DNA data and morphology suggest an occurrence of Dendrolimus sibiricus Tschetverikov, 1908 (Lepidoptera: Lasiocampidae) instead of D. superans Butler, 1877, in South Korea
Abstract
In the present study, specimens of Dendrolimus superans collected from South Korea suggest the presence of D. sibiricus, instead of D. superans. Comparisons of the wing morphology, female and male genitalia, 3′-end region of the cytochrome c oxidase I (COI) gene sequence, and internal transcribed spacer (ITS) 2 sequence of the Korean specimens with those of the D. superans specimens from Japan consistently supported the presence of D. sibiricus in South Korea. Phylogenetic analyses of the concatenated sequences of COI and ITS2 from the available sequence types of D. sibiricus and D. superans, along with South Korean specimens, were conducted using the Bayesian inference (BI) and maximum likelihood (ML) methods. These phylogenetic analyses placed the South Korean specimens with the Russian D. sibiricus as an inclusive group, excluding the Japanese D. superans, indicating the distribution of D. sibiricus in South Korea. Nevertheless, D. superans formed a distinct group only by BI analysis (Bayesian posterior probabilities = 0.89), whereas D. sibiricus, including the South Korean samples, formed a distinct group only by the ML analysis (99%), suggesting a low genetic divergence between the two species.
Introduction
The larch caterpillar Dendrolimus superans Butler 1877 had gained notoriety as a destructive pest of pine (Kuanyu 1994; Park et al. 1999) and is highly similar to its Siberian sister species D. sibiricus Tschetverikov 1908, which also damages the leaves of several coniferous trees, including pine (Rozhkov 1963; European and Mediterranean Plant Protection Organization (EPPO) 2005). Both species are similar in having a brown–grey larval color and a white distal spot at the center of the forewing. However, D. superans and D. sibiricus differ in the lengths of both the female (60–90 and 77–102 mm, respectively) and male (40–60 and 60–79 mm, respectively) wingspans (EPPO 2005). In addition, the moths of D. superans are mostly richer in brownish or rusty hues than D. sibiricus (Mikkola & Ståhls 2008). Furthermore, the male genitalia differs between the two species, with the harpe being stouter in D. superans (Mikkola & Ståhls 2008).
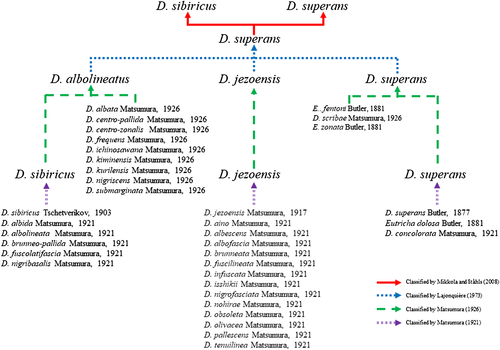
In the past, D. sibiricus and D. superans have long been confused with D. jezoensis and D. albolineatus, but Matsumura (1921, 1926) eventually revised this complex into three species, resulting in five species of the genus Dendrolimus in the Palearctic region (Fig. 1). Later, these three species were combined into a single species, D. superans, consequently resulting in 12 species of the genus Dendrolimus in the Palearctic region (D. pini, D. rex, D. sericus, D. spectabilis, D. atrilineis, D. himalayanus, D. suffuscus, D. superans, D. monticola, D. punctata, D. angulata, and D. kikuchii; Lajonquière 1973). Later, however, Mikkola and Ståhls (2008) divided D. superans into D. superans and D. sibiricus and added a new species, D. kilmez, that is close to D. pini. Later Zhang et al. (2004a,b,c) added an additional species, D. houi, resulting in 15 Dendrolimus species in the Palearctic region.
Dendrolimus sibiricus is known to be distributed in Russia (from the west of the Ural Mountains in the European part of Russia to the Primorsky Krai in the Russian Far East), Kazakhstan, Mongolia, China (in the provinces of Jilin, Liaoning, Beijing, and Neimenggu), and both South and North Korea (Hou 1987; EPPO 2005; Hardin & Suazo 2012). Conversely, D. superans is restricted to Japan (Hokkaido and Northern Honshu) and Russia (the Sakhalin and Kuril Islands, as well as some regions of the Russian Far East; Fukuyama 1978; Maeto 1991; EPPO 2005; Hardin & Suazo 2012). Although Hou (1987), EPPO (2005) and Hardin and Suazo (2012) recorded the distribution of D. sibiricus in South Korea, these references provide a mere list, without any taxonomic description. In fact, this species is not listed in any Korean record (Park et al. 1999, 2014; Shin 2001; Paek et al. 2010; Lim et al. 2012). Instead, D. superans and D. spectabilis have been listed as the South Korean species (Park et al. 1999; Shin 2001). Thus, it is not clear which Dendrolimus species is distributed in South Korea.
To understand the species delimitation and phylogenetic relationships of Dendrolimus, the DNA barcoding region of the mitochondrial cytochrome c oxidase I (COI) gene and the nuclear internal transcribed spacer (ITS) 1 and ITS2 sequences have been used individually, as well as in combination (Dai et al. 2012). As a result, three distantly related species, namely D. superans, D. houi, and D. kikuchii, were recovered as distinct species by using both single and combined sequences, whereas three closely related species, namely D. punctatus, D. tabulaeformis, and D. spectabilis, were not recovered as distinct species (Dai et al. 2012). Using the full-length mitochondrial genome sequences, Qin et al. (2015) further identified three distinct species: D. spectabilis forms an independent lineage separately from D. punctatus, whereas D. tabulaeformis has a very close relationship with D. punctatus, indicating genetic similarity in some species of the genus Dendrolimus.
Mikkola and Ståhls (2008) sequenced the 3′-end region of a partial mitochondrial COI gene (729 bp of the 3′-end region) and the ITS2 sequences of D. sibiricus and D. superans from the European foothills of the Ural Mountains, Russia, along a few closely related species, and found that D. sibiricus shared the COI haplotypes with D. pini, whereas D. pini shared the ITS2 sequences with another species, D. kilmez, indicating a genetic resemblance between some species of the genus Dendrolimus. Conversely, all D. superans haplotypes and sequences were distinct from the other Dendrolimus taxa, but the parsimony-based phylogenetic tree presented a minimal phylogenetic signal, showing D. superans + D. kilmez as an inclusive group with no resolution among the D. superans + D. kilmez group, D. sibiricus, and D. pini according to the COI sequence. However, the ITS2-based analysis provided a status of distinct species to D. sibiricus, whereas the other species were ambiguous (Mikkola & Ståhls 2008). Nevertheless, the haplotypes or sequence types of D. sibiricus and D. superans did not form a close sister relationship with each other (Mikkola & Ståhls 2008).
Against this background, we collected 29 specimens of Dendrolimus (28 males, one female) that have officially been recorded as D. superans from five localities in South Korea and two specimens of D. superans from Japan. These specimens were examined for their external morphology, including male and female genitalia, to identify the South Korean specimens of Dendrolimus and the results were compared with the published data of Mikkola and Ståhls (2008), which provides a detailed description of the morphology of both D. superans and D. sibiricus.
In order to generate comparative sequences to the previous molecular study of Mikkola and Ståhls (2008), both the 3′-end region of the COI gene (729 bp) and the ITS2 region were sequenced. The sequences of each gene (or region) were analyzed for genetic distance and phylogenetic relationships by comparing them with the available homologous congeneric sequence data obtained from the National Center for Biotechnology Information (NCBI) GenBank database through a Basic Local Alignment Search Tool (BLAST) search (http://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed 8 March 2017) and the Barcode of Life Data (BOLD) system (http://boldsystems.org, accessed 8 March 2017; Ratnasingham & Hebert 2007, 2013).
Materials and methods
Specimens and morphology
Twenty-nine specimens of Dendrolimus were collected from five Korean localities (i.e. Yeongwol, Mt Taebaeksan, Mt Maebongsan, Jeongseon, and Mt Jirisan) in Jeollanam-do Province between 2011 and 2015. A single female specimen was collected only from Mt Taebaeksan (sample DS16188; Table 1). For D. superans, one male and one female specimen were collected from Nagano and Sazikirindo in Japan in 2015. For morphological examination, dry specimens and genitalia slides were prepared using conventional methods (Brown 1955; Scoble 1992; Kulakova & Tatarinov 2011). Furthermore, the congeneric D. spectabilis specimen collected from South Korea was sequenced for use as an outgroup for phylogenetic analysis. In all, the 3′-end region of the COI gene and the ITS2 region were sequenced for 32 specimens (Table 1). Voucher specimens have been deposited at Mokpo National University (MNU) and Chonnam National University (CNU), Republic of Korea.
| Species | Source (sample name) | Country | COI ID (haplotype) | ITS2 ID (sequence type) | COI + ITS2 sequence type |
|---|---|---|---|---|---|
| D. sibiricus | Present study (DSI4629) | Yeongwol, South Korea | MF178173 (DSICOI01) | MF178205 (DSIITS01§) | DSICOIITS01 |
| Present study (DSI4631) | Yeongwol, South Korea | MF178174 (DSICOI01) | MF178206 (DSIITS01§) | DSICOIITS01 | |
| Present study (DSI4632) | Yeongwol, South Korea | MF178175 (DSICOI01) | MF178207 (DSIITS01§) | DSICOIITS01 | |
| Present study (DSI4634) | Yeongwol, South Korea | MF178176 (DSICOI01) | MF178208 (DSIITS01§) | DSICOIITS01 | |
| Present study (DSI4635) | Yeongwol, South Korea | MF178177 (DSICOI01) | MF178209 (DSIITS01§) | DSICOIITS01 | |
| Present study (DSI4636) | Yeongwol, South Korea | MF178178 (DSICOI01) | MF178210 (DSIITS01§) | DSICOIITS01 | |
| Present study (DSI4706) | Mt Taebaeksan, South Korea | MF178179 (DSICOI02) | MF178211 (DSIITS01§) | DSICOIITS02 | |
| Present study (DSI4707) | Mt Taebaeksan, South Korea | MF178180 (DSICOI01) | MF178212 (DSIITS01§) | DSICOIITS01 | |
| Present study (DSI4708) | Mt Taebaeksan, South Korea | MF178181 (DSICOI01) | MF178213 (DSIITS02) | DSICOIITS03 | |
| Present study (DSI4709) | Mt Taebaeksan, South Korea | MF178182 (DSICOI02) | MF178214 (DSIITS01§) | DSICOIITS02 | |
| Present study (DSI4710) | Mt Taebaeksan, South Korea | MF178183 (DSICOI01) | MF178215 (DSIITS01§) | DSICOIITS01 | |
| Present study (DSI4711) | Mt Taebaeksan, South Korea | MF178184 (DSICOI01) | MF178216 (DSIITS01§) | DSICOIITS01 | |
| Present study (DSI4712) | Mt Taebaeksan, South Korea | MF178185 (DSICOI01) | MF178217 (DSIITS01§) | DSICOIITS01 | |
| Present study (DSI4713) | Mt Taebaeksan, South Korea | MF178186 (DSICOI01) | MF178218 (DSIITS01§) | DSICOIITS01 | |
| Present study (DSI4714) | Mt Taebaeksan, South Korea | MF178187 (DSICOI01) | MF178219 (DSIITS01§) | DSICOIITS01 | |
| Present study (DSI4715) | Mt Taebaeksan, South Korea | MF178188 (DSICOI01) | MF178220 (DSIITS01§) | DSICOIITS01 | |
| Present study (DSI4716) | Mt Taebaeksan, South Korea | MF178189 (DSICOI01) | MF178221 (DSIITS01§) | DSICOIITS01 | |
| Present study (DSI4717) | Mt Taebaeksan, South Korea | MF178190 (DSICOI01) | MF178222 (DSIITS01§) | DSICOIITS01 | |
| Present study (DSI4718) | Mt Taebaeksan, South Korea | MF178191 (DSICOI01) | MF178223 (DSIITS01§) | DSICOIITS01 | |
| Present study (DSI4719) | Mt Taebaeksan, South Korea | MF178192 (DSICOI01) | MF178224 (DSIITS01§) | DSICOIITS01 | |
| Present study (DSI4720) | Mt Taebaeksan, South Korea | MF178193 (DSICOI01) | MF178225 (DSIITS01§) | DSICOIITS01 | |
| Present study (DSI4724) | Mt Jirisan, South Korea | MF178194 (DSICOI03†) | MF178226 (DSIITS01§) | DSICOIITS04 | |
| Present study (DSI5585) | Mt Maebongsan, South Korea | MF178195 (DSICOI01) | MF178227 (DSIITS01§) | DSICOIITS01 | |
| Present study (DSI5586) | Mt Maebongsan, South Korea | MF178196 (DSICOI01) | MF178228 (DSIITS03) | DSICOIITS05 | |
| Present study (DSI5729) | Jeongseon, South Korea | MF178197 (DSICOI01) | MF178229 (DSIITS01§) | DSICOIITS01 | |
| Present study (DSI5730) | Jeongseon, South Korea | MF178198 (DSICOI01) | MF178230 (DSIITS01§) | DSICOIITS01 | |
| Present study (DSI5731) | Jeongseon, South Korea | MF178199 (DSICOI01) | MF178231 (DSIITS01§) | DSICOIITS01 | |
| Present study (DSI5732) | Jeongseon, South Korea | MF178200 (DSICOI01) | MF178232 (DSIITS01§) | DSICOIITS01 | |
| Present study (DSI6188) | Mt Taebaeksan, South Korea | MF178201 (DSICOI04) | MF178233 (DSIITS04) | DSICOIITS06 | |
| MZH_KM1 | Cherdyn, Perm, Russia | AM946694 (KM1SIBIRICUS†) | AM946744 (KM1SIBIRICUS§) | DSICOIITS04 | |
| MZH_KM2 | Cherdyn, Perm, Russia | AM946695 (KM1SIBIRICUS†) | AM946745 (KM1SIBIRICUS§) | DSICOIITS04 | |
| MZH_KM3 | Solikamsk, Perm, Russia | AM946696 (KM3SIBIRICUS) | AM946746 (KM1SIBIRICUS§) | DSICOIITS07 | |
| MZH_KM5 | Kilmez, Udmurtia, Russia | AM946697 (KM1SIBIRICUS†) | AM946756 (KM1SIBIRICUS§) | DSICOIITS04 | |
| MZH_KM6 | Kilmez, Udmurtia, Russia | AM946698 (KM1SIBIRICUS†) | – | ||
| MZH_KM7 | Cherdyn, Perm, Russia | – | AM946747 (KM1SIBIRICUS§) | ||
| MZH_KM8 | Cherdyn, Perm, Russia | – | AM946748 (KM1SIBIRICUS§) | ||
| MZH_KM9 | Cherdyn, Perm, Russia | AM946699 (KM1SIBIRICUS†) | – | ||
| MZH_KM12 | Cherdyn, Perm, Russia | – | AM946749 (KM1SIBIRICUS§) | ||
| MZH_KM13 | Cherdyn, Perm, Russia | – | AM946750 (KM1SIBIRICUS§) | ||
| MZH_KM19 | Kilmez, Udmurtia, Russia | AM946701 (KM1SIBIRICUS†) | AM946751 (KM1SIBIRICUS§) | DSICOIITS04 | |
| MZH_KM20 | Kilmez, Udmurtia, Russia | AM946702 (KM1SIBIRICUS†) | AM946752 (KM1SIBIRICUS§) | DSICOIITS04 | |
| MZH_KM21 | Kilmez, Udmurtia, Russia | – | AM946753 (KM1SIBIRICUS§) | ||
| MZH_KM31 | Baikal, Burjatia, Russia | AM946703 (KM1SIBIRICUS†) | AM946754 (KM1SIBIRICUS§) | DSICOIITS04 | |
| MZH_KM32 | Baikal, Burjatia, Russia | AM946704 (KM1SIBIRICUS†) | AM946755 (KM1SIBIRICUS§) | DSICOIITS04 | |
| D. superans | Present study (DSU5741) | Nagano, Japan | MF178202 (DSUCOI01) | MF178234 (DSUITS01) | DSUCOIITS01 |
| Present study (DSU5742) | Sazikirindo, Japan | MF178203 (DSUCOI02‡) | MF178235 (DSUITS02) | DSUCOIITS02 | |
| MZH_KM_J1 | Tazawa, Minami-Azumi, Nagano, Honshu Japan | AM946712 (J1SUPERANS) | AM946735 (J1SUPERANS) | DSUCOIITS03 | |
| MZH_KM_J2 | Tazawa, Minami-Azumi, Nagano, Honshu Japan | AM946713 (J2SUPERANS‡) | AM946736 (J2SUPERANS) | DSUCOIITS04 | |
| MZH_KM_J5 | Tazawa, Minami-Azumi, Nagano, Honshu Japan | AM946714 (J5SUPERANS) | AM946734 (J5SUPERANS) | DSUCOIITS05 | |
| MZH_KM_J6 | Tazawa, Minami-Azumi, Nagano, Honshu Japan | AM946715 (J1SUPERANS) | AM946731 (J5SUPERANS) | DSUCOIITS06 | |
| MZH_KM_J7 | Tazawa, Minami-Azumi, Nagano, Honshu Japan | AM946716 (J5SUPERANS) | AM946732 (J5SUPERANS) | DSUCOIITS05 | |
| MZH_KM_J12 | Idenemaizawa, Kawabe, Akita Prefecture, Japan | AM946717 (J12SUPERANS) | AM946737 (J12SUPERANS) | DSUCOIITS07 | |
| MZH_KM_J13 | Moriyashizawa, Moriyoshi, Akita Prefecture, Japan | AM946718 (J12SUPERANS) | AM946738 (J5SUPERANS) | DSUCOIITS08 | |
| MZH_KM_J14 | Moriyashizawa, Moriyoshi, Akita Prefecture, Japan | AM946719 (J12SUPERANS) | AM946739 (J14SUPERANS) | DSUCOIITS09 | |
| MZH_KM_J15 | Moriyashizawa, Moriyoshi, Akita Prefecture, Japan | AM946720 (J12SUPERANS) | AM946740 (J5SUPERANS) | DSUCOIITS08 | |
| MZH_KM_J27 | Japan | AM946721 (J27SUPERANS) | – | ||
| D. spectabilis | Present study (DSP4704) | Ulleungdo Island, South Korea | MF178204 (DSPCOI01) | MF178236 (DSPITS01) | DSPCOIITS01 |
- The haplotype names for COI and the sequence types for ITS2 are presented in the parentheses.
- –, not available; ID, GenBank accession number.
- † KM1SIBIRICUS = DSICOI03;
- ‡ J2SUPERANS = DSUCOI02;
- § KM1SIBIRICUS = DSIITS01.
DNA extraction, amplification, and sequencing
The genomic DNA of the collected specimens was extracted from one or two legs using a commercially available DNA purification kit (Bioneer, Daejeon, Korea) according to the manufacturer's instructions. To amplify the 729-bp 3′-end region of the COI gene, the following primer pair was adapted from Simon et al. (1994): C1-J-2183, 5′-CAACATTTATTTTGATTTTTTGG-3′ and tl2-n-3014, 5′-TCCAATGCACTAATCTGCCATATTA-3′. To sequence the ITS2 region, the following primers were adapted from Beebe and Saul (1995): ITS2A, 5′-TGTGAACTGCAGGACACAT-3′ and ITS2B, 5′-TATGCTTAAATTCAGGGGGT-3′. Polymerase chain reaction (PCR) was conducted using the AccuPower PCR PreMix (Bioneer) under the following conditions: initial denaturation at 94°C for 7 min, followed by 35 cycles of denaturation at 94°C for 1 min, annealing at 51–52°C for 1 min, and extension at 72°C for 1 min, with a subsequent final 7-min extension at 72°C. Electrophoresis was performed to confirm successful DNA amplification using 0.5× TAE buffer on 1% agarose gel. The PCR products were purified using a PCR purification Kit (Bioneer) and sequenced from both ends using the ABI PRISM BigDye Terminator ver. 3.1 Cycle Sequencing kit with an ABI 3100 Genetic Analyzer (PE Applied Biosystems, Foster City, CA, USA).
Sequence analysis
The boundary of each directional sequence was delimitated using ChromasPro ver. 1.5 (http://www.technelysium.com.au/ChromasPro.html, accessed 8 March 2017), and both directional sequences of an individual were aligned using CLUSTAL OMEGA (http://www.ebi.ac.uk/Tools/msa/clustalo/, accessed 8 March 2017) to obtain the complete individual sequences. The haplotype and sequence type designations were applied to new sequences as they were discovered, namely DSICOI01–DSICOI04 or DSUCOI01–DSUCOI02 for the 3′-end region of COI of the 29 South Korean specimens of Dendrolimus and D. superans, respectively, and DSIITS01–DSIITS04 and DSUITS01–DSUITS02 for the ITS2 region of the 29 South Korean specimens of Dendrolimus and D. superans, respectively (Table 1). The concatenated sequences of the 3′-end COI and ITS2 were designated as DSICOIITS01, DSICOIITS02, DSICOIITS04 etc. for the 29 South Korean specimens of Dendrolimus and DSUCOIITS01, DSUCOIITS02, DSUCOIITS03 etc. for D. superans.
Database search
Complete individual sequences of the 3′-end of COI and ITS2 were searched for among available GenBank (http://www.ncbi.nlm.nih.gov/genbank/, accessed 8 March 2017)-registered sequences using a BLAST search to obtain the corresponding sequences for estimation of sequence divergence and phylogenetic analysis. We found that all 10 of the 3′-end COI sequences for D. sibiricus originated from Russia, whereas that for D. superans originated from Japan (Table 1). Furthermore, 13 ITS2 sequences were obtained for D. sibiricus originating from Russia, whereas nine ITS2 sequences were obtained for D. superans originating from Japan (Table 1).
Sequence divergence and phylogenetic analysis
The degree of divergence among and between D. sibiricus and D. superans, including the 29 South Korean specimens of Dendrolimus, was obtained from the unrooted pairwise distances using PAUP* v4.01b10 (Swofford 2002). The nucleotide sequences were translated based on the invertebrate mitochondrial (mt) DNA genetic code. For phylogenetic analysis, each well-aligned conserved block of the concatenated sequences of the 3′-end of the COI gene and ITS2 was selected using GBLOCKS 0.91b (Castresana 2000), with the minimum length of a block set to 9 and the allowed gap positions set to none for both COI and ITS2.
The GTR + GAMMA + I model was selected for the 3′-end of the COI gene and the GTR + I model was selected for ITS2 using Modeltest ver. 3.7 (Posada & Crandall 1998). These models were applied for both the Bayesian inference (BI) and maximum likelihood (ML) methods using MrBayes ver. 3.2.6 (Ronquist et al. 2012) and RAxML-HPC2 on XSEDE ver. 8.0.24 (Stamatakis 2014), respectively, which were implemented on the CIPRES Portal ver. 3.1 (Miller et al. 2010). For conducting BI analysis, two independent runs of the four incrementally heated Markov and Monte Carlo chains (one cold, three hot chains) were run simultaneously for one million generations, with tree sampling conducted at every 100 generations, and the first 25% of the sampled trees discarded as burn-in. The average split frequency of <0.01 was used as the decision criterion for the convergence of two simultaneous runs. The confidence values of the BI tree are presented as the Bayesian posterior probabilities (BPP). For conducting ML analysis, 1000 non-parametric iterations were run under default parameter settings. The D. spectabilis specimen sequenced in the present study was used as an outgroup for the phylogenetic analysis. The phylogenetic trees were visualized using FigTree ver. 1.42 (http://tree.bio.ed.ac.uk/software/figtree/, accessed 8 March 2017).
Systematics
Order Lepidoptera Linnaeus, 1758
Family Lasiocampidae Harris, 1841
Genus Dendrolimus Germar, 1812
Dendrolimus Germar, 1812, Dissertatio sistens Bombycum species secundum oris partum diversitatem in nova genera distributas. Section II: p. 48. Type species: Lasiocampa pini Linnaeus, 1758.
Odonestis Germar, 1812, Dissertation sistens Bombycum species. Section II: pp. 48–49. Type species: Odonestis pruni Linnaeus, 1758.
Eutricha Stephens, 1829, A systematic catalogue of British insects. p. 49. Type species: Bombyx pini Linnaeus, 1758.
Ptilorhina Zetterstedt, 1839, Insecta lapponica. p. 925. Type species: Bombyx pini Linnaeus, 1758.
Dendrolimus sibiricus Tschetverikov, 1908 (Korean name: Sol-song-na-bang) Figs 2A, B, 3A, B
Dendrolimus sibiricus Tschetverikov, 1908, Russkoe entomologicheskoe obozrenie. 8: 1–7. Type locality: [Russia] Ural, Sayan and Bureya [Buretian] Mts.
Odonestis superans Butler, 1877, The annals and magazine of natural history, including Zoology, Botany, and Geology. 20: 481. Type locality: [Japan] Yokohama.
Dendrolimus albolineata Matsumura, 1921, Thousand Insects Japan Additamenta. Vol. IV, p. 918, pI. LXVIII, f.10. Type locality: [Russia] Saghalin.
Material examined
Six males, Yeongwol-gun, Gangwon-do Province, 37°17.60′N, 128°31.08′E, 23 June 2012, MNU. S. S. Kim; 15 males and one female, Mt Taebaeksan, Gangwon-do Province, 37°05.32′N, 128°57.92′E, 6 August 2012, MNU. S. S. Kim; two males, Mt Maebongsan, Gangwon-do Province, 37°11.53′N, 128°58.17′E, 23 June 2015, CNU. J. S. Jeong; four males, Jeongseon-gun, Gangwon-do Province, 37°21.77′N, 128°38.15′E, 6 August 2015, CNU. J. S.; one male, Mt Jirisan, Jeollanam-do Province, 35°17.52′N, 127°29.65′E, 3 August 2011, MNU. S. S. Kim.
Diagnosis
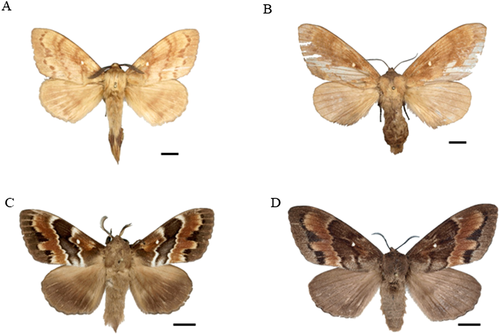
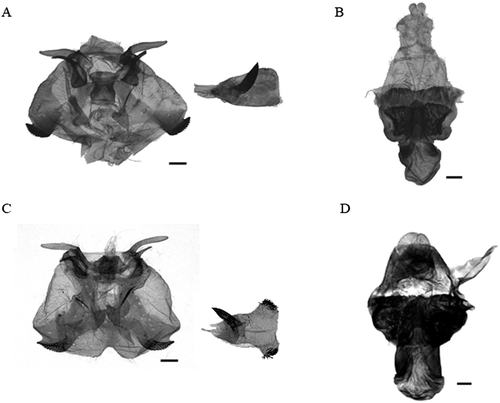
Wingspan males 51–70 mm, females 58–68 mm. A morphological comparison between the two species of Dendrolimus indicates that the 29 South Korean specimens of Dendrolimus from South Korea were slightly smaller and the transverse line on forewings not obvious compared with the D. superans samples collected from Japan. In particular, the outer line on the forewings is crooked at the CuA1. Compared with the D. sibiricus described by Mikkola and Ståhls (2008), the Korean samples are pale brown rather than gray (Fig. 2A,B). The male genitalia of D. sibiricus are characterized by a less bent and ankle-shaped harpe, wider and flatter cubiles, aedeagus with fewer fine hairs, and a thick bird-bill process compared with D. superans (Fig. 3A,C). The female genitalia of the 29 South Korean specimens of Dendrolimus are characterized by smaller concave plates at the sclerotized region of each end in the corpus bursae, and smaller and convex anales papillae compared with D. superans (Fig. 3B,D).
Hosts
Abies sibirica, Abies nephrolepis, Pinus sibirica, Pinus koraiensis, Larix gmelinii, Larix sibirica, Picea ajanensis, and Picea obovata (Rozhkov 1963).
Distribution
Russia (all Asian Russia, except for the extreme north, the Sakhalin and Kuril islands), Kazakhstan, China (Heilongjiang, Jilin, Liaoning, and Neimenggu), North Korea, South Korea, and Mongolia (North) (EPPO 2005).
Ecology
The full life cycle usually takes 2 years. However, in the southern parts of the natural range in Russia, one generation can develop in a single year, whereas in the northern regions the completion of a generation can sometimes take 3 years (Rozhkov 1963; Galkin 1993; Vorontsov 1995).
Dendrolimus superans Butler, 1877: Figs 2C, D, 3C, D
Odonestis superans Butler, 1877, The Annals and Magazine of Natural History. 20: 481. Type locality: [Japan] Yokohama.
Eutricha zonata Butler, 1881, Transactions of the Royal Entomological Society of London. 29: 17. Type locality: [Japan] Tokei (Tokyo).
Eutricha dolosa Butler, 1881, Transactions of the Royal Entomological Society of London. 29: 16–17. Type locality: [Japan] Tokei (Tokyo).
Eutricha fentoni Butler, 1881, Transactions of the Royal Entomological Society of London. 29: 17–18. Type locality: [Japan] Tokei (Tokyo).
Dendrolimus jezoensis Matsumura, 1917, Applied Entomology. p. 687, fig. 4 in pI. XXXIX and fig. 1 in pI. XL. Type locality: [Japan] Sapporo.
Material examined
One male, Nagano Prefecture, Japan, 36°32.52′N, 138°14.40′E, 23 August 2015, MNU. Y. Kishida; one female, Gunma Prefecture, Japan, 37°06.20′N, 139°33.23′E, 10 August 2015, MNU. Y. Kishida.
Diagnosis
Mikkola and Ståhls (2008) described the species as follows:
Very polymorphic. Forewing markings, if visible, similar to those of D. sibiricus, but background color mostly rusty brownish or greyish with brownish hue. Antemedian line mostly turning inward at costa, but does not end medially of discal dot. Hind bulge of subterminal line more irregular in shape. Male genitalia show a long, daggerlike, basally wide, and tapering harpe in the apical direction. Lateral extensions of vesica with very strong cornuti.
Hosts
Pinus pumila, Larix kamtschatica, Picea ajanensis and Abies sachalinensis (Rozhkov 1963).
Distribution
Japan (Hokkaido and northern Honshu) and Russia (the Sakhalin and Kuril Islands) (Matsumura 1926; Rozhkov 1963).
Ecology
The life cycle may take 1 or 2 years (or even more) depending on the climatic conditions; development takes much longer in the north of Sakhalin than in the south (Bushmelev & Yurchenko 1989) and Japan.
Molecular identification
The sequencing of the 729-bp 3′-end region of the COI gene from the South Korean specimens of Dendrolimus revealed four haplotypes (Table 1; DSICOI01–DSICOI04) with a sequence divergence ranging from 0.14% (1 bp) to 0.41% (3 bp; Table 2). When the 10 GenBank-registered COI homologous sequences of D. sibiricus originating from Russia (Mikkola & Ståhls 2008) were aligned with our sequences, two haplotypes (KM1SIBIRICUS and KM3SIBIRICUS) were obtained, and KM1SIBIRICUS was found to be identical to our DSICOI03. Altogether, the sequence divergence of the five haplotypes ranged from 0.14% (1 bp) to 0.55% (4 bp; Table 2), showing a high similarity of the South Korean specimens of Dendrolimus to D. sibiricus (hereafter termed potential D. sibiricus specimens). From the two D. superans individuals collected from Japan, two COI haplotypes (DSUCOI01 and DSUCOI02) were obtained with 1-bp difference (0.14%; Table 2). When the 10 GenBank-registered COI homologous sequences of D. superans originating from Japan were aligned with our sequences, five haplotypes were obtained (J1SUPERANS, J2SUPERANS, J5SUPERANS, J12SUPERANS, and J27SUPERANS). When the five haplotypes of D. superans were compared with the present data, J2SUPERANS turned out to be identical to our DSUCOI02. Altogether, the sequence divergence of the six haplotypes ranged from 0.14% (1 bp) to 0.82% (6 bp; Table 2). A comparison between the two species revealed a sequence divergence ranging from 0.41% (3 bp) to 1.24% (9 bp; Table 2), presenting a slight overlap of the between-species divergence (0.41–1.24%) with the within-species divergence (0.14–0.55% in D. sibiricus and 0.14–0.82% in D. superans). Nevertheless, the haplotypes of the potential D. sibiricus specimens collected from South Korea are either identical or closer to those of D. sibiricus originating from Russia rather than D. superans originating from Japan.
| Number | Species (country, haplotype) | Pairwise divergence | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| 1 | D. sibiricus (S. Korea, DSICOI01) | – | 0.14 | 0.27 | 0.41 | 0.41 | 0.96 | 1.10 | 1.10 | 0.69 | 1.10 | 0.82 | 6.31 |
| 2 | D. sibiricus (S. Korea, DSICOI02) | 1 | – | 0.41 | 0.27 | 0.55 | 1.10 | 1.24 | 1.24 | 0.82 | 1.24 | 0.96 | 6.45 |
| 3 | D. sibiricus (S. Korea & Russia, DSICOI03) | 2 | 3 | – | 0.14 | 0.14 | 0.69 | 0.82 | 0.82 | 0.41 | 0.82 | 0.55 | 6.31 |
| 4 | D. sibiricus (S. Korea, DSICOI04) | 3 | 2 | 1 | – | 0.27 | 0.82 | 0.96 | 0.96 | 0.55 | 0.96 | 0.69 | 6.45 |
| 5 | D. sibiricus (Russia, KM3SIBIRICUS) | 3 | 4 | 1 | 2 | – | 0.55 | 0.69 | 0.96 | 0.55 | 0.96 | 0.69 | 6.45 |
| 6 | D. superans (Japan, DSUCOI01) | 7 | 8 | 5 | 6 | 4 | – | 0.14 | 0.69 | 0.55 | 0.69 | 0.69 | 6.72 |
| 7 | D. superans (Japan, DSUCOI02) | 8 | 9 | 6 | 7 | 5 | 1 | – | 0.82 | 0.69 | 0.82 | 0.82 | 6.72 |
| 8 | D. superans (Japan, J1SUPERANS) | 8 | 9 | 6 | 7 | 7 | 5 | 6 | – | 0.69 | 0.82 | 0.82 | 6.45 |
| 9 | D. superans (Japan, J5SUPERANS) | 5 | 6 | 3 | 4 | 4 | 4 | 5 | 5 | – | 0.41 | 0.69 | 6.72 |
| 10 | D. superans (Japan, J12SUPERANS) | 8 | 9 | 6 | 7 | 7 | 5 | 6 | 6 | 3 | – | 0.82 | 6.86 |
| 11 | D. superans (Japan, J27SUPERANS) | 6 | 7 | 4 | 5 | 5 | 5 | 6 | 6 | 5 | 6 | – | 6.58 |
| 12 | D. spectabilis (S. Korea, DSPCOI01) | 46 | 47 | 46 | 47 | 47 | 49 | 49 | 47 | 49 | 50 | 48 | – |
- The text within parentheses indicates the origin of species and the sequence type of the COI haplotype. The numbers above the diagonal are mean distance values; the numbers below the diagonal are absolute distance values.
The sequencing of the 393- to 399-bp long ITS2 from the potential D. sibiricus specimens collected from South Korea revealed four ITS2 sequence types (Table 1; DSIITS01–DSIITS04), with the alignment length of 406 bp, including gaps. These four sequences had a sequence divergence ranging from 0.25% (1 bp) to 0.49% (2 bp; Table 3). When the only GenBank-registered ITS-sequence type of D. sibiricus originating from 13 Russian individuals (Mikkola & Ståhls 2008) was aligned with the present data, it was found to be identical to our DSIITS01 (Table 3). From the two D. superans individuals sequenced in the present study, two ITS2 sequence types were obtained with an 8-bp difference (1.97%; Table 3). When the five GenBank-registered ITS2 homologous sequence types of D. superans originating from Japan were included, the sequence divergence ranged from 0.25% to 1.97% (8 bp; Table 3). Such slightly higher sequence divergence was detected in our DSUITS02, which contained a unique 5-bp deletion compared with the others. A comparison between the two species revealed a sequence divergence ranging from 0.99% (4 bp) to 2.71% (11 bp), presenting a slight overlap of the within-species divergence of D. superans (0.25–1.97%) with the magnitude of the between-species divergence (0.99–2.71%), but not with the within-species divergence of D. sibiricus (0.25–0.49%; Table 3). Similarly, the ITS2 sequences also indicate that the potential D. sibiricus specimens collected from South Korea are either identical or closer to the D. sibiricus specimens originating from Russia rather than the D. superans specimens originating from Japan.
| Number | Species (country, haplotype) | Pairwise divergence | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||
| 1 | D. sibiricus (S. Korea & Russia, DSIITS01) | – | 0.25 | 0.25 | 0.25 | 1.48 | 2.46 | 0.99 | 1.23 | 1.48 | 1.23 | 10.59 |
| 2 | D. sibiricus (S. Korea, DSIITS02) | 1 | – | 0.49 | 0.49 | 1.23 | 2.71 | 1.23 | 1.48 | 1.72 | 1.48 | 10.84 |
| 3 | D. sibiricus (S. Korea, DSIITS03) | 1 | 2 | – | 0.49 | 1.72 | 2.71 | 1.23 | 1.48 | 1.72 | 1.48 | 10.84 |
| 4 | D. sibiricus (S. Korea, DSIITS04) | 1 | 2 | 2 | – | 1.72 | 2.71 | 1.23 | 1.48 | 1.72 | 1.48 | 10.84 |
| 5 | D. superans (Japan, DSUITS01) | 6 | 5 | 7 | 7 | – | 1.97 | 0.49 | 0.74 | 0.49 | 0.74 | 9.61 |
| 6 | D. superans (Japan, DSUITS02) | 10 | 11 | 11 | 11 | 8 | – | 1.48 | 1.72 | 1.97 | 1.72 | 11.08 |
| 7 | D. superans (Japan, J5SUPERANS) | 4 | 5 | 5 | 5 | 2 | 6 | – | 0.25 | 0.49 | 0.25 | 9.61 |
| 8 | D. superans (Japan, J2SUPERANS) | 5 | 6 | 6 | 6 | 3 | 7 | 1 | – | 0.74 | 0.49 | 9.85 |
| 9 | D. superans (Japan, J12SUPERANS) | 6 | 7 | 7 | 7 | 2 | 8 | 2 | 3 | – | 0.74 | 9.61 |
| 10 | D. superans (Japan, J14SUPERANS) | 5 | 6 | 6 | 6 | 3 | 7 | 1 | 2 | 3 | – | 9.85 |
| 11 | D. spectabilis (S. Korea, DSPITS01) | 43 | 44 | 44 | 44 | 39 | 45 | 39 | 40 | 39 | 40 | – |
- The text in parentheses indicates the origin of the species and sequence types of the ITS2 haplotypes. The numbers above the diagonal are mean distance values; the numbers below the diagonal are absolute distance values.
When the COI and ITS2 sequences of the potential D. sibiricus specimens collected from the South Korean localities were concatenated individually, a total of six sequence types was obtained (Table 1; DSICOIITS01–DSICOIITS06) with a sequence divergence ranging from 0.09% (1 bp) to 0.44% (4 bp; Table 4). When these six concatenated sequences were aligned with the GenBank-registered sequences of D. sibiricus (from Russia), two sequence types were found, one of which was identical to our DSICOIITS04. The sequence divergence of these seven sequence types ranged from 0.09% (1 bp) to 0.44% (5 bp; Table 4). From the two D. superans individuals sequenced in the present study, we also obtained two concatenated sequences, named DSUCOIITS01 and DSUCOIITS02, with a 9-bp difference (0.79%; Table 4) between them. When these two concatenated sequences were aligned with the GenBank-registered sequence of D. superans (from Japan), seven additional sequence types were obtained (DSUCOIITS03–DSUCOIITS09), which had a sequence divergence ranging from 0.09% (1 bp) to 0.79% (9 bp; Table 4). When all D. superans sequence types were compared, the sequence divergence ranged from 0.09% (1 bp) to 1.15% (13 bp; Table 4). A comparison between the two species revealed a sequence divergence ranging from 0.62% (7 bp) to 1.59% (18 bp), presenting a slight overlap of the within-species divergence of D. superans (0.09–1.15%) with the magnitude of the between-species divergence, but not with the within-species divergence of D. sibiricus (0.09–0.44%; Table 4). Collectively, the concatenated sequence analysis also indicates that the potential D. sibiricus samples collected from South Korea are immediately closer to the D. sibiricus samples originating from Russia rather than D. superans. This, in turn, supports the notion that the Dendrolimus species examined herein are D. sibiricus rather than D. superans.
| Number | Species (country, haplotype) | Pairwise divergence | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | ||
| 1 | D. sibiricus (S. Korea, DSICOIITS01) | – | 0.09 | 0.09 | 0.18 | 0.09 | 0.35 | 0.35 | 1.50 | 1.23 | 1.23 | 1.15 | 0.79 | 1.06 | 1.23 | 1.06 | 1.15 | 7.75 |
| 2 | D. sibiricus (S. Korea, DSICOIITS02) | 1 | – | 0.18 | 0.26 | 0.18 | 0.26 | 0.44 | 1.59 | 1.32 | 1.32 | 1.23 | 0.88 | 1.15 | 1.32 | 1.15 | 1.23 | 7.84 |
| 3 | D. sibiricus (S. Korea, DSICOIITS03) | 1 | 2 | – | 0.26 | 0.18 | 0.44 | 0.44 | 1.59 | 1.32 | 1.32 | 1.23 | 0.88 | 1.15 | 1.32 | 1.15 | 1.23 | 7.84 |
| 4 | D. sibiricus (S. Korea & Russia, DSICOIITS04) | 2 | 3 | 3 | – | 0.26 | 0.18 | 0.18 | 1.32 | 1.06 | 1.06 | 0.97 | 0.62 | 0.88 | 1.06 | 0.88 | 0.97 | 7.75 |
| 5 | D. sibiricus (S. Korea, DSICOIITS05) | 1 | 2 | 2 | 3 | – | 0.44 | 0.44 | 1.59 | 1.15 | 1.15 | 1.23 | 0.88 | 1.15 | 1.32 | 1.15 | 1.23 | 7.84 |
| 6 | D. sibiricus (S. Korea, DSICOIITS06) | 4 | 3 | 5 | 2 | 5 | – | 0.18 | 1.50 | 1.23 | 1.23 | 1.15 | 0.79 | 1.06 | 1.23 | 1.06 | 1.15 | 7.93 |
| 7 | D. sibiricus (Russia, DSICOIITS07) | 4 | 5 | 5 | 2 | 5 | 2 | – | 1.32 | 1.06 | 1.23 | 0.97 | 0.79 | 1.06 | 1.23 | 1.06 | 1.15 | 7.93 |
| 8 | D. superans (Japan, DSUCOIITS01) | 17 | 18 | 18 | 15 | 18 | 17 | 15 | – | 0.79 | 1.15 | 0.71 | 0.88 | 0.97 | 1.15 | 0.97 | 1.06 | 8.19 |
| 9 | D. superans (Japan, DSUCOIITS02) | 14 | 15 | 15 | 12 | 13 | 14 | 12 | 9 | – | 0.53 | 0.26 | 0.62 | 0.71 | 0.71 | 0.71 | 0.79 | 7.67 |
| 10 | D. superans (Japan, DSUCOIITS03) | 14 | 15 | 15 | 12 | 13 | 14 | 14 | 13 | 6 | – | 0.79 | 0.62 | 0.18 | 0.71 | 0.71 | 0.79 | 7.49 |
| 11 | D. superans (Japan, DSUCOIITS04) | 13 | 14 | 14 | 11 | 14 | 13 | 11 | 8 | 3 | 9 | – | 0.53 | 0.62 | 0.79 | 0.62 | 0.71 | 7.75 |
| 12 | D. superans (Japan, DSUCOIITS05) | 9 | 10 | 10 | 7 | 10 | 9 | 9 | 10 | 7 | 7 | 6 | – | 0.44 | 0.44 | 0.26 | 0.35 | 7.67 |
| 13 | D. superans (Japan, DSUCOIITS06) | 12 | 13 | 13 | 10 | 13 | 12 | 12 | 11 | 8 | 2 | 7 | 5 | – | 0.71 | 0.53 | 0.62 | 7.49 |
| 14 | D. superans (Japan, DSUCOIITS07) | 14 | 15 | 15 | 12 | 15 | 14 | 14 | 13 | 8 | 8 | 9 | 5 | 8 | – | 0.18 | 0.26 | 7.75 |
| 15 | D. superans (Japan, DSUCOIITS08) | 12 | 13 | 13 | 10 | 13 | 12 | 12 | 11 | 8 | 8 | 7 | 3 | 6 | 2 | – | 0.09 | 7.75 |
| 16 | D. superans (Japan, DSUCOIITS09) | 13 | 14 | 14 | 11 | 14 | 13 | 13 | 12 | 9 | 9 | 8 | 4 | 7 | 3 | 1 | – | 7.84 |
| 17 | D. spectabilis (S. Korea, DSPCOIITS01) | 88 | 89 | 89 | 88 | 89 | 90 | 90 | 93 | 87 | 85 | 88 | 87 | 85 | 88 | 88 | 89 | – |
- The text within parentheses indicates the origin of the species and sequence type of the ITS2 haplotypes. The numbers above the diagonal are mean distance values; the numbers below the diagonal are absolute distance values.
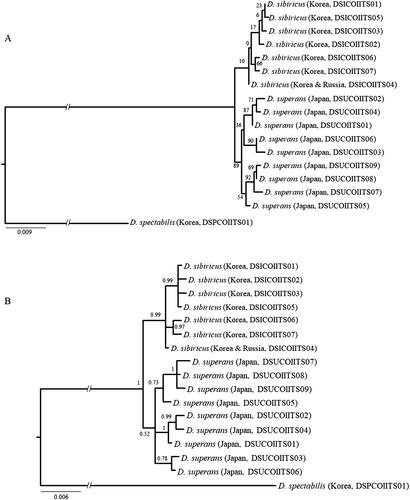
Phylogenetic analyses of the concatenated sequences of 3′-end of COI and ITS2 from D. sibiricus and D. superans showed that the nine D. superans sequence types collected from Japan (DSICOIITS01–DSICOIITS09) formed a relatively strong group with a nodal support of 89%, whereas the D. sibiricus sequence types poorly formed an inclusive group with a nodal support of 10% according to the ML analysis (Fig. 4A). The BI-based analysis was slightly better for D. sibiricus in that the D. sibiricus sequence types were strongly supported at 0.99 in BPP, whereas the D. superans sequence types formed a mere inclusive group with the BPP at 0.52 (Fig. 4B). Although each species was not supported as a complete independent group in any analysis, it is obvious that the D. sibiricus samples collected from South Korea grouped together with the Russian samples of D. sibiricus, rather than the Japanese samples of D. superans, supporting the distribution of D. sibiricus in South Korea rather than D. superans. Although we did not present an individual sequence-based analysis, the overall resolution for each species was roughly similar to that obtained from the present concatenated sequence-based analysis.
Discussion
When we compared specimens of the two closely related species D. sibiricus and D. superans collected from South Korea and Japan, the wing-pattern elements on fore- and hindwings and the genitalia obviously differed between the two species (Figs 2,3 ). Nevertheless, complete separation of the two species based on the data of the two concatenated sequences was impossible either using BI or ML methods (Fig. 4). Further, no pairwise sequence divergence of the individual or concatenated sequences of COI and ITS2 provided enough distance to distinguish the two species confidently (Tables 2-4). The sequence divergence of the DNA barcoding region from all individuals used in the present study was almost similar to that of the 3′-end region of COI (three haplotypes and four variable sites in the DNA barcoding region vs. four haplotypes and three variable sites in the 3′-end region of COI; data not shown). This indicated that neither the 3′-end region of COI nor the DNA barcoding region provided enough resolution to solve the status of these two species.
The DNA barcoding sequence has been widely used to identify and delimitate animal species, including insects (Hebert et al. 2003; Hajibabaei et al. 2006), but difficulties separating one species from another have also been reported among closely related species. This could happen in cases of relatively larger intraspecific variation and smaller interspecific divergence, particularly where intra- and interspecific variations overlap (Meyer & Paulay 2005; Song et al. 2008). Similar challenges discriminating several Dendrolimus species have been reported. For example, an uncertainty of the species status of D. punctatus, D. tabulaeformis, and D. spectabilis has been reported, with the latter two species treated as subspecies of the former (Zhao et al. 1992, 1999), whereas a morphology-based good species status has also been suggested other studies (Zhang et al. 2004a,b,c). Nevertheless, DNA barcoding sequence-based phylogeny provided unresolved relationships among them (Dai et al. 2012).
To overcome this difficulty, we further used the ITS2 sequence in accordance with previous studies that used this sequence in addition to the 3′-end of the COI sequence (Mikkola & Ståhls 2008; Dai et al. 2012). However, the use of the ITS2 sequence alone (data not shown) or in combination with the 3′-end of the COI sequence (Fig. 4) still could not clearly separate the two species. A similar resolution was reported in a recent study of Dendrolimus phylogeny, which used the nucleotide sequences of the 3′-end of COI and ITS2 sequences for several geographic samples of D. sibiricus and D. superans (Kononov et al. 2016).
Dendrolimus superans and D. sibiricus have long been named as several different species, but the two species were once combined into a single species, D. superans, due to their morphological similarity (Lajonquière 1973). However, they are treated as two independent species, D. superans and D. sibiricus (Mikkola & Ståhls 2008). Owing to the morphological similarity resulting from the intrinsic similarity between the two species caused by a rather incipient status of speciation, no clear genetic distance (Table 2) or phylogenetic separation of the two species (Fig. 4) was obtained from the current DNA data. Nevertheless, our current morphological analyses indicate that the potential D. sibiricus specimens collected from South Korea are truly D. sibiricus rather than D. superans. In particular, our two different tree-building methods (BI and ML) consistently indicated that the Dendrolimus samples collected from South Korea form a rather inclusive group with the D. sibiricus specimens collected from Russia, rather than with D. superans (Fig. 4), indicating previous misidentification of D. sibiricus as D. superans in South Korea. In future studies, variable molecular markers (e.g. microsatellite DNA or single nucleotide polymorphism markers) are needed to clarify the status of these two species with other congeneric species of the genus Dendrolimus.
Acknowledgments
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science, and Technology (2015R1D1A3A03018119). The authors thank Yasunori Kishida, an entomologist from Tokyo, Japan, for providing specimens of Dendrolimus superans.




