An overview of insect innate immunity
Abstract
Due to the exposure of insects to various sources of pathogens during different stages of their life cycle and their vulnerability towards subsequent infections, moreover lack of morality issues, economical breeding and short-term-life cycle, insect's immunity has been considered as a high-potential candidate to study underlying mechanisms of defense responses against all sort of invaders, as well as pandemic diseases. Currently, the world is enduring monumental pressure to meet global food production demands. One viable option would be to mass rear edible insects, such as coleopteran meal worm Tenebrio molitor, which is thought to be a substantial protein source. In addition, using antimicrobial peptides as an alternative for antibiotic source against multidrug-resistant pathogens, makes insects a valuable option to solve this health issue. As a consequence, sufficient knowledge of insect immunity will lead us to reach advanced diagnostic and treatment technologies. To accomplish these goals, crucial importance of identification and functional characterization of the main signaling pathways, such as Toll and immune deficiency (IMD), prompted us to review the mechanisms of the signaling pathways involved in immune response in well-known insect models.
Introduction
Insects are considered as the conquerors over 400 million years of evolution, from the Devonian period until today, with the largest species diversity (more than 10 million species), having territories in almost all ecological niches (Adams et al. 2000; Ferrandon et al. 2004). It is well-known that there are several reasons for this dominance, such as exoskeleton, flight ability, metamorphosis, adaptation and immunity. The radical importance of insect immunology is due to the identification and characterization of immunological mechanisms and disease etiology and also approaching developed strategies in parasitoids and pest control (Beckage 2011). As using vertebrates, such as mice, in basic scientific and clinical studies raises ethical issues, and is limited by restriction such as economical expenses and long-term protocols, using an alternative study model has been raised drastically. Therefore, among all the distinct options, insects have been chosen frequently in order to be used in different biological studies (Hendriksen 2009; Giacomotto & Ségalat 2010; Canteri De Souza et al. 2018). The lack of antibody production and a complex innate immune system comprising cellular and humoral immunity in insects, makes them a potential candidate for a wide range of scientific experiments (Balls 1994; Doke & Dhawale 2015; Franco 2013; Rollin 2003). Besides, insects are exposed to various pathogenic and parasitic invaders during different phases of their life cycle; speciifically they are highly susceptible to pathogenic infections over the molting period and during ecdysis, when the external cuticle, foregut and hindgut lining are discarded (Beckage 2011). Further to the absence of ethical limitations, comparatively to mammalian models, insect breeding is considerably more economical and sophisticated laboratory equipment is not required. Additionally, large-scale experiments in a short term would be possible due to the short life cycle of insects (Canteri De Souza et al. 2018).
Innate immunity is the initiator of the immune response to pathogenic invaders and constitutes the first line host defense in all metazoans (Shia et al. 2009; Sun et al. 2016; Yang et al. 2018). Microorganism identification via mechanisms involved in proteolytic cascade activation and antimicrobial peptide (AMP) production are the focal points of the innate immune response. Unlike this immune response, adaptive immunity is benefited by memory (Hoffmann 2003). As previously mentioned, insects do not have adaptive immunity; instead, their innate immune response includes both cellular and humoral immune responses. In insects, phagocytosis, nodulation, and encapsulation form a part of the cellular innate immune response, and are performed by hemocytes (insect blood cells). The humoral innate immune response is responsible for clotting, melanin synthesis, and AMPs production, mediated by soluble plasma proteins or fat body (the mammalian liver equivalent) (Hoffmann 2003).
Apparently AMP triggering is highly precise and their induction and expression appear to be noticeably exclusive and dependent on specific infections (Tauszig et al. 2000). For instance, filamentous fungi activate production of drosomycin and metchnikowin, while the production of antibacterial compounds such as diptericin, drosocin, cecropin and attacin is triggered by mostly Gram-negative bacteria. The only example of AMPs produced in response to Gram-positive bacteria is defensin (Hoffmann 2003; Keshavarz et al. 2019; Park et al. 2019; Tauszig et al. 2000). Genetic analysis has revealed that promoter regions of insect AMP genes resemble the binding sites of NF-κB/Rel in mammals as they contain the same nucleotide motifs (Ferrandon et al. 2004; Hoffmann 2003). Hence, two distinctive NF-κB-like signal transduction pathways, Toll and immune deficiency (IMD), are responsible for AMP inductions, activated by fungal and Gram-positive and Gram-negative bacterial infections, respectively (Imler & Zheng 2004).
In the light of all the above matter, being benefited by enhanced knowledge regarding insect immunity is critical and the necessity of a collective review has been felt. Therefore, in the following section, we have reviewed our current understanding of all the ligands and molecular mechanisms mediating immune responses, their structure, mode of action and the signaling pathways they trigger.
Insects and vertebrates' immunology background: similarities and differences
Among Arthropoda, insects, with over a million species, are the largest hexapod group. Various cell/component/process-involved antimicrobial responses rapidly are produced in insects upon infection. The antimicrobial response in insects seems to bear resemblance to the mammalian innate immunity. A group of soluble proteins and receptors, called pattern recognition receptors (PRRs) and proteins, respectively, are responsible for activating the innate immune system. Pathogen-associated molecular patterns (PAMPs) are conserved motifs which are part of the cell wall components, include lipopolysaccharide (LPS) and meso-diaminopimelic acid (DAP)-type peptidoglycan (PGN) of Gram-negative bacteria, lipoteichoic acid (LTA) and lysine (Lys)-type PGN of Gram-positive bacteria, and β-1,3-glucan of fungi were suggested to be sensed by mentioned pattern recognition molecules (Janeway 1989). In addition to its complexity, due to many reasons, the innate immune system differs from the adaptive immune system in many respects. One of these differences is that regardless of any organism or predecessor, antibodies directly bind to the epitopes through epitope-specific binding. Over ontogeny, through clonal selection, self-recognizing cells which produce antibodies are removed and ultimately the basis of immunological memory is produced by antibody-producing immune cells. All the aforementioned phenomena are exclusive to the vertebrate adaptive immune system and cannot be found in innate immunity of vertebrates or invertebrates. By being exposed to various damages over years and many generations, recognition proteins develop through multiple evolutionary adaptations. As a result of splicing and genetic recombination, diverse proteins can be found greatly, albeit complex mechanisms, including removal of self-binding proteins and elongation of pathogen-binding proteins portraits innate adaptation processes. The unforeseen results of these events are that the developed immune status can be transferred to latter generations. This maternal effect hints that specific epigenetic mechanisms makes the transmission happen (Sadd & Schmid-Hempel 2006). Regardless of the mechanism, insects are highly resistant to injuries, and as a result of their enhanced immune status, they subsequently pass this trait to the next generation (Rahman et al. 2004).
Cellular innate immune response in insects
The key premier guard to host physiological or environmental challenge is inflammatory response. This response, comprising humoral and cellular immunity, is one of the initial lines of defense to fight with potential threats. Various consequences of this response contribute to host defense against invading microbes, but they also result in undesired damage to host tissue(s) (Tokusumi et al. 2018). The cell-mediated defense is executed by immune-activated hemocytes. The immune response activated by hemocytes combats external pathogens, via various processes and pathways such as phagocytosis, encapsulation, and nodulation (Hong et al. 2018; Kim et al. 2017). In mammals as well as insects, phagocytosis is considered a major cellular innate immune response. Given that phagocytosis is essential, it is involved in various complex biological events, including pathogenic microbe's elimination, apoptotic cells discard, tissue remodeling, and innate and adaptive immune responses induction. Insect hemocytes are categorized as crystal cells, lamellocytes, and plasmatocytes (Jutras & Desjardins 2005). Plasmatocytes, as the major circulating hemocytes, are small round phagocytic cells. Prophenol-oxidase (proPO), which is involved in melanization, is carried by crystal cells. Last but not the least, there are lamellocytes, large flat adherent cells, which are hard to find under routine physiological and developmental conditions. Although, multiple defensive lamellocytes are induced under challenge conditions such as wasp parasitization, wherein the action of encapsulation is taken towards the foreign invader (Tokusumi et al. 2018). Due to the vast range of the functional and biochemical studies regarding phagocytic receptors, ligands and components of intracellular signaling pathways, it has been demonstrated that six elaborate receptors in phagocytosis in Drosophila have been described as follows: (1) Croquemort (catcher of death), in Drosophila embryos, is in charge of apoptotic cells clearance; (2) PGN recognition protein-LC (PGRP-LC) triggers Gram-negative bacteria phagocytosis; (3) Draper is a receptor involved in the apoptotic cells phagocytosis; (4) Eater, which is a receptor on the surface of the cell having 32 epidermal growth factor (EGF) repeats, mediates Escherichia coli and Staphylococcus aureus phagocytosis regulations; (5) Nimrod C1 (NimC1) contributes to bacterial phagocytosis; and (6) scavenger receptor class C1 (dSR-C1), involved in both Gram-positive and Gram-negative bacterial recognition, is a multi-domain modular membrane protein consisting of 609 amino acid residues and several well-known sequences motifs. These include two complement control protein (CCP) domains, a somatomedin B domain, a domain found in Meprin, A5 antigen (MAM) domain, and receptor-type tyrosine-protein phosphatase (RPTP) Mu and mucin-like domains (Stuart & Ezekowitz 2008). In spite of a few existing reports, SR-C remains an uncharacterized gene in innate immune functions in insect studies. In the coleopteran insect Tenebrio molitor, these receptors have a great importance, especially as this insect has been used as a practical model in immune signaling pathway studies. It has been proposed that TmSR-C acts in the microorganisms phagocytosis such as Escherichia coli, Staphylococcus aureus, and Candida albicans (Kim et al. 2017). Additionally, studies have revealed that Drosophila crystal cells are required for melanization, and plasmatocytes are involved in phagocytosis. However, lamellocytes are responsible for encapsulation. Once foreign bodies that are too large to be engulfed are surrounded, another cellular defense response called encapsulation occurs. Encapsulation-related morphological analysis revealed that blood cells react to the invasion of foreign bodies, such as wasp eggs, bind to them and create a multi-layered cellular capsule. However, encapsulation during cellular immune responses characterization in insects remains unknown (Gilbert 2012).
Humoral immune response in insects
The insect and mammalian innate immune systems comprise humoral and cellular responses. Insect hemocytes have similar structural and functional characteristics as mammalian neutrophils. In both insect and mammalian humoral responses, processes like melanization, clotting and AMPs secretion are implicated. Importantly, mammals hold an adaptive immune system that first evolved in jawed fish 500 million years ago, after separation of the vertebrate and invertebrate. However, as insects do not possess an adaptive immune response, but have elevated humoral and cellular responses towards any microbial insult, they have succeeded having enhanced survival capability (Sheehan et al. 2018).
The effector AMPs produced from fat body, constitute the humoral immune system in the hemolymph. Additionally, these proteins participate in the proPO cascade and reactive oxygen and nitrogen species, including these peptides. Humoral immune signaling pathways primarily comprise the Toll and IMD pathways. Upon sensation of PAMPs such as LPS, LTA, DAP and Lys-type PGNs, and β-1,3-glucan by PRRs, the aforementioned signaling pathways will be initiated (Kim et al. 2017). Through recognition, diverse signaling cascades, like gene expression induction involved in the innate immune response, are activated by PRRs, for instance, inflammatory cytokines or AMPs (Gilbert 2012).
Toll signaling pathway
The Toll-like receptors (TLRs), among all of the identified PRRs, are one of the oldest classes, with a high capability of spotting pathogens (Nie et al. 2018; Vasselon & Detmers 2002). Initially, the role of the Toll pathway was defined during Drosophila embryo development in the establishment of dorsal-ventral polarity (Belvin & Anderson 1996). Toll receptors, having a key function in development and immunity, are type I transmembrane proteins (Imler & Zheng 2004) and a huge number of them have been recognized across a wide variety of invertebrate and vertebrate species. According to the phylogenetic analyses, it has been signified that in a wide range of organisms, from Porifera to mammals, TLRs are critically conserved in signaling pathways initiated by them or related adaptor proteins, including intracellular Toll-Interleukin receptor (TIR) and extracellular leucine-rich repeats (LRRs) domains (Imler & Zheng 2004; Janeway 1989). TLR-related receptors, termed as TIRs, contain a 150-amino-acid intracytoplasmic domain, and are characterized as being shared with members of the interleukin-1 receptor (IL-1R) family and plant disease resistance (R) genes. Base on the TIR domain phylogenetic assay, these proteins are classified into three groups: IL-1R, Toll/TLR, and cytosolic TIR proteins. All members of these three groups are transmembrane proteins with an intracellular TIR domain. The presence of three extracellular immunoglobulin domains is the particularity of the vertebrate-specific group, the IL-1R protein. A dimeric receptor comprising two structurally related subunits, the IL-1R accessory protein (IL-1RAcP) and the type I IL-1 receptor (IL-1RI), that the cytokine IL-1 interacts with initiates intracellular signaling events , ultimately conducting to inflammation. Via pinpoint of an extracellular domain comprised of amino-terminal LRRs cysteine clusters on the c-terminal (CF motif) or N-terminal (NF motif) side of LRRs in the vertebrate and invertebrate Toll/TLR group, is characterized. The last group, cytosolic LRRs, has got different molecules from vertebrates, invertebrates and plants. Nearly same number, about 10, of TLR receptors are encoded by the genomes of mammals and dipteran insects: nine Toll-related genes in Drosophila, ten in human and the malaria vector Anopheles gambiae, twelve in mouse, and seven TLRs from T. molitor (unpublished) have been recognized (Anthoney et al. 2018; Imler & Zheng 2004). As a part of the mammalian role in recognition of pathogens, cells implicated in the first line of host defense, like dermal endothelial cells, macrophages, neutrophils, mucosal epithelial cells and dendritic cells express TLR family member (Armant & Fenton 2002; Kondo et al. 2017; Mclaughlin et al. 2019). The expression pattern of Tolls and TLRs in Drosophila and mammals is significantly different. All the forenamed Tolls and their homologs in other insects play roles in development. One radical distinction between the insect and mammalian pathways is that TLRs in mammals are PRRs; thus, pathogens have direct interactions with them, while unlike mammals, insects require the ligand Spaetzle (Spz), a cysteine-knot growth factor, for the TLR to be activated. Signaling cascades that initiate via Toll upstream pathogen pattern recognition, lead to the conjugation of activated Spz to Toll (Edosa et al. 2020b; Jo et al. 2017; Keshavarz et al. 2019). Keeping this in mind, it seems that the Toll/TLR immune functions are not a consequence of similar ancestry, but may reflect an evolutionary convergence (Imler & Zheng 2004).
The cytokine Spaetzle as a Toll receptor ligand
Considering data mentioned erstwhile, it has been verified that the Spz gene that encodes the cysteine-knot growth factor, is Toll upstream inquiries. A serine protease processes the premature synthesized form of this gene. The cysteine-knot motif of the freed carboxyl-terminal fragment binds to the Toll receptor and leads to its activation (Alpar et al. 2018; Imler & Zheng 2004). Spz cleavage and activation during Drosophila development is proceeded by protease Easter which is activated by protease Snake, but neither of these proteases are needed for Toll pathway activation involved in the immune response (Hong et al. 2018; Nonaka et al. 2018; Parthier et al. 2014). Although, in order for this integration to happen, the immature Spz must be activated by the serine protease, Spz processing enzyme (SPE), which itself is activated by three protease cascades. Through recognition of the cell wall components of Gram-positive bacteria (Lys-type PGN) and fungi (β-1,3-glucan), two of these cascades are initiated by the modular serine protease (ModSP) and the Grass protease. Moreover, different serine proteases, known as Spirit, Spheroide, and Sphinx 1/2, are assumed to lie in the pathway between Grass and SPE. Once microbial proteases or distress signals activate the Persephone (Psh) protease, this leads to the third cascade (Lewis et al. 2013; Nonaka et al. 2018; Weber et al. 2003). Presumably, Gram-positive bacteria or fungi are able trigger the direct cleavage of Psh. In summary, all said modes of action conducts to SPE downstream activation, although it is yet to be determined what protease is in charge of direct SPE activation in Drosophila; even so in T. molitor, an SPE-activating enzyme (TmSAE) has been identified (Park et al. 2019).
IMD signaling pathway
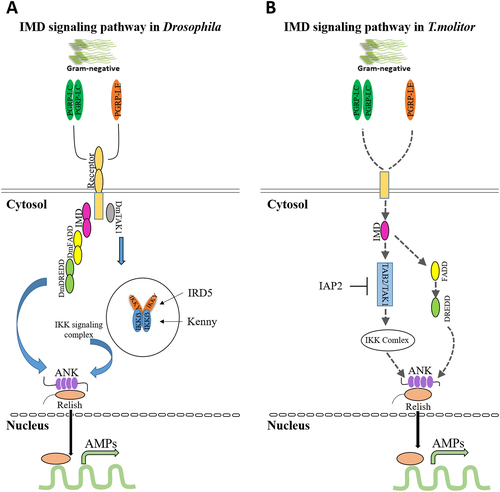
The IMD pathway, subjected to the DAP-type PGN, which consists of the Gram-negative bacteria cell wall and also some of the Gram-positive bacteria such as Bacillus and Listeria species, originally named after Drosophila mutant flies, called IMD (Edosa et al. 2020a; Gilbert 2012; Kleino & Silverman 2014). Like mammalian receptor interacting protein, tumor necrosis factor receptor (TNFR), a death-domain-containing protein, is encoded by the IMD gene in insects (Gilbert 2012). Discovery of the other pathway elements has been distinguished by different research teams. These elements include the nuclear factor-κB (NF-κB) transcription factor Relish, the Drosophila inhibitor of κB kinase (IKK) complex, the caspase-8 homolog Death-related ced-3/Nedd2-like protein (DREDD), Drosophila Fas-associated protein with Death domain (dFADD), transforming growth factor (TGF)-β activated kinase 1 (TAK1), and finally the PGRP-LC receptor. Another receptor has been identified and subsequently supplemented to the IMD pathway; PGRP-LE, TAK1-associated binding protein 2 (TAB2), the ubiquitination machinery components inhibitor of apoptosis 2 (IAP2), Bendless (Ubc13), Uev1a, and Effete (Ubc5), and the transcription cofactor Akirin. Likewise, some negative regulators have also been defined later, which function at different stages of the pathway (Kleino & Silverman 2014). Both PGRP-LC receptor located on the plasma membrane, and intracellular PGRP-LE, which is exclusive to DAP-type PGN, are involved in the IMD pathway. Through dimerization or multimerization after PGN association, the intracellular signal is conducted to the adaptor protein IMD and afterwards, IMD engages with dFADD and the caspase DREDD. Following the ubiquitination of K63, Dredd is activated and leads to IMD cleavage. In reference to the current understanding and the illustrated model, through the TAB2 ubiquitin-binding domain, TAK1 is activated by K63-polyubiquitin chains recruit (Chen 2012). The c-Jun N-terminal kinase (JNK) and IKK/Relish branches of the IMD pathway are actuated by TAK1. Once Relish is cleaved by DREDD, the N-terminus region of Relish translocates to the nucleus by its nucleus localization sequence (NLS) and initiates genes transcription. In T. molitor, similar to Drosophila, we have identified IMD pathway components, including the IMD receptor, PGRP-LE/LC, FADD, DREDD, TAB2, TAK1 and the IKK signaling complex, comprising TmIKK-β, −γ and -ε (unpublished data), which are proposed to associate with Relish phosphorylation and translocation to the nucleus, leading to AMP genes expression (Keshavarz et al. 2020b; Keshavarz et al. 2020c; Keshavarz et al. 2020d). Moreover, the IMD pathway participates in immune response against some RNA viruses, such as Drosophila X virus (DXV) infection, but in a different way from responding to bacterial, which is lack of strong induction of AMPs expression (Beckage 2011; Kleino & Silverman 2014). Thereupon, the underlying mechanisms of aiming IMD pathway by viruses and the way this signaling activation conducts the viral infection resistance remains unknown (Kleino & Silverman 2014).
Mode of action for the IMD and Toll pathway after bacterial and fungal infection
As has been pointed out formerly, Toll pathway activation is not concluded by direct microbial contact, and it is activated through the cytokine-like polypeptide Spz cleavage. Due to the genetic analysis, it has been indicated that Gram-positive-dependent Toll requires two genes, Semmelweis and Osiris. The PGRPs and Gram-negative-binding proteins (GNBPs), first characterized in larger insect species through their intriguing ability to either bind to bacteria or to motifs of bacteria structure, are encoded by each of the mentioned genes. Additionally, a hemolymph trypsin-like protease, encoded by Psh, mediates fungal-activated Spz cleavage and subsequently Toll activation (Adelaja & Hoffmann 2019; Hoffmann 2003). Once the said recognition signals initiate the most upstream serine protease of the Drosophila Toll cascade, ModSP, it activates the proteolytic serine protease cascade. The role between ModSP and SPE is played by several death-domain-containing serine proteases, such as Grass and Spirit. Serine–threonine kinase Pelle activation is processed upon binding processed Spz, Toll/IL-1 Receptor (TIR) and/or the death-domain-containing adaptor molecules dMyD88 and Tube (Gilbert 2012; Hopkins & Sriskandan 2005; Modlin 2002). This kinase leads to Cactus degradation (Ferrandon et al. 2004; Gilbert 2012; Hoffmann 2003; Means et al. 1999). However, Pelle does not conduct Cactus phosphorylation directly and the identity of the Cactus kinase dwells vague (Hoffmann 2003). Degradation of the Cactus kinase leads to translocation of the Rel transcriptional factors, Dorsal-related immunity factor (Dif) and Dorsal, to the nucleus. Interaction of these factors with the NF-κB-response component induces transcriptional activation of AMP genes, such as drosomycin (Fig. 1A) (Gilbert 2012).
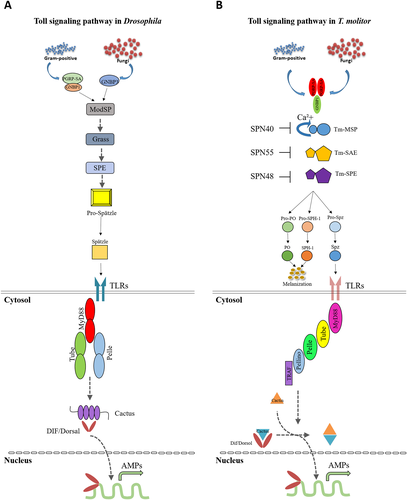
Diversely, presumed transmembrane proteins, PGRP-LC and PGRP-LE, which are required for IMD pathway activation, also recognize DAP-type PGN of Gram-negative bacteria (Gilbert 2012; Hoffmann 2003) and latterly, these signals are transferred to the cytoplasm where IMD is located. The intracellular domain of PGRP-LCs engages with IMD upon the monomeric or polymeric DAP-type PGN association with the PGRP-Lcx/Lca heterodimer and PGRP-1Lcx homodimer, respectively. Then, IMD binds to dFADD and the caspase Dredd interacts with Relish, leading to cleavage of phosphorylated Relish. The phosphorylation process itself is assumed to be performed by an IKK complex containing immune response deficient 5 (Ird5) and Kenny. Then, the phosphorylated Relish translocate to the nucleus, where the Rel domain recruits NF-κB response elements and alternatively activates the transcription of AMP genes, such as diptericin (Fig. 2A) (Chowdhury et al. 2019; Gilbert 2012).
In T. molitor, due to the activation of Lys-type PGN recognition signaling pathway towards Gram-positive bacteria, the PGRP-SA/GNBP1/modular serine protease (MSP)/SAE/SPE/Spz cascade is inevitable (Gilbert 2012; Keshavarz et al. 2020a; Kim et al. 2008). Similar to Drosophila, the Toll signaling pathway in T. molitor consists of a three step of proteolytic cascade. Once the PGRP-SA/GNBP1 complex and GNBP3 recognize Lys-type PGN and β-1,3-glucan, which cause the induction of three serine protease downstream (Gilbert 2012). We have identified the MyD88 (Patnaik et al. 2014), Tube, Pelle, Pellino and TNF receptor associated factor (TRAF) genes in T. molitor (unpublished data); therefore, subsequent to pro-Spz cleavage after SPE activation, the triggered Toll cascade eventually leads to Tenebrio AMPs like tenecin 1 and 2 production (Gilbert 2012). Considering the similar characteristics of TmCactin with Drosophila Cactin, based on analogy data, it may be possible that TmCactin regulates the translocation of Tenebrio NF-κB-like proteins to the nucleus to activate AMP expression (Fig. 1B). Controversially, it has been shown that even notable levels of Cactus inhibitors do not suppress active NF-κB transcription factors Dorsal and Dif in TollD (gain-of function) mutant flies. Regarding the said ambiguity, among various explanations one of which is that the NF-κB factors activity depends on the Cactus degradation intensity as much as Cactus protein level (Hoshino et al. 1999). Nicolas and colleagues have suggested that due to modification of either or both of the NF-κB and Cactus proteins, free Cactus cannot suppress NF-κB proteins once it disjoins from the NF-κB (Dif and/or Dorsal) complex (Hoshino et al. 1999). There are also other possible scenarios that after Cactus/NF-κB proteins dissociation, due to the conformation alteration Cactus is unable to bind to the NF-κB complex again. Lin and colleagues have shown that eventually, degradation of Cactus may increase by Cactin (Brightbill et al. 1999). However, the correlation between Cactus and Cactin expression level and their precise mutual interaction is yet to be figured. Taken all the previous results together, it can be concluded that unlike human Toll signaling, Cactin acts as a positive regulator of Toll signaling in both Drosophila and T. molitor (Vasselon & Detmers 2002).
Since we identified the IMD pathway components in T. molitor (unpublished data), it is assumed that signal transduction into the cell occurs through IMD, which contains a death domain that recruits FADD (TAK1 activator) and Dredd (a caspase). Active TAK1 is a protein kinase that triggers Relish phosphorylation (TmIKK-β, −γ and -ε). Eventually the Rel domain moves into the nucleus and leads to AMP genes transcription (Fig. 2B).
Conclusion
Enlighted by all the results of advanced molecular and biochemical studies, also in spite of all achieved comprehensions and discoveries, there are yet many gaps in our knowledge regarding insect immunity which must be narrowed down. By having a valid perspective in mind, Toll/TLRs and IMD pathways and all engaged elements in the signaling process in a vast range of inflammatory and innate immune responses have been evident in insects. Notwithstanding, identification research regarding mechanisms characterization beneath the formerly mentioned responses illustrate a bright future towards developing novel therapeutics and clinical trials.
Acknowledgments
This research was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through the Export Promotion Technology Development Program (Grant no. 617077-5), funded by the Ministry of Agriculture, Food, and Rural Affairs (MAFRA).
Conflicts of Interest
The authors declare no conflict of interest.




