Molecular cloning and expression analysis of the highly conserved eukaryotic translation initiation factor 5A (eIF-5A) from Antheraea pernyi
Abstract
Eukaryotic initiation factor 5A (eIF-5A) is a highly conserved protein found in all eukaryotic organisms that plays a key role in the regulation of many cellular processes including translation elongation, cell proliferation, programmed cell death, mRNA turnover and decay, and abiotic stress responses. In this study, the eIF-5A gene from the Chinese oak silkworm Antheraea pernyi (Lepidoptera: Saturniidae) was characterized. The full-length ApeIF-5A cDNA of 1056 bp includes a 5′-untranslated region (UTR) of 138 bp, a 3′-UTR of 435 bp, and an open reading frame of 483 bp encoding a polypeptide of 160 amino acids. The deduced ApeIF-5A protein shares 99 %, 82 %, and 72 % sequence identity with orthologs in Bombyx mori, Drosophila melanogaster and Homo sapiens, indicating high conservation during animal evolution. Real-time quantitative reverse transcription PCR revealed expression in all four developmental stages and in all nine tissues tested, consistent with an important role in development. After challenge with lipopolysaccharide, the expression levels of ApeIF-5A were markedly upregulated. Phylogenetic analysis of amino acid sequences revealed A. pernyi eIF-5A was closely related to B. mori eIF-5A, consistent with traditional classification and other molecular data. The results indicate the potential value of eIF-5A in phylogenetic analysis.
Introduction
Eukaryotic translation initiation factor 5A (eIF-5A), present in all eukaryotic cells, is a small acidic polypeptide with a molecular weight of 17–21 kDa that is the only protein known to contain the unique polyamine-derived amino acid hypusine, formed by a novel post-translational modification involving two enzymatic steps (Kapp & Lorsch 2004; Mandal et al. 2014). EIF-5A takes part in the first stage of peptide bond formation during translation, and experimental evidence implicates it as a universally conserved translation elongation factor (Dever et al. 2014). The eIF5A translation initiation factor was originally identified in and isolated from rabbit reticulocytes, and characterized by in vitro assays (Kemper et al. 1976). EIF-5A proteins play key roles in many biological processes including translation elongation (Kang & Hershey 1994), cancer metastasis (Fujimura et al. 2015; Mathews & Hershey 2015), cell proliferation (Chatterjee et al. 2006), programmed cell death (Seko et al. 2015), mRNA turnover and decay (Liu et al. 2008), immune response (Martinez-Rocha et al. 2016), and abiotic stress responses (Xu et al. 2011).
The eIF-5A family is highly conserved and has been studied in various organisms including insects, plants, and mammals (Rossi et al. 2014). The eIF-5A gene has been isolated and characterized from many insect species such as Spodoptera exigua and S. frugiperda (van Oers et al. 1999), Bombyx mori (Hu et al. 2005), and Aedes albopictus (Shih et al. 2010). The amino acid sequence identity among insect eIF-5A ranges from ~82 % for distantly related species to 100 % for more closely related insects. The eIF-5A gene in B. mori is expressed in four developmental stages and in various tissues including the fat body, Malpighian tube, silk gland, midgut, testis, and ovary (Duan et al. 2009). Insect eIF-5A genes are involved in many biological processes, and is essential for cell growth, autophagy and protein synthesis in Drosophila melanogaster (Patel et al. 2009). eIF-5A plays a role in preventing mosquito cells from dying following viral infection by facilitating continued viral growth and persistent infection (Shih et al. 2010). In addition, eIF-5A has been implicated in the immune response against pathogen injection in insects (Hugo et al. 2013; Liu et al. 2013).
The Chinese oak silkworm Antheraea pernyi (Lepidoptera: Saturniidae) is a semi-domesticated species that is commercially cultivated in China, India, and Korea for silk production and as a food source (Liu et al. 2010a). In this study, the eIF-5A gene from A. pernyi was cloned and characterized, and its expression pattern during various developmental stages and different tissues was investigated. Finally, eIF-5A proteins from various organisms were evaluated for their potential value to phylogenetic study.
Materials and methods
Insects
Chinese oak silkworm strain No. 101 was collected from the Henan Province and reared on oak leaves until pupation. Nine different tissues (fat body, hemocytes, testis, ovary, brain, midgut, integument, Malpighian tubules and silk glands) were dissected from day 3 fifth instar larvae. Eggs, larvae, pupae, and adults were immediately frozen in liquid nitrogen and stored at −80°C until needed. To determine immune response, A. pernyi were randomly divided into two groups: one group was challenged by injecting 10 μL lipopolysaccharide (LPS; 1 μg/μL) into the pupae, the control group was injected with 10 μL PBS. After treatment, fat body were collected at 3, 6, 12, 24, 36, and 48 h.
RNA extraction and cDNA synthesis
Total RNA was extracted using the EASYspin Total RNA Extraction Kit (Aidlab, Beijing, China) according to the manufacturer's instructions. RNase-free DNase I was used to remove contaminating genomic DNA (Promega, Madison, WI, USA). The purity and quantity of extracted RNA were quantified by the OD260/OD280 ration using a NanoDrop 1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). First strand cDNA was generated from 1 μg of total RNA per sample using a TUREscript cDNA Synthesis Kit (Aidlab). For rapid-amplification of cDNA ends (RACE)-PCR, single-stranded cDNAs were synthesised using the SMART RACE cDNA Amplification kit (Clontech, Mountain View, CA, USA).
Cloning of the ApeIF-5A gene
A suppression subtractive hybridization of pupal cDNA library of A. pernyi has been constructed in our lab (Liu et al. 2013). An EST encoding eIF-5A homolog was isolated by random EST sequencing. Oligonucleotide primers (Table 1) were designed to amplify the full-length cDNA based on eIF-5A sequences from insects using Primer premier 5.0 software and RT-PCR and RACE-PCR approaches. RC3 and RC5 primers were used for RACE-PCR with cycling parameters as follows: 5 min at 94°C followed by 5 cycles of 94°C for 1 min, 65°C for 2 min, and then 30 cycles of 94°C for 1 min, 60°C for 40 s, 72°C for 50 s. PCR products were analysed on 1 % agarose gels, purified, ligated into the T-vector (Sangon, Shanghai, China) and sequenced commercially (Sunbiotech, Beijing, China).
| Primer | Primer sequence (5′-3′) | Experiment |
|---|---|---|
| RC5 | CGACCAAGTGAACCTTAGCG | RACE-PCR |
| RC3 | TGTGATCGCGGTCAAAGCA | RACE-PCR |
| F1 | CAAACACGGTCACGCTAAG | qPCR |
| R1 | TTGCCGCTATCGAAATCAG | qPCR |
| F18S | CGATCCGCCGACGTTACTAC | qPCR |
| R18S | GTCCGGGCCTGGTGAGATT | qPCR |
Sequence analysis of ApeIF-5A
DNASTAR software (DNASTAR Inc., Madison, WI, USA) was used to identify the open reading frame (ORF) and deduce the amino acid sequence. The sequence was subjected to a BLAST search (http://www.ncbi.nlm.nih.gov/blast.cgi) and functional domains were predicted using SMART (http://smart.embl-heidelberg.de/). Signal peptide prediction was performed using the SignalP server online tool (http://www.cbs.dtu.dk/services/SignalP/). The isoelectric point (pI) and molecular weight (MW) of the deduced amino acid sequences were predicted using the Compute pI/MW Tool at the Expert Protein Analysis System (ExPAsy) site (http://web.expasy.org/compute_pi/).
Homologous alignment and phylogenetic analysis
Amino acid sequences of eIF-5A proteins from selected organisms were downloaded from the GenBank database and used for phylogenetic analysis. Multiple sequence alignments were carried out using Clustal X software (Thompson et al. 1997). A phylogenetic tree was constructed by MEGA version 6.06 using the neighbour-joining method to generate phylogenetic trees with 1000 bootstrap repetitions. The JJT + G model was found to be appropriate for the amino acid sequence dataset (Tamura et al. 2013).
Expression analysis of ApeIF-5A gene
Expression levels of the eIF-5A gene were verified by qPCR during four developmental stages and in nine tissues. The 18S rRNA gene of A. pernyi was used as an internal reference (GenBank No. DQ347469). Primers for qPCR were designed using Primer premier 5.0 software, and qPCR was performed using a StepOnePlus Real-Time PCR System with a 2× SYBR Green qPCR Mix Kit (Aidlab, Beijing, China). Reaction mixtures (20 μL) contained 10 μL 2× SYBR Green qPCR Mix, 1 μL forward and reverse primers, 1 μL cDNA, and 7 μL RNase-free H2O. Cycling parameters were as follows: 95°C for 10 s followed by 40 cycles of 95°C for 15 s, 55°C for 15 s, and 72°C for 20 s. At the end of the reaction, a melting curve was produced by monitoring the fluorescence continuously while slowly heating the sample from 60 to 95°C. Each independent experiment was conducted in triplicate, and relative expression levels were determined as described previously by Livak and Schmittgen (2001) and analysed by qPCR. The expression level in the lowest group was set to 1.0. Data are expressed as the mean fold change (mean ± SE, n = 3).
Results and discussion
Cloning and sequence analysis of ApeIF-5A
The eIF-5A gene was isolated from the fat body of A. pernyi. A full-length cDNA was obtained by RT-PCR and RACE-PCR. The nucleotide and deduced amino acid sequences of ApeIF-5A are shown in Figure 1. The obtained 1056 bp cDNA sequence contains a 138 bp 5′-untranslated sequence, a putative ORF of 483 bp encoding a polypeptide of 160 amino acids, a 435 bp 3′-untranslated region, and a 19 bp poly-A tail. However, a putative AATAAA polyadenylation signal was not found in ApeIF-5A. The cDNA sequence of eIF-5A was submitted to NCBI under GenBank accession number KM979431. Based on the entire amino acid sequence, the molecular weight and isoelectric point were predicted to be 17.52 kDa and 5.10. Signal peptide prediction using SMART indicated no signal peptide, suggesting ApeIF-5A is not secreted. Motif scanning indicated that ApeIF-5A contains an amidation site at position 67–70, five casein kinase II phosphorylation sites at positions 7–10, 128–131, 132–135, 143–146, and 156–159, an N-myristoylation site at position 15–20, three protein kinase C phosphorylation sites at positions 47–49, 50–52, and 132–134, and a eukaryotic initiation factor 5A hypusine signature at position 50–57. Using SMART software, conserved domain prediction indicated a N-terminal at position 18–84, and a C-terminal at position 85–153. These domains are involved in a wide range of biological processes including translation elongation, cell proliferation, programmed cell death, mRNA turnover and decay, and abiotic stress responses (Kang & Hershey 1994; Chatterjee et al. 2006; Liu et al. 2008; Xu et al. 2011; Mathews & Hershey 2015; Seko et al. 2015; Martinez-Rocha et al. 2016).
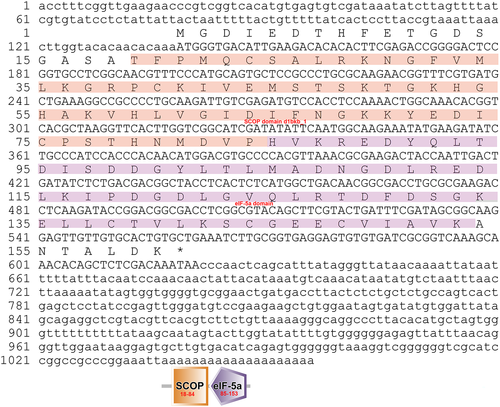
Alignment of homologous sequences from related species
A database search using the ApeIF-5A sequence as a query identified homologs in various eukaryotic organisms including both invertebrates and vertebrates. Of all eIF-5A sequences available to date, several are from Lepidopteran species, and the order Lepidoptera is the second largest insect order and includes the most damaging agricultural pests as well as beneficial insects. To assess the relatedness between eIF-5A and its homologs in other organisms, nine eIF-5A protein sequences were aligned using Clustal X software. As shown in Figure 2, multiple sequence alignment revealed that the deduced ApeIF-5A protein sequence has close homologs in various organisms. BLASTP analysis revealed that the predicted ApeIF-5A protein sequence shares 99 % sequence identity with B. mori eIF-5A (AAZ80490) and 82 % with D. melanogaster eIF-5A (AAG17032). The results showed that A. pernyi eIF-4A shares 82–99 % sequence identity with eIF-5As in closely related species, and 72–73 % sequence identity with eIF-5As in vertebrates. Sequence identity of >60 % indicates high conservation during the evolution of eukaryote organisms following divergence from a common ancestor (Embley & Martin 2006; Chen et al. 2014). Furthermore, sequence alignment and prediction of functional domains revealed that the amino acid sequences of the conserved features of ApeIF-5A, including the N-terminal and C-terminal domain, were even more highly conserved (Kapp & Lorsch 2004). These results strongly implicate ApeIF-5A as a translation elongation factor (Kang & Hershey 1994).
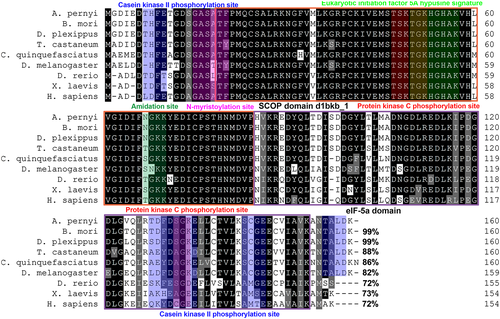
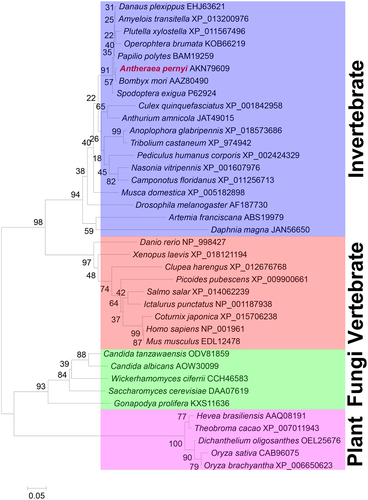
Phylogenetic analysis
A total of 28 eIF-5A amino acid sequences from eukaryotic organisms were obtained from the NCBI and used to construct a phylogenetic tree by the neighbour-joining method (Fig. 3). The results indicated that A. pernyi is most closely related to B. mori, and sequences could be divided into two distinct invertebrate and vertebrate groups (with high bootstrap support). Within the invertebrate group, insects were clustered into one branch. Crustacea are closely related to insect, and the clustering followed the expected evolutionary trend (Embley & Martin 2006). An increasing number of housekeeping genes are being identified, including myosin heavy chain type II genes (Ruiz-Trillo et al. 2002), ribosomal proteins (Hou et al. 2008), myosin light chains 1 and 2 (Liu et al. 2010b; Liu et al. 2015a), enolase (Liu et al. 2010c), phosphoserine aminotransferase (Wang et al. 2013), sans fille (Li et al. 2013), and 14–3-3 zeta (Liu et al. 2015b), and these are providing new molecular tools to assess evolutionary relationships. Phylogenetic analysis based on eIF-5A family amino acid sequences resulted in a clear separation between vertebrates and invertebrates, consistent with traditional classification and other molecular data (Liu et al. 2016). This suggests that eIF-5A might also be useful for phylogenetic analysis.
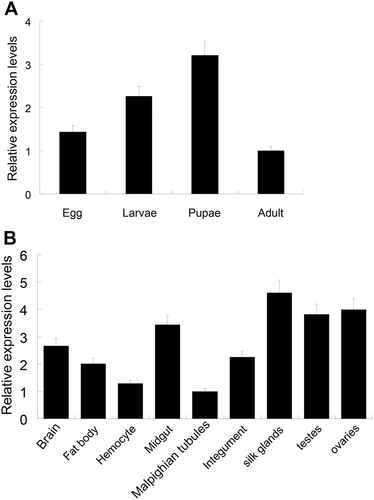
Expression of ApeIF-5A during different developmental stages and in different tissues
In this study, qRT-PCR was used to measure the expression pattern of ApeIF-5A during different developmental stages and in different tissues in day 3 fifth instar larvae. As shown in Figure 4A, ApeIF-5A mRNA was detected during four developmental stages (egg, larvae, pupae, and adult), indicating a potentially important role throughout the entire life cycle. Among the four developmental stages, ApeIF-5A mRNA levels were highest in pupae. The tissue distribution of ApeIF-5A expression in day 3 of fifth instar larvae was determined (Fig. 4B). Expression was detected in all nine tissues tested (fat body, hemocytes, testis, ovary, brain, midgut, integument, Malpighian tubules and silk glands). Expression was highest in silkgland, testis, and ovary tissue, and lowest in Malpighian tubules and hemocytes. The qPCR results revealed no significant differences in mRNA levels among these tissues, suggesting that the eIF-5A gene might not be differentially expressed in particular organs and may therefore act as a housekeeping gene, consistent with findings in other insects. A genome-wide microarray study using SilkDB showed that the B. mori eIF-5A gene is widely expressed in testis, ovary, fat body, integument, blood, midgut, Malpighian tubules, and silk gland tissue (Duan et al. 2009). The A. pernyi qRT-PCR results were also consistent with the B. mori microarray data.
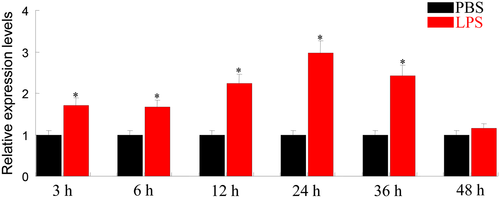
Expression of ApeIF-5A in response to LPS challenge
The expression levels of ApeIF-5A were identified in fat body at different time points in response to LPS challenge. After LPS challenge, the expression of ApeIF-5A was upregulated significantly compared to the controls (Fig. 5). The peak of ApeIF-5A mRNA transcripts was observed 24 h post-injection. EIF-5A proteins play key roles in many biological processes. eIF-5A from mosquito cells may stimulate the overexpression in response to viral infection, which facilitates a reduction in cell death in infected C6/36 cells (Shih et al. 2010). Post-translational hypusination of the EIF-5A plays a key role in regulating Fusarium graminearum virulence (Martinez-Rocha et al. 2016). EIF-5A from soybean acts as a key element in Rsv1-mediated lethal systemic hypersensitive response to soybean mosaic virus infection (Chen et al. 2017). All these results imply that EIF-5A plays an important role in immune response.
Acknowledgments
The authors declare no competing interests. This work was supported by the National Natural Science Foundation of China (31640074 and 31672267), the Natural Science Foundation of Jiangsu Province (BK20160444), the Natural Science Research General Program of Jiangsu Provincial Higher Education Institutions (15KJB240002, 16KJA180008, and 12KJA180009), the Special Guide Fund Project of Agricultural Science and Technology Innovation of Yancheng city (YKN2014022, YKN2013013, and YKN2014019), the Jiangsu Provincial Key Laboratory of Coastal Wetland Bioresources and Environmental Protection (JLCBE14006), the Jiangsu Provincial Key Laboratory for Bioresources of Saline Soils (JKLBS2014013 and JKLBS2015004).




