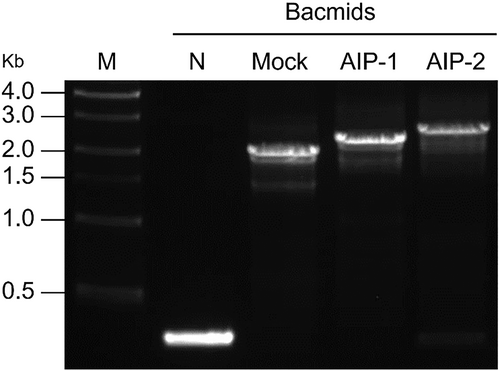
AIP-1 and AIP-2 were cloned into pFastBac1 vector under the control of polyhedrin promoter (PPH) and SV40 polyadenylation signal (Fig. 1(A), 1(B)). The size of these genes was approximately 510 bp and 822 bp for the AIP-1 and AIP-2, respectively (Fig. 1(C), 1(D)). The baculovirus-insect cell expression vector carrying the AIP-1 and AIP-2 gene expression cassettes were transposed into the baculovirus genome. To confirm whether a recombinant bacmid contained the AIP-1 and AIP-2 genes, PCR was performed according to manufacturer recommendation. PCR bands were confirmed at all experimental groups. The PCR band for Bacmid-AIP-1 and Bacmid-AIP-2 were approximately 2,800 bp and 3,100 bp, respectively. However, in negative control (N), a PCR band was observed at approximately 300 bp. The bacmid of the empty vector for the gene of interest in pFastBac1 (Mock) yielded a PCR band at approximately 2,300 bp. This is the expected size for the empty vector without any inserted gene. These results suggest that Bacmid-AIP-1 and Bacmid-AIP-2 contained the inserts that increased its size compared to the empty vector (Fig. 2).
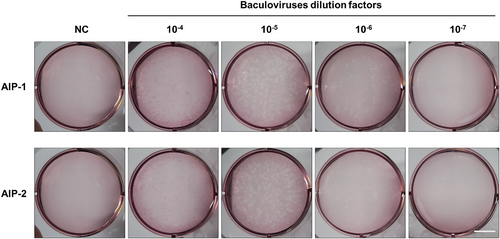
Determination of AIP-1 and AIP-2 P2 baculovirus titration values
Plaque assay was performed to determine the baculovirus titration values of the AIP-1 and AIP-2 P2 baculovirus stocks. In 10−6 dilution factor, the number of AIP-1 P2 baculovirus plaques were 39, and AIP-2 P2 baculovirus plaques were 25. As a result of calculation based on a known formula, the obtained AIP-1 P2 baculovirus titer value was 3.9 × 107 pfu/mL, and AIP-2 P2 baculovirus titer value was 2.5 × 107 pfu/mL (Fig. 3).
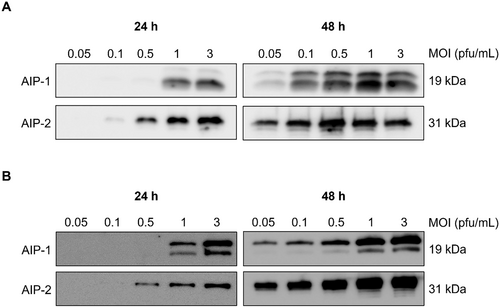
Optimization of MOI and time for the expression of AIP-1 and AIP-2 in Sf9 and High-five insect cells
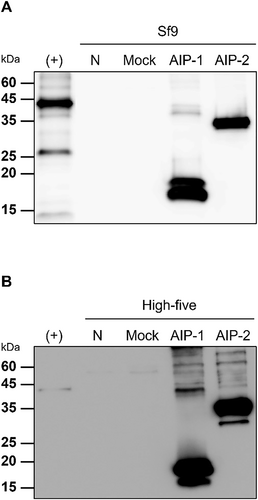
AIP-1 and AIP-2 were detected in Sf9 and High-five insect cell lysates, with variations based on MOI and time conditions (24 h or 48 h). Both Sf9 and High-five cell lysates, AIP-1 expression was predominantly observed when an MOI of 1 was applied at 24 h post-infection, and AIP-2 expression was mainly detected when an MOI of 0.5 was applied at 24 h post-infection (Fig. 4). Concerning protein expression levels, AIP-1 and AIP-2 were consistently present in both Sf9 and High-five cell lysates, showing higher expression levels at 48 h post-infection compared to 24 h. In the comparison of protein expression levels for AIP-1 and AIP-2 within each cell line, both proteins were expressed more in the High-five cell line than in the Sf9 cell line (Fig. 4). The experiment suggested a time-dependent increase in AIP-1 and AIP-2 expression under these conditions.
The conditions for the highest expression of AIP-1 and AIP-2 in the cell lysate of each cell line were identified. Western blot analysis was performed to confirm the expression conditions of both proteins expressed in Sf9 and High-five insect cell lysates. The best conditions for high expression of AIP-1 were an MOI of 1 at 48 h post-infection in Sf9 cells, and an MOI of 3 at 48 h post-infection in High-five cells (Fig. 5). In AIP-2, the MOI of 0.5 at 48 h post-infection in Sf9 cells, and the MOI of 3 at 48 h post-infection in High-five cells were the best conditions (Fig. 5).
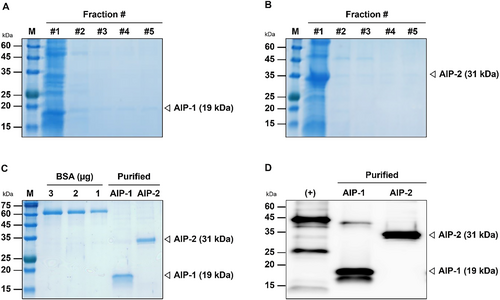
Purification of AIP-1 and AIP-2 proteins by Immobilized Metal Affinity Chromatography (IMAC)
AIP-1 and AIP-2 were purified and fractionated through Immobilized Metal Affinity Chromatography (IMAC). SDS-PAGE showed that the fractionated protein sizes of the AIP-1 and AIP-2 were approximately 19 and 31 kDa, respectively (Fig. 6(A), 6(B)). The protein bands of the purified AIP-1 and AIP-2 were compared with 1, 2, and 3 μg of BSA (Fig. 6(C)). In Western blot, multi-tag included 6x histidine and insect cell-derived AIP-1 and AIP-2 proteins samples were loaded (Fig. 6(D)). The amount of AIP-1 and AIP-2 protein compared to BSA were 1.351 mg/mL and 0.746 mg/mL, respectively. Protein bands observed in the purified AIP-1 sample appeared slightly stronger than those observed in the multi-tag (50 ng) sample. This suggests that AIP-1 exhibits stronger affinity for the anti-polyhistidine tag-specific antibody used compared to the multi-tag protein. On the other hand, protein bands observed in the purified AIP-2 sample appeared slightly weaker than those observed in the multi-tag (50 ng) sample. This indicates potentially lower binding affinity for the anti-polyhistidine tag-specific antibody compared to the multi-tag protein.
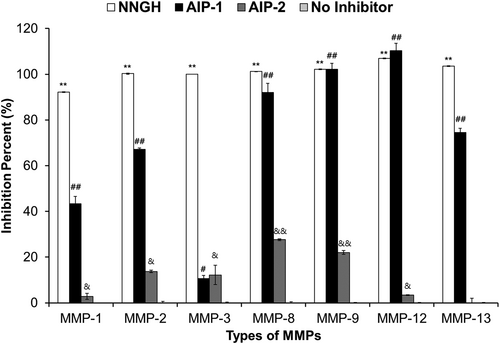
Effect of insect cell-derived AIP-1 and AIP-2 proteins on inhibition of human MMPs activity
To examine the specificity of AIP-1 and AIP-2 against a panel of human MMPs, inhibition activity of the purified insect cell-derived AIP-1 and AIP-2 were measured by MMP inhibitor profiling kit. NNGH as positive control was included as a prototypic control inhibitor. When compared inhibition activity the AIP-1 to AIP-2, AIP-1 showed stronger than AIP-2. AIP-1 showed the strongest inhibitory activity against human MMP-12, the weakest activity against human MMP-3. In contrast, AIP-2 showed the strongest inhibitory activity against human MMP-8, the weakest activity against human MMP-13. AIP-1 demonstrated inhibition activity on MMP-8, MMP-9, and MMP-12, which was similar to that of NNGH, the positive control inhibitor. However, for MMP-1, MMP-2, MMP-3, and MMP-13, the inhibition activity of AIP-1 was slightly lower compared to the NNGH treatment group. In AIP-2, inhibition activity against human MMPs was observed to be approximately 20%. The negative control group, which lacks an inhibitor, showed nearly 0% inhibition activity on human MMPs serves as an important baseline control (Fig. 7). These results indicated that the insect cell-derived AIP-1 and AIP-2 proteins inhibit the activity of mostly human MMPs.
Discussion
The current study demonstrated that AIP-1 and AIP-2 proteins known as human TIMP-like protein in Ancylostoma caninum ES products were produced in BEVS under the optimized best expression conditions, and they inhibited the human MMPs activity. Recombinant baculoviruses carrying AIP-1 and AIP-2 genes were generated by transfection and infection in Sf9 insect cells. The plaque assay was performed to confirm the baculovirus titration values. Virus titers were estimated for infection of Sf9 and High-five insect cells (Table 1). The obtained baculoviruses were infected to establish the best expression conditions of both proteins. The best conditions for AIP-1 and AIP-2 were the MOI of 1 and 0.5 at 48 h post-infection in Sf9 cells, respectively. In High-five cells, the best conditions of both proteins were the MOI of 3 at 48 h post-infection.
The insect cell-derived AIP-1 and AIP-2 were successfully purified using IMAC in baculovirus-infected High-five cells. In IMAC purification analysis, AIP-1 (19 kDa) and AIP-2 (31 kDa) were fractionated, these samples were detected in SDS-PAGE and western blot analyses. Previous studies showed that AIP-1 and AIP-2 proteins are similar with human TIMP from a structural perspective (Zhan et al., 2002; Zhan et al., 2008). Thus, in this study, human MMPs inhibition activity of insect cell-derived AIP-1 and AIP-2 proteins was measured. Insect cell-derived AIP-1 generally showed strong inhibitory activity against human MMPs, whereas insect cell-derived AIP-2 revealed weak inhibitory activity against human MMPs. These findings suggest that both AIP-1 and AIP-2 display distinct inhibitory effects on different human MMPs. AIP-1 demonstrates comparable activity to NNGH for certain MMPs and slightly diminished activity for others. On the other hand, AIP-2 generally exhibits lower inhibitory activity across the board. These results offer valuable insights into the prospective utilization of AIP-1 and AIP-2 as MMP inhibitors.
A. caninum ES products have therapeutic effects in various inflammatory diseases (Cobos et al., 2022). A. caninum ES products are reported that limits intestinal pathology and proinflammatory cytokine expression during dextran sodium sulfate (DSS)-induced colitis. These results indicated that injection of A. caninum ES products in mice induces a robust antigen-specific type 2 cytokine response, including the emergence of a distinct CD4 + T cell population that coexpresses IL-4 and IL-10 and the recruitment of macrophages and eosinophils to the site of injection (Ferreira et al., 2013). The loss of protective effects observed in colitis following the denaturation of A. caninum ES products implies that the immunomodulatory properties of A. caninum ES products are likely attributed, at least in part, to a protein component.
In previous study, treatment of AIP-1 with a single intraperitoneal dose at 1 mg kg−1 in mice was released that colitic inflammation assessed by weight loss, colon atrophy, oedema, ulceration, and necrosis, as well as abdominal adhesion was significantly suppressed. Therefore, AIP-1 treatment promoted the production of colon interleukin (IL)-10, transforming growth factor (TGF)-β and thymic stromal lymphopoietin (TSLP) (Ferreira et al., 2017). In previous study of AIP-2, AIP-2 treatment suppressed airway inflammation in a mouse model of asthma, reduced expression of co-stimulatory markers on human dendritic cells (DCs) and suppressed proliferation ex vivo of T cells from human subjects with house dust mite allergy (Navarro et al., 2016). These results indicated that AIP-1 and AIP-2 can apply to the other inflammatory diseases, and the insect cell-derived AIP-1 and AIP-2 proteins could be applied for inflammatory diseases such as asthma and colitis (Ferreira et al., 2017; Navarro et al., 2016).
In conclusion, the insect cell-derived AIP-1 and AIP-2 were effectively expressed and purified using BEVS demonstrating inhibition activity against human MMPs. These findings suggest that AIP-1 and AIP-2 derived from insect cells hold promise for the treatment of diseases related to the MMP-TIMP axis. For future research, the anti-inflammation effect of insect cell-derived AIP-1 protein should be investigated in inflammatory animal model.
Conflict of Interest
The authors declare no conflict of interest.
Funding
This work was supported by a National Research Foundation of Korea grant funded by the Korean Government (MEST) (NRF- 2023R1A2C100473512).




