A review of entomopathogenic fungi as a potential tool for mosquito vector control: A cost-effective and environmentally friendly approach
Abstract
Mosquitoes around the world spread diseases like malaria, dengue, zika, lymphatic filariasis and arboviruses, which are dangerous to human health and the economy. Eventually, mosquitoes develop resistance to synthetic chemical insecticides and, moreover, these insecticides have adverse environmental impacts, accumulating in soils and in the food chain. So, researchers are searching for better vector control tools from biological sources such as plants, bacteria, fungi, viruses and other predators. Eco-friendly methods that use entomopathogenic fungi to reduce vector-borne disease burdens are becoming more popular because they are selective and safe for the environment. Based on existing literature, several microbial agents show potential for the biocontrol of mosquitoes. With advances in genetic recombination and transformation techniques, in the ongoing battle against insecticide-resistant mosquitoes, genetically engineered fungal biopesticides represent a cutting-edge solution. These biopesticides are the result of novel genetic changes that improve the ability of fungi to target and kill mosquitoes. These fungi can effectively combat mosquito populations by introducing genes that produce insecticidal proteins or toxins. This method has several advantages, including a lower environmental impact, because the fungi are highly specific to mosquitoes and are harmless to non-target organisms. It also helps to reduce the problem of insecticide resistance because the fungi have a unique mode of action. These biopesticides hold great promise for reducing mosquito-borne diseases while minimizing environmental damage and combating resistance. This review article discusses various entomopathogenic fungal pathogens that can act as biocontrol agents and their mode of action against mosquitoes. We discus recent advances in entomopathogenic fungi-secreted effector molecules for suppressing host immunity and progress in the development of transgenic mosquito-killing fungi.
Introduction
An overview of mosquito-borne diseases and their public health implications
Malaria is one of the most lethal mosquito-borne diseases, caused by Plasmodium parasites spread by Anopheles mosquitoes. The World Health Organization (WHO 2021) estimates that there will be 241 million malaria cases and 627 000 malaria-related deaths in 2020, highlighting the ongoing burden of the disease (World Malaria Report 2021). Dengue fever is caused by the dengue virus and is spread by Aedes mosquitoes (Kumar et al. 2021). According to the US Centers for Disease Control and Prevention, dengue fever is a significant global health threat, with an estimated 390 million infections occurring each year (Kumar et al. 2021). Because of its potential link to birth defects, the Zika virus, which is primarily transmitted by Aedes mosquitoes, has gained international attention. Despite the fact that Zika outbreaks have decreased, the disease remains a public health concern (Hills et al. 2021). Chikungunya is a virus spread by Aedes mosquitoes that causes severe joint pain and fever. The prevalence of this disease has increased in recent years in many regions, including Asia and the Americas (Mourad et al. 2022). Yellow fever is spread by Aedes mosquitoes in cities, and by Haemagogus or Sabethes mosquitoes in the jungle. It can cause severe illness and outbreaks of the disease have serious health and economic consequences (Gaythorpe et al. 2021). West Nile virus is primarily transmitted by Culex mosquitoes and is a growing public health concern, with outbreaks occurring in North America and other parts of the world. Thousands of cases and deaths have been reported in the USA, for example (Lorenz et al. 2022).
These diseases place a significant burden on healthcare systems, disrupt economies and impede development efforts, particularly in endemic areas. Furthermore, urbanization, climate change and international travel all contribute to the emergence and re-emergence of mosquito-borne diseases, posing ongoing challenges for public health officials. Mosquito control, public awareness campaigns, and the development of vaccines and antiviral treatments are all part of the fight against these diseases. New control methods, such as genetically engineered entomopathogenic fungi, are being studied in the hopes of developing more effective strategies to reduce the burden of mosquito-borne diseases and improve public health outcomes (Fig. 1).
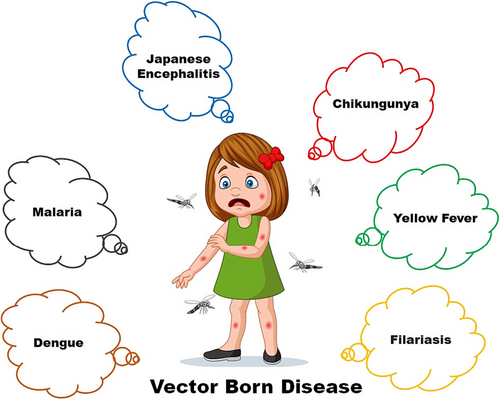
Limitations of existing mosquito control methods, such as insecticides
Mosquitoes can develop resistance to commonly used chemical insecticides (Liu 2015; Vivekanandhan et al. 2021). It has been discovered that Aedes mosquitoes, which spread diseases such as dengue and Zika, are mostly resistant to pyrethroid insecticides (Ranson et al. 2010). Non-target organisms, such as beneficial insects, aquatic life and other wildlife, can be harmed by chemical insecticides (Sánchez-Bayo 2012). Insecticides are harmful to aquatic communities, according to a study conducted by Bunzel et al. (2013). In England, long-term population changes in wild bees have been observed with neonicotinoid use (Woodcock et al. 2016). The widespread use of chemical insecticides contributes to pollution in the environment. According to Vivekanandhan et al. (2021), Anopheles mosquitoes can develop resistance to temephos, a non-systemic organophosphorus insecticide that often provides only short-term relief and may not provide long-term mosquito control. The limitations of insecticide-based mosquito control and the need for integrated approaches are discussed by Chaudhry et al. (2019). Concerns have been raised about the impact of chemical insecticides on public health. A study investigated the link between pesticide exposure and adverse health effects (Gunier et al. 2017). Maintaining and applying insecticides can be expensive and may not be a long-term solution.
Hemingway (2018) discussed the need for more cost-effective and long-term vector control strategies for mosquito-borne diseases. Because of these issues, we require new and long-lasting mosquito control methods, such as genetically modified entomopathogenic fungi, which can kill mosquitoes specifically and for an extended period of time, while having fewer negative effects on the environment and people's health than traditional insecticides.
An overview of genetically modified entomopathogenic fungi as a possible substitute
In recent years, genetically engineered entomopathogenic fungi have emerged as a promising and novel mosquito control option worldwide. Precision mosquito targeting is made possible by genetically engineered entomopathogenic fungi such as Beauveria and Metarhizium species. Researchers can improve the ability of these fungi to infect and kill specific mosquito species responsible for disease transmission, such as Aedes, Anopheles and Culex mosquitoes, by modifying them (Lacey et al. 2015). Engineered fungi have a distinct mode of action. They infect mosquitoes and other insect pests, eventually killing them. This biological control method lowers the risk for the development of resistance, which is a common issue with chemical insecticides (Lovett et al. 2019a). Fungi that have been genetically engineered are environmentally friendly and biodegradable. They have low toxicity to non-target organisms and do not persist in the environment, making them a safer and more sustainable alternative to chemical pesticides (St. Leger & Wang 2020).
Implementing these fungi can reduce the reliance on chemical insecticides, thereby reducing the negative environmental and public health consequences of pesticide use (Yakubu et al. 2021). Entomopathogenic fungi that have been genetically engineered can provide long-term mosquito control. They can survive in the environment, infecting and killing mosquitoes for an extended period of time, and potentially reducing disease transmission (Wang et al. 2023). The use of these fungi has the potential to significantly reduce the burden of mosquito-borne diseases, particularly in endemic areas. Genetic engineering is a rapidly evolving field. The efficacy, safety and target specificity of these fungi are constantly being improved by researchers, paving the way for more effective mosquito control methods (Fang & St. Leger 2010). Finally, genetically engineered entomopathogenic fungi appear to be a promising alternative to traditional mosquito control methods. These biopesticides represent an innovative approach to combating mosquito-borne diseases because of their precision, unique mode of action and low environmental impact. Ongoing research and development in this field is propelling advancement and demonstrating the potential for significant public health benefits and long-term mosquito control.
Entomopathogenic fungi: an overview and their use in pest management
Entomopathogenic fungi are a type of entomopathogen, or organism, that causes disease in insects (Krutmuang et al. 2023; Perumal et al. 2023a, 2023b). Unlike many other insect pathogens, entomopathogenic fungi are only found in insects. They are members of several fungal genera, the most well-known of which are Beauveria, Cordyceps and Metarhizium (Krutmuang et al. 2023; Perumal et al. 2023a, 2023b). These fungi co-evolved with insects and developed a variety of strategies for infecting and parasitizing their hosts.
Mode of action
Entomopathogenic fungi typically attach to the cuticle (exoskeleton) of the insect host using specialized adhesive structures, such as conidia (spores) or appressoria. Once attached, the fungus penetrates the insect's cuticle through mechanical pressure or the secretion of cuticle-degrading enzymes. This early infection stage allows the fungus to enter the insect's body and then multiply (Altinok et al. 2019). The fungus colonizes the insect's hemocoel, or circulatory system, and releases enzymes and toxins that break down the insect's tissues and converts them into nutrients for the fungus. In the end, the infection kills the host and new fungal structures, such as conidiophores, which produce infectious spores, emerge (Altinok et al. 2019).
Diversity and classification
Entomopathogenic fungi are extremely diverse and have been discovered in a wide range of environments, from forests to agricultural fields. They are classified into several genera. Beauveria species are used in biological control and are effective against a wide range of insects, including beetles, mosquitoes, moths and aphids (Rajula et al. 2020; Zimmermann 2007). Metarhizium is another well-studied genus containing species that are effective against a wide range of insect pests. Commercial biopesticides frequently contain Metarhizium species (Perumal et al. 2023a, 2023b). Cordyceps fungi are well known for their entomopathogenic behavior, and they are especially notable for their host specificity. Different species of Cordyceps specifically target certain insect species (Zimmermann 2007, 2008).
Ecological importance
Entomopathogenic fungi are important in natural ecosystems (Meyling & Eilenberg 2007). They aid in the regulation of insect populations and maintain balance in insect communities. Furthermore, they are regarded as important components of soil ecology, where they can survive as saprophytes, feeding on organic matter and debris in the soil (Meyling & Eilenberg 2007).
Agricultural and forestry applications
Entomopathogenic fungi have long been recognized as potential biological control agents. These fungi provide an environmentally friendly and sustainable alternative to chemical pesticides in agriculture. They are used as biopesticides, killing a variety of crop-damaging insects like aphids, thrips and whiteflies. Entomopathogenic fungi, when used correctly, can reduce pest populations and minimize the negative impacts of conventional chemical pesticides on beneficial insects and the environment (Bamisile et al. 2021).
Entomopathogenic fungi have been used in forestry to control forest pests such as bark beetles. These pests can devastate forest ecosystems and cause economic losses in the timber industry. The use of entomopathogenic fungi provides a targeted and effective method of pest management (Bamisile et al. 2021).
Public health significance
Entomopathogenic fungi play an important role in public health in addition to agriculture and forestry. Several entomopathogenic fungal species are used in mosquito control programs. Genetically engineered strains of Beauveria and Metarhizium have been developed to increase their pathogenicity against disease-transmitting mosquito species. This breakthrough holds great promise for reducing mosquito-borne diseases, particularly in endemic areas.
Insect-pathogenic fungi and mosquito control
The action of entomopathogenic fungi against several mosquito species have been documented under laboratory conditions. The mosquitocidal activity of soil-borne entomopathogenic fungal spores and their secondary metabolites was recently investigated (Vivekanandhan et al. 2020a, 2020b; Vivekanandhan et al. 2022). Entomopathogenic fungal virulence and mosquito pathogenicity were noted (Table 1). Entomopathogenic fungi are classified as aquatic or soil-dwelling microorganisms. The divisions of entomopathogenic fungi are Ascomycota, Chytridiomycota, Deuteromycota, Oomycota and Zygomycota. The most frequent genera of insect pathogenic fungi are Zygomycota or Hyphomycetes/Deuteromycota (Scholte 2004; Vivekanandhan et al. 2018a). Several studies have investigated the action of entomopathogenic fungi against mosquitoes, including Ascocarpic sp., Beauveria sp., Culicinomyces sp., Hirsutella sp., Isaria sp., Lagenidium sp., Lecanicillium sp., Metarhizium sp., Myriangiales sp., Paecilomyces sp. and Tolypocladium sp. (Samson et al. 1988; Vivekanandhan et al. 2018a, 2018b; Balumahendhiran et al. 2019; Logeswaran et al. 2019; Vivekanandhan et al. 2020a; Vivekanandhan et al. 2022). The entomopathogenic fungi mentioned above are virulent in the field and under laboratory conditions against mosquito larvae such as Aedes sp., Anopheles sp. and Culex sp. (Scholte et al. 2003; Scholte et al. 2004; Greenfield et al. 2015; Vivekanandhan et al. 2020b; Hamama et al. 2022). Today, only a few (Beauveria and Metarhizium species) entomopathogenic fungi-based effective insecticides have been commercialized worldwide (Li et al. 2010).
| Fungal species | Mosquito host | Infection stage | Reference |
|---|---|---|---|
| Metarhizium anisopliae | Aedes aegypti | Pupae | Carolino et al. (2019) |
| Metarhizium anisopliae | Aedes aegypti | Larvae | Butt et al. (2013) |
| Beauveria bassiana, Lecanicillium lecanii and Metarhizium anisopliae | Aedes aegypti, Anopheles stephensi and Culex quinquefasciatus | Larvae | Vivekanandhan et al. (2020a) |
| Aspergillus niger, Aspergillus fumigatus, Nattrassia mangiferae, Penicillium sp. and Trichoderma sp. | Anopheles maculipennis, Culex deserticola, Culex perexiguus and Culiseta longiareolata | Larvae | Moosa-Kazemi et al. (2021) |
| Metarhizium anisopliae | Anopheles gambiae and Culex quinquefasciatus | Adult | Seye et al. (2012) |
| Metarhizium anisopliae | Anopheles gambiae | Adult | Scholte et al. (2008) |
| Metarhizium robertsii | Aedes aegypti | Larvae | Noskov et al. (2019) |
| Beauveria bassiana | Anopheles coluzzii | Adult | Valero-Jiménez et al. (2014) |
| Beauveria bassiana | Anopheles stephensi | Adult | Kanzok and Jacobs-Lorena (2006) |
| Beauveria bassiana, Isaria farinosa, Isaria flavovirescens, Isaria fumosorosea, Lecanicillium sp. and Metarhizium anisopliae | Aedes aegypti | Adult | Darbro et al. (2011) |
| Beauveria bassiana and Metarhizium robertsii | Anopheles gambiae | Larvae | Fang and St. Leger (2012) |
| Beauveria bassiana | Culex quinquefasciatus | Larvae | Vivekanandhan et al. (2018a) |
| Metarhizium anisopliae | Aedes aegypti and Aedes albopictus | Adult | Scholte et al. (2007) |
| Metarhizium anisopliae | Anopheles gambiae | Adult | Mnyone et al. (2011) |
| Beauveria bassiana | Anopheles gambiae | Adult | Yassine et al. (2012) |
In the future, more research must be carried out on Beauveria, Isaria, Metarhizium and Verticillium species because these are the most potentially effective agents for controlling pests. So far a few genera of entomopathogenic fungi have been focused upon, based on the interests of the researchers: Metarhizium anisopliae (Butt et al. 2013; Carolino et al. 2019); Trichoderma (Sundaravadivelan & Padmanabhan 2014); Tolypocladium (Rocha et al. 2015); Beauveria bassiana (Valero-Jiménez et al. 2014); Aspergillus fumigatus, Aspergillus niger, Nattrassia mangiferae, Penicillium sp. and Trichoderma sp. (Moosa-Kazemi et al. 2021); Metarhizium robertsii (Noskov et al. 2019); B. bassiana, Isaria farinosa, Isaria flavovirescens, Isaria fumosorosea, and Lecanicillium sp. and M. anisopliae (Darbro et al. 2011); and B. bassiana and M. robertsii (Fang & St. Leger 2012) (Table 1). The four most attractive fungal species are M. anisopliae, Metarhizium brunneum, Metarhizium pingshaense and M. robertsii, because of their enormous traits, high efficacy against insect pests, safety with respect to non-target species, easy production and tolerance to a wide range of temperatures (0–30°C) (Chang et al. 2021; Vivekanandhan et al. 2022, 2023a, 2023b). Also, to control malarial disease-causing mosquitoes, entomopathogenic fungi like B. bassiana and Metarhizium species have been widely used (Ortiz-Urquiza et al. 2015; Pattnaik et al. 2018; Accoti et al. 2021).
Important entomopathogenic fungi genera for mosquito control
Metarhizium
Metarhizium fungal species (Hypocreales: Clavicipitaceae) are common ascomycetous fungi that are well known as biocontrol agents for different kinds of insects (Vivekanandhan et al. 2022; 2023a, 2023b, 2023c). Species of this genus are commonly found in soils and plants endophytically, where they function primarily as soil rhizosphere microorganisms and are pathogenic to insect pests. The species Metarhizium acridum, M. anisopliae, M. brunneum, Metarhizium globosum, Metarhizium guizhouense, Metarhizium lepidiotae, Metarhizium majus, M. pingshaense and M. robertsii demonstrated high pathogenicity against mosquitoes and agricultural insect pests (Bischoff et al. 2009; Mayerhofer et al. 2015; Vivekanandhan et al. 2018b).
According to Mongkolsamrit et al. (2020) and Thanakitpipattana et al. (2020), the host range of these fungal species has been described as having a high degree of variability. For example, M. anisopliae and M. robertsii have the ability to infect or colonize a wide variety of hosts. These hosts include mosquitoes, lepidopteran insect species, wireworms, plants (as endophytes), fruit flies and herbivorous arthropods (Luz et al. 2019; Mathulwe et al. 2022). Metarhizium fungal species can produce conidia (asexual spores) on conidiophores in adult insects and larvae, thereby initiating mycosis. During the infection stage, certain Metarhizium fungal species produce toxins known as cyclic hexadepsipeptides. These toxins are responsible for a high mortality rate in a variety of insect pests.
Beauveria
The genus Beauveria (Ascomycota) contains four species: Beauveria amorpha, B. bassiana, Beauveria brongniartii and Beauveria caledonica (Bustamante et al. 2019). Among these species, B. bassiana has excellent pathogenic properties for over 700 insects, including arthropods and disease vectors (such as mosquito species) (Vivekanandhan et al. 2018b; de Souza et al. 2020). Beauveria entomopathogenic fungi species are commonly responsible for white muscardine disease in insect pests. When the infections have taken hold, the host exoskeleton is completely covered with mycelium and synnemata structures. Beauveria fungi species produce virulent spores, which can be more pathogenic to mosquitoes and other insect pests (Bustamante et al. 2019; Krutmuang et al. 2023). Beauveria fungi species produce toxins through secondary metabolism, which increases their virulence against the host (Vivekanandhan et al. 2018b; Krutmuang et al. 2023). Bassiatin, bassianolide, beauvericin, beauverolides, oosporein and tenellin are some examples of these toxins (de Souza et al. 2020). Habitat, soil properties (e.g., soil moisture, pH, organic matter and chemical properties), humidity and temperature all influence the prevalence and infectivity of this genus in agroecosystems (Altinok et al. 2019; Bamisile et al. 2021).
Isaria
The genus Isaria (Ascomycota) was taxonomically revised from the genus Paecilomyces in 2005 owing to similar morphological properties (Luangsa-Ard et al. 2005; Zimmermann 2008; Mongkolsamrit et al. 2018). This genus currently contains five species: Isaria cateniannulata, I. farinosa, I. flavovirescens, I. fumosorosea and Isaria javanica. Among these, I. fumosorosea and I. javanica have received the most attention and have been used as mycoinsecticides to control soft-skinned insect species such as mosquitoes and other pests (Luangsa-Ard et al. 2005; Zimmermann 2008; Mongkolsamrit et al. 2018; Altinok et al. 2019). Isaria species are found all over the world, in various environmental conditions (e.g., water and soil), and infect a diverse range of hosts (e.g., Diptera, Coleoptera, Lepidoptera, mealy bugs and others) (Shahid et al. 2023). The conidia of this group are typically ellipsoidal to fusiform–elliptical. However, there is little information in the literature about the metabolites and toxins produced by Isaria fungi species.
Lecaninicillium
In 2001, the genus Lecanicillium (formerly Verticillium) was reclassified, and now contains five species: Lecanicillium attenuatum, Lecanicillium lecanii, Lecanicillium longisporum, Lecanicillium muscarium and Lecanicillium nodulosum (Ravensberg 2011). The Lecanicillium fungi species have been isolated from various insect cadavers and have wide range of insect hosts (Woo et al. 2020).
Entomophthora
Entomophthora (Entomophthoromycota) is made up of 21 highly host-specific species that cause epizootics in insects like aphids, beetles, bugs, flies, fungus gnats and midges (Guizar-Guzman & Sanchez-Peña 2013). The most well-known species in this genus is Entomophthora muscae, which can only affect the adult housefly, Musca domestica. Members of this genus generally infect the host, manipulate the host's behavior, and produce or disperse their spores from the living and/or dead host. Members of this genus have not been known to produce mycotoxins. Although Entomophthora is thought to be host-specific, under laboratory conditions members of this genus can infect other hosts. For example, E. muscae has been shown to infect wild Drosophila melanogaster (Guizar-Guzman & Sanchez-Peña 2013; Becher et al. 2018).
Mode of action of entomopathogenic fungi
The globally accepted mode of action of pathogenic microbes in mosquitoes is through their asexual spores, which attach to the exoskeleton of the host body, degrade the cuticle layer of insects containing chitin by the secretion of enzymes and produce mechanical forces to enter the hemocoel of insects (Fig. 2) (Anwar et al. 2017). A detailed study of cuticle penetration by fungi and oomycetes was reported by Shen et al. (2020a). The oral ingestion of microbes by insects as an alternative mode of action has also been studied (Mannino et al. 2019). We support this method of entry into insects based on a number of studies on the oral ingestion of microbes by insects. Conidia of entomopathogenic fungi enter the midgut of mosquito larvae after ingestion (Angleró-Rodríguez et al. 2016). When the conidia and oomycetes were ingested by mosquitoes via water, the digestive tract of the mosquitoes was filled with conidia (Alves et al. 2002).
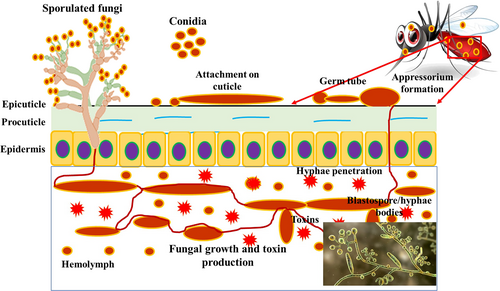
It has been observed that the mycelia of Pythium guiyangense are ingested by Culex pipiens larvae with the feed. This resulted in the destruction of internal tissues and caused the death of the mosquito larvae (Su 2008; Shen et al. 2019; Wang et al. 2019). It confirmed that the ingestion of conidia or mycelia has a unique pathway mechanism. The entomopathogenic fungi M. brunneum can produce conidia, which are asexual spores that can enter mosquitoes in a variety of ways. Mosquito cuticles may become infested with fungal spores upon contact with fungal conidia. They may then penetrate via physical pressure, cuticle-degrading proteins or enzymes. In contrast, mosquitoes have the capacity to consume conidia while feeding on contaminated surfaces, including water or plant matter, that harbor fungal spores. Upon ingestion, the conidia are capable of withstanding the severe conditions of the digestive tract, ultimately infecting the mosquito internally.
This results in the successful invasion and multiplication of the fungal pathogen within the host (Alkhaibari et al. 2016). Several entomopathogenic fungal genes contribute to mosquito death in different developmental stages. Mosquito mortality is facilitated through the encoding of proteins and enzymes by fungal genes that participate in multiple phases of the fungal infection process. The presence of these genes is critical for the entomopathogenic fungi B. bassiana and M. anisopliae to act as pathogenic species towards mosquitoes. Certain pivotal fungal genes are implicated in the control of mosquitoes (de Paula et al. 2008; Kassa et al. 2008; Xiao et al. 2012; Valero-Jiménez et al. 2016). The role of these genes is to degrade enzymes like chitinase, peptidase and proteases to manipulate the insect's immune response and toxin production; ultimately, the insect dies. The factors that contribute to the pathogenicity were MADS-box transcription factor Mcm1, stress sensor protein MrMID2 and actin-regulating kinase MrArk1, which have been investigated in a few studies (Shen et al. 2020a).
The virulent mechanisms involving the secreted proteins, protease inhibitors and protein kinases were revealed during the ingestion of P. guiyangense into the mosquito larvae (Shen et al. 2019). A few family genes, such as kinase and AGC kinase, play critical roles in the virulence of the host regulatory pathway (Hogan & Sundstrom 2009; Peixoto et al. 2010). In P. guiyangense, the silencing of the glycoside hydrolase 18 family genes of chitinase resulted in a reduction in the virulence of the fungi (Shen et al. 2020b). The pathogenicity of glycoside hydrolase 20 and elicitor genes was described previously (Olivera et al. 2016). The Crinkler (CRN) effectors of P. guiyangense were delivered to the host mosquito to induce host cell death (Shen et al. 2019). The A. niger 1-1,3-glucan and M. robertsii metalloprotease inhibit the insect phenoloxidase enzyme system (Butt et al. 2016).
Employing genetic engineering to control mosquitoes with entomopathogenic fungi
With millions of people afflicted by illnesses such as chikungunya, dengue fever, malaria and Zika virus, mosquito-borne diseases present a substantial threat to global health. Conventional approaches to mosquito management, such as the application of chemical insecticides, have encountered many problems, including the emergence of insecticide resistance and apprehensions regarding the potential consequences for the environment and public health. In light of these concerns, genetic engineering has surfaced as a potentially effective method to augment the effectiveness of entomopathogenic fungi in the context of mosquito management. The potential of genetically engineering entomopathogenic fungi to develop sustainable and innovative mosquito control strategies is examined in this article.
Entomopathogenic fungi: an effective green solution
Fungi that kill insects, like some species of Beauveria and Metarhizium, are known to be effective biological pest control agents against a wide range of insect pests, such as mosquitoes. Specialized mechanisms have been developed by these fungi to infect and parasitize insects, rendering them an inherent option for the management of pests. However, in an effort to improve their efficacy in the control of mosquitoes, scientists have resorted to genetic engineering.
Genetically modified entomopathogenic fungi
Entomopathogenic fungi can be genetically altered or given new genes to increase their virulence, effectiveness in killing particular hosts, ability to withstand harsh conditions and other desirable characteristics. By using this method, scientists can modify the fungi to make them more potent against the mosquito species that spread disease while having the least negative effects on non-target organisms.
Boosting virulence
Enhancing the virulence of an entomopathogenic fungus against mosquito vectors is one of the main objectives of genetic engineering. Scientists have inserted genes into entomopathogenic fungi that encode insecticidal proteins. These proteins interfere with the vital biological functions of mosquitoes, and this can kill them when they become infected. For instance, Bacillus thuringiensis (Bt) protein expression can increase the pathogenicity of fungi against mosquitoes (Wang et al. 2022). By altering the metabolic pathways of the fungi through genetic engineering, compounds that increase virulence can be produced. Increased spore production and more effective insect killing are possible outcomes of this strategy (Fang & St. Leger 2010).
Improved host specificity
Researchers have worked to increase the host specificity of genetically modified entomopathogenic fungi to reduce the impact on non-target organisms. This is especially important for biocontrol applications where targeting only certain species of insects is necessary. Promoters, like pheromones or cuticular compounds, that are only activated when certain host insect cues are met can be used to restrict the type of mosquito that the fungi infect, allowing a more targeted attack (Lacey et al. 2015). To reduce the number of unintended consequences, genetic modifications can also be made to ensure that the fungi only infect specific organs or tissues in the insect host (Xu et al. 2018).
Environmental tolerance
Enhancing the resistance of entomopathogenic fungi to environmental stresses is a crucial component of genetic engineering. This entails strengthening their resistance to unfavorable environmental factors like desiccation, UV rays and temperature extremes.
UV resistance
Even in the presence of direct sunlight, genetic modifications that increase UV resistance can keep fungi alive and contagious (Fang & St. Leger 2010).
Improved sporulation
According to Fang and St. Leger (2010), genetic engineering has the potential to enhance sporulation, thereby augmenting the capacity of the fungus to endure and proliferate within its surroundings.
Research and innovations continue
Genetic engineering for entomopathogenic fungi is an ever-evolving field. To improve the effectiveness of these fungi for mosquito control, scientists are looking into different genetic modifications, novel approaches and gene editing techniques like CRISPR-Cas9 (Lovett et al. 2019b). Current investigations center on the following areas.
Enhancement of genetic frameworks
Scientists are trying to make genetic structures that allow entomopathogenic fungi to make proteins that kill insects and develop other traits to enhance the pathogenic action of entomopathogenic fungi.
Field application
Experiments and studies are being conducted in areas where diseases are prevalent to see how well genetically modified fungi work at reducing mosquito populations and preventing disease spread (Wang et al. 2022).
Environmental impact assessment
In addition, ongoing research is investigating the ecological impact of genetically engineered fungi on non-target organisms and ecosystems to ensure their safety and sustainability (Lovett et al. 2019a).
The development and deployment of genetically engineered entomopathogenic fungi for mosquito control must take into account regulatory and safety issues. To ensure the safety and efficacy of these biocontrol agents, regulatory agencies in various countries may require rigorous testing and approval processes. To avoid unintended consequences, ethical and environmental concerns must also be addressed. The genetic engineering of entomopathogenic fungi holds great promise for mosquito control. These modified fungi provide an environmentally friendly and targeted alternative to chemical insecticides. They have the potential to reduce the burden of mosquito-borne diseases, particularly in endemic regions (Lovett et al. 2019a).
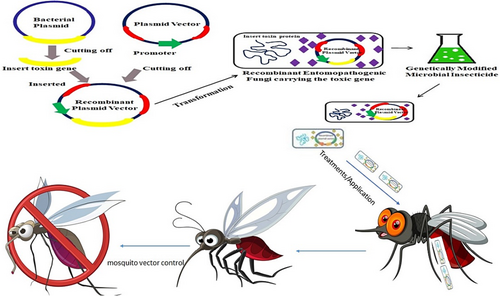
Native microbial strains have not met the World Health Organization Pesticide Evaluation Scheme (WHOPES) qualification for use as a vector control because they have a slow killing mechanism, the dosage requirement is higher and they have no tolerance to environmental stress (Stączek et al. 2020). The researcher's interest in gene recombination and genetic transformation has led to genetically modified entomopathogenic fungi (Lovett & St. Leger 2018; Vogel 2019). In laboratory experiments, transgenic entomopathogenic fungi are used to eliminate mosquitoes in order to prevent malarial transmission and enhance the virulence mechanism of fungal genes to various stresses (Zhao et al. 2016; Karabörklü et al. 2018; Lovett et al. 2019b). Protein engineering can make insect-killing proteins with new properties by combining functional domains from different genes derived from entomopathogenic fungi and other organisms (Zhao et al. 2016). The B. thuringiensis toxin Cyt2Ba was inserted into B. bassiana to improve the killing efficacy of mosquitoes such as Aedes aegypti and Aedes albopictus (Deng et al. 2019). The sodium voltage-gated channel AaIT from the scorpion Androctonus australis was inserted into the fungus M. anisopliae using collagen protein and resulted in a ninefold increase in toxicity against A. aegypti (Vogel 2019; Shen et al. 2020b). The spider venom toxin Mp-hybrid (which blocks calcium-activated potassium channels and voltage-gated calcium channels) was engineered into M. pingshaense (Bilgo et al. 2017). AaIT genes and mixed spider toxins kill mosquitoes at a low dose and are lethal to insecticidal-resistant mosquitoes. Mycoinsecticide has less viability than asexual spores in natural conditions. To improve the tolerance efficacy of fungal biopesticides to different biological stressors and other stressors, genetic engineering was used (Lovett & St Leger 2015). The genes inserted were reviewed, including Halobacterium salinarum CPD photolyase, the dihydroxynaphthalene synthesis pathway of Alternaria alternata, tyrosinase from Aspergillus fumigatus and heat-shock protein (Lovett & St. Leger 2018). When pyruvate and reactive oxygen species (ROS) scavengers were overexpressed in M. robertsii, conidial thermotolerance was increased (Wu & Özkan 2019). In a malaria-endemic area in Burkina Faso, Metarhizium sp., a genetically engineered entomopathogenic fungal biopesticide, was investigated in semi-field conditions. Genetically modified entomopathogenic fungi make Metarhizium species more virulent and eliminate insecticide-resistant malaria mosquitoes effectively, according to the study (Lovett et al. 2019b). Genetically modified Metarhizium species were considered an optimal bioinsecticide as they produce spider-venom toxins and were controlled by a stage-specific promoter, MCL1, which allowed the toxins to only transcribe inside mosquitoes (Lovett et al. 2019b). Employing genetically modified entomopathogenic fungi is the right choice to control disease-transmitting mosquito species in the near future (Fig. 3).
Conclusion, with a look towards the future
Several newly discovered entomopathogenic fungi species have been highlighted in this review. The potential application of an invasion pathway through oral ingestion has drawn particular attention. There has been extensive discussion of the recently discovered pathogenic virulence genes that modulate the immunity of mosquitoes to fungi. Several transgenic mosquito-pathogenic fungi with increased viability have been demonstrated to control mosquito populations in the field (Lovett et al. 2019b). Despite the recent discovery and advancement of mosquito entomopathogenic fungi, some considerations for future mycoinsecticide studies remain: (i) B. bassiana and M. anisopliae are the only native and genetically modified mosquito control fungi that have been extensively studied; and (ii) field application should consider employing transgenic strains of soil-borne entomopathogenic fungi and oomycetes together to target different developmental stages of mosquitoes for better efficiency in the future. In this study, genetically engineered entomopathogenic fungi have been discovered that destroy mosquito defense mechanisms by secreting toxins into mosquitoes. This genetically modified entomopathogenic fungus lends insight into the development of a new mosquito control strategy by disabling mosquito defense mechanisms.
Author contributions
Conceptualization: Vivekanandhan Perumal, Swathy Kannan, Sarayut Pittarate, and Patcharin Krutmuang. Data observation: Vivekanandhan Perumal and Swathy Kannan. Formal analysis: Vivekanandhan Perumal, Swathy Kannan, Sarayut Pittarate, and Patcharin Krutmuang. Investigation: Vivekanandhan Perumal and Patcharin Krutmuang. Resources: Vivekanandhan Perumal and Patcharin Krutmuang. Supervision: Vivekanandhan Perumal and Patcharin Krutmuang. Validation: Vivekanandhan Perumal, Swathy Kannan, Sarayut Pittarate, and Patcharin Krutmuang. Visualization: Vivekanandhan Perumal, Swathy Kannan, Sarayut Pittarate, and Patcharin Krutmuang. Writing—original draft: Vivekanandhan Perumal, Swathy Kannan, Sarayut Pittarate, and Patcharin Krutmuang. Writing—review and editing: Vivekanandhan Perumal, Swathy Kannan, Sarayut Pittarate, and Patcharin Krutmuang.
Acknowledgments
The authors would like to express their gratitude to the Office of Research Administration, Chiang Mai University, and the Department of Entomology and Plant Pathology, Faculty of Agriculture, Chiang Mai University, Thailand, for their support in providing the necessary facilities for this review.
Conflict of interest statement
The authors declare that they have no conflicts of interest associated with this work.
Open Research
Data availability statement
All the review data are presented in the review articles.




