Quaternary vicariance of tiger beetle, Cicindela chinensis, in Ryukyu, Japan, Taiwan and Korea–China
Abstract
We show vicariance of Cicindela chinensis in Okinawa, Japan (differentiated within Japan) and Korea–China through construction of Bayesian inference trees by BEAST2. Calibration was done using an assumption of the MRCA expansion of C. chinensis at 1.55 Ma (=geologically obtained formative time of the Ryukyu islands) following the protocol of BEAUti. We derived substitution rates for mitochondrial COI (1.66%/m.y.) and nuclear 28SrRNA (0.109%/m.y.) of analyzed Cicindela. Cicindela ferriei is a sister of C. chinensis, and these two species differentiated from each other at ca. 3 Ma before the expansion of C. chinensis. However, they are not strongly differentiated between Amami-Oshima and Tokuno-shima, although they display different color. Vicariance at 1.55 Ma is also recognized between Cicindela batesi in Taiwan and Cicindela aurulenta and virgula in continental China. From the sequence data we obtained, it is also evident that C. c. okinawana recently colonized Ishigaki-jima from Okinawa-jima, as did C. batesi in Iriomote-jima from Taiwan.
Introduction
Absolute age calibration of a phylogenetic tree is only possible if an accurately dated geologic event can be reasonably interpreted to have caused a branching event by creating barriers to migration of species. This allows precise phylogenetic age determination independent of an assumed molecular clock. However, very few geologic events of this type have been robustly dated and/or characterized to the current research standard in geology. The Quaternary geologic event that formed the present Ryukyu, Japan and Taiwan archipelagoes is an exception, and it offers the potential for robust geologically based age calibration of phylogenetics (Fig. 1; Osozawa et al. 2012).
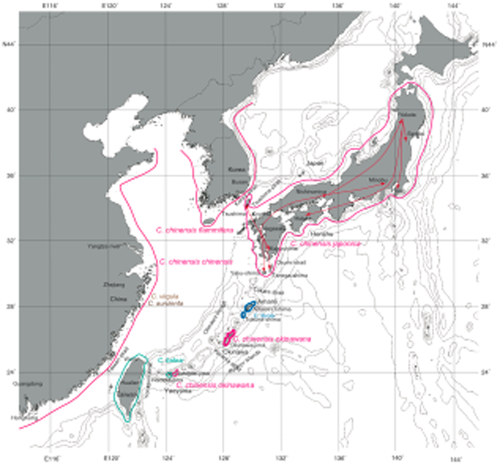
Distribution map of Cicindela (tiger beetle) in the Ryukyu islands and neighboring regions. Arrows in Japan: diversion routs inferred from COI haplotype networks indicated by Figure 4. (notice: this inference is not the case).
The Okinawa trough began to open at about 1.55 Ma, separating and isolating the Ryukyu islands from the Chinese continent to form the eastern Asia archipelagoes along with the islands of Japan and Taiwan (Fig. 1; Osozawa et al. 2012). As these islands were rifted from the mainland, major straits between them also formed, so the time of opening of the Okinawa trough also corresponds to the creation of the major straits between islands or island groups. These straits include the Kerama Strait separating northern and southern Ryukyu islands, the Tokara Strait separating the Japanese islands and northern Ryukyu islands, the Yonaguni Strait separating southern Ryukyu islands and Taiwan island, the Tsushima Strait separating southwest Japan islands and Korea and the Taiwan Strait separating Taiwan island and China (Fig. 1; Osozawa et al. 2012).
These straits acted as strong barriers, even for flying insect species, and generated endemic species from the most recent common ancestral species (MRCA) that were originally distributed widely on the continent (Osozawa et al. 2013, 2015a,b; Osozawa & Wakabayashi 2015). Polytomy of phylogenetic trees may be a characteristic of this type of simultaneous vicarant speciation, rather than time-transgressive and step-like branching topology expected in island chains such as the Hawaiian islands as a consequence of a stepping-stone-like process of dispersion and colonization (e.g. Bess et al. 2014). For Ryukyu island endemic species, calibration for polytomy can be accurately and reasonably done by assigning 1.55 Ma to the simultaneous multi-branched basal node (basal splitting node from the MRCA).
Among tiger beetles distributed in eastern Asia, Cylindera species have been extensively studied by Sota et al. (2011) using mitochondrial cytochrome oxidase subunit I (COI) and nuclear 28SrDNA sequences. However, Ryukyu endemic Cicindela species, the main target of the present study, have not been investigated in detail. In addition, the previously published model of phylogenetic evolution for these species was based on an obsolete geological model for the formation of the Ryukyu islands, which featured a major land bridge (e. g., Kimura 2000). Detailed geologic evidence, however, shows that such a land bridge did not exist (so dispersion by way of such a bridge would not be plausible), and the phylogenetic evolution appears much more compatible with our new geological model (Osozawa et al. 2012) as we show here.
This paper presents evidence for 1.55 Ma vicariance of Cicindela chinensis, particularly the differentiation into its subspecies, by construction of Bayesian inference trees of COI and 28SrRNA and their haplotype network analyses. We also genetically investigate the origin of the recently introduced Cicindela species in the Yaeyama islands.
Materials and methods
Cicindela distribution and sampling
Endemic subspecies of Cicindela chinensis live in Japan, Ryukyu and Korea–China (Fig. 2). Cicindela chinensis japonica Thunberg, 1781 resides in Japan, including islets like Tsushima, Tanega-shima and Yaku-shima, but excluding Hokkaido (Fig. 1). A similar form, Cicindela chinensis okinawana Nakane, 1957, lives on Okinawa-jima in the Ryukyu islands, and has been recently (in 2005) found in Ishigaki-jima (Sato & Takagi 2006). We collected C. c. okinawana at the same locality from Ishigaki-jima in 2010 and 2013 (Table S1). We also collected both Korean and Chinese specimens. A nominotypical subspecies in Korea is Cicindela chinensis flammifera Horn, 1921, and the host species in China is Cicindela chinensis chinensis DeGeer, 1774.
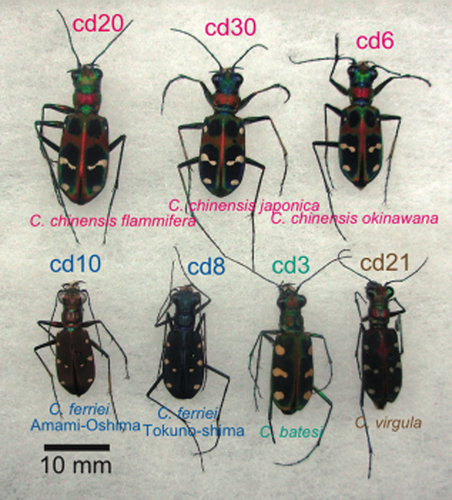
Cicindela specimens: C. chinensis okinawana is morphologically similar to the other C. chinensis subspecies; C. ferriei in Amami-Oshima and Tokuno-shima are differently colored. Eight spots in the elytra is a basic pattern common to Cicindelidae.
On Amami-Oshima and Tokuno-shima, the endemic species is Cicindela ferriei Fleutiaux, 1894. Each island yields subspecies of a brownish Cicindela ferriei ferriei Fleutiaux, 1894, and a bluish Cicindela ferriei indigonacea Miwa, 1935, respectively (Fig. 2).
Cicindela batesi Fleutiaux, 1894 = Cosmodela batesi (Fleutiaux, 1893) was found on Iriomote-jima in 2002 (Hori 2002), and spread over the island. This species is endemic to Taiwan (Fig. 2; Lin & Okuyama 2014), but probably recently colonized in Iriomote-jima. We collected C. batesi at the Gunkan-iwa boat boarding point, at the mouth of the Urauchi-gawa River, Iriomote-jima, as well as in Taiwan. A similar species, Cicindela aurulenta Fabricius, 1801, is widespread in China and Indochina. We collected that species in Hong Kong. In China, Cicindela (Cosmodela) virgula (Fleutiaux, 1894) co-occurs, and we also collected this species (Fig. 2).
As outgroup species, we selected Cylindera ovipennis (GenBank/DDBJ; Sota et al. 2011).
DNA sequence data of Cicindela are registered in the DDBJ/GenBank. Species isolate, country, accession number and collection date are shown in Table S1.
DNA extraction and polymerase chain reaction amplification
Collected leg muscle samples used for analyses were stored in 1.5 mL tubes filled with 99% ethanol, with the remaining material stored at −30°C in a freezer.
The COI gene has a relatively high substitution rate, and it has proved useful for subspecies level systematics. The 28SrRNA gene has a relatively low substitution rate, and is suitable to analyze for higher level phylogeny (Sagegami-Oba et al. 2007).
DNA extraction was done using the GenElute Mammalian Genomic DNA Miniprep Kit (Sigma-Aldrich, Tokyo, Japan).
Amplification was done using the same primers as Sota et al. (2011). COI primers were C1-J-2195 (5-TTG ATT TTT TGG TCA TCC AGA AGT-3) and TL-2N-3014 (5-TCC AAT GCA CTA ATC TGC CAT AT TA-3) (Simon et al. 1994). Nuclear 28SrRNA primers were 28S-01 (5-GAC TAC CCC CTG AAT TTA AGC AT-3) and 28S-R01 (5-GAC TCC TTG GTC CGT GTT TCA AG-3) (Kim et al. 2000).
COI amplification was done using GoTaq G2 Green Master Mix (Promega, Tokyo, Japan). The temperature of incubation was 94°C for 60 s, with denaturation at 94°C for 30 s, annealing at 50°C for 60 s, extension at 72°C for 60 s, cycled 35 times, and final extension was at 72°C for 5 min. 28SrRNA amplification was done using Tks Gflex DNA Polymerase (Takara Bio Inc., Kusatsu, Japan). The temperature of incubation was 94°C for 120 s, with denaturation at 98°C for 10 s, annealing at 56°C for 15 s, extension at 68°C for 60 s, cycled 30 times, and final extension was at 72°C for 10 min.
The PCR product was purified using Wizard SV Gel and PCR Clean-Up System (Promega, Tokyo, Japan). Sequencing was done by Macrogen Japan.
Sequence alignment
Sequence alignment was done using ClustalW incorporated in MEGA5 (Tamura et al. 2011). No gap was observed in our aligned COI sequences, and an aligned COI sequence of 811 bp is available to analyze. Systematic gaps reflecting species were recognized in the 28SrRNA sequences of 863 to 872 bp, and such positions containing gaps were eliminated in analyses. Codon translation for the COI sequence was checked by the ExPASy-Translate tool (Swiss Institute of Bioinformatics, Lausanne, Switzerland), and such data is reflected in our registered data in the DDBJ/GenBank. The sequences were also checked by basic local alignment search tool (BLAST) offered by DDBJ/GenBank.
Phylogenetic analyses by BEAST2
Bayesian inference trees were constructed using a series of software for Bayesian Evolutionary Analysis Sampling Trees 2 (BEAST2; Bouckaert et al. 2014), running BEAUti, BEAST, (Tracer), (LogCombiner) and DensiTree (or TreeAnnotator + FigTree), in ascending order.
In BEAST analyses, as much as possible, we avoided paralogy where two terminals belonging to the same species are not concatenated, because otherwise they must have significant overlapping sequences if treated separately.
We considered that the MRCA of Cicindela chinensis began to differentiate at 1.55 ± 0.15 Ma, following the geologic evidence for island separation presented in Osozawa et al. (2012). For the time to the MRCA for C. chinensis, a date of 1.55 Ma was input into BEAUti.
The following software settings were used in BEAUti. Import Alignment (fasta files for COI and 28SrRNA sequences were converted into nexus files by ClustalW offered by DDBJ, appeared data in Partitions), Site Model (Substitution Rate: check box, Gamma Category Count: 4, Shape: check box, Substitution Model: HKY, Frequencies: Empirical; for each gene), Clock Model (Strict Clock), Priors (press + button, incorporate data, named, select Normal, tmrca for C. chinensis: Mean: 1.55 Ma, Sigma: 0.15 Ma) and Markov chain Monte Carlo methods (MCMC: length of chain: 10 000 000; if not passed indicated in Tracer, increased as 20 000 000).
Running BEAST2 was done by incorporating each xml input file made by BEAUti.
Two tree files made by BEAST2 for each gene could be combined into one combined file using LogCombiner.
The consequent tree was drawn by DensiTree. Alternatively, the tree files were input into TreeAnnotator and the tree could be drawn using FigTree. The 95% highest posterior density for confidence intervals of ages and posterior probability are shown in FigTree.
A tracer was used to record the mean base substitution rate of each gene, incorporating log input file made by BEAST2. Effective sample size values were selected to be >200, and if they were not, an extended run was set in MCMC in BEAUti.
Haplotype network analyses
The relationships among COI and also 28SrRNA haplotypes were visually assessed by constructing statistical parsimony networks with a 95% connection limit using TCS v1.21 (Clement et al. 2000). The sequence file was converted into phylip format using ClustalW offered by DDBJ.
Results
Bayesian inference trees
The Bayesian inference tree based on the mitochondrial COI region of Cicindela tiger beetles is shown in Figure 3. The topology made by combined analysis of COI and 28SrRNA is concordant to that in Figure 3.
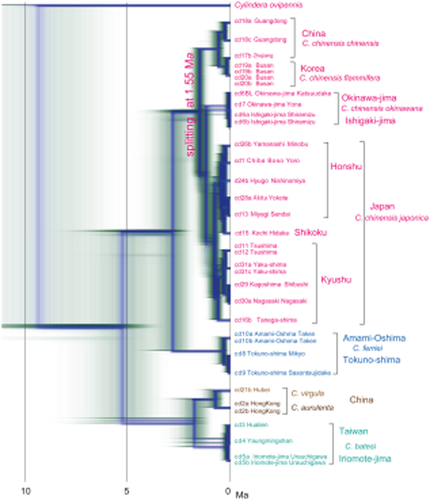
Bayesian inference tree of Cicindela species using COI gene sequence (811 bp). Output by DensiTree. Tree for combined genes has similar topology.
Three major lineages are recognized: (i) Cicindela chinensis; (ii) C. ferriei; and (iii) C. batesi + aurulenta + virgula.
The C. chinensis lineage is subdivided into China–Korea and Japan–Ryukyu major clades (Fig. 3). In addition, C. c. japonica and okinawana constitute clades as a sister group, but C. c. japonica is multifurcated within Japan. The C. c. japonica clade consists of Kyushu subclade including those of Tsushima, Yaku-shima and Tanega-shima, and the remaining Honshu and Shikoku subclade. C. c. okinawana has not diverged between Okinawa-jima and Ishigaki-jima.
The C. ferriei lineage constitutes C. f. ferriei (Amami-Oshima) and C. f. indigonacea (Tokuno-shima) subclades (Fig. 3), but theses are not deeply diversified.
The C. batesi (Iriomote-jima and Taiwan) and C. aurulenta + C. virgula (China) subclades constitute a sister group with relatively deep divergence (Fig. 3). The COI sequences of the Taiwan and Iriomote-jima populations of C. batesi are almost common.
For splitting of ancestral C. chinensis, we have assigned an age of 1.55 Ma, reflecting the age of isolation of the islands from the mainland (Fig. 3). The COI substitution rate is estimated as 1.66%/m.y., and the rate for 28SrRNA rate is estimated as 0.109%/m.y. As indicated in FigTree for combined tree, the branching age between C. chinensis and C. ferriei is estimated at 2.8 Ma, and that between these and C. batesi + aurulenta + virgula is at 5.3 Ma. The branching age between the China populations of C. aurulenta + virgula and the Taiwan + Iriomote-jima populations of C. batesi, constituting a sister group, is 1.7 Ma.
Haplotype networks
COI networks (Fig. 4) reflect subspecies or their areal populations. The Chinese populations of C. chinensis chinensis show higher genetic diversity than do Korean populations of C. c. flammifera. The Japanese populations of C. c. japonica are very diverse, particularly within Kyushu, and are differentiated into Kyushu and Honshu–Shikoku populations.
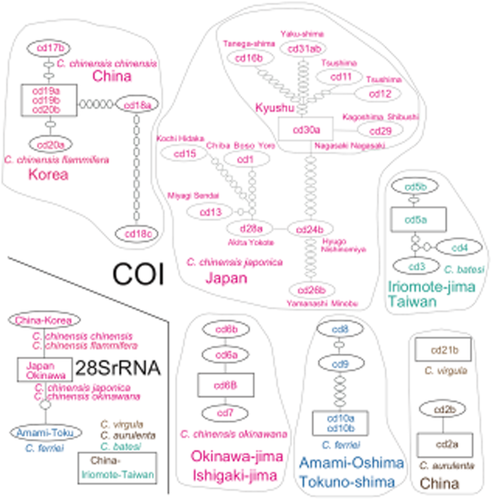
Haplotype networks of Cicindela species using the COI sequence (811 bp) and the nuclear 28SrRNA gene sequence (863 to 872 bp). Large circles are sampled haplotypes (square = root and ancestral haplotype); small circles are unsampled intermediate haplotypes; lines indicate single sequence differences (mutations) joining haplotypes. These networks reflect clades and subclades of the phylogenetic tree.
In the 28SrRNA network (Fig. 4), C. chinensis and C. c. japonica and okinawana constitute a single network. A haplotype of C. ferriei is also connected as one of a haplotype network of the species C. chinensis. In addition, only one haplotype was observed from three species: C. batesi, C. aurulenta and C. virgule.
Discussion
Vicariance vs dispersion of Cicindela
We calibrated the Bayesian inference tree by setting a splitting date of the MRCA of C. chinensis at 1.55 Ma, following protocol of BEAUti. We propose that strong vicariance between China–Korea and Japan–Ryukyu for C. chinensis began at 1.55 Ma corresponding to the date of separation of the islands from the mainland (Osozawa et al. 2012).
The migration barrier was the Korea strait between Korea and Tsushima (Fig. 1), because the Tsushima population is included in the Japan population (Figs 3, 4); a similar scenario was proposed for Ephoron mayfly (Sekine et al. 2015). This case differs from that of the Pyrocoelia firefly, for which the migration barrier was the Tsushima Strait between Tsushima island and Japan (Osozawa et al. 2015b). The reason for this difference is uncertain.
The barrier for C. c. japonica in Japan and C. c. okinawana in Okinawa-jima was the Tokara Strait (Fig. 1). These two subspecies were vicariantly speciated at 1.2 Ma (Fig. 3), lagging slightly behind the 1.55 Ma formative age of the Tokara Strait.
In a wide area including central-southern China and Korea, vicariance is not evident (Fig. 3; the Zhejiang and Guangdong populations constitute sister populations, but the Zhejiang and Busan population also constitute sister populations; thus differentiation is shallow).
In contrast, extensive vicariance of C. c. japonica within Japan may reflect development of barriers on smaller scale within Japan, similar to the case of barriers for carabid beetles in Japan (Su et al. 1998; Osozawa et al. 2016). The Kyushu main island population constitutes the Kyushu clade with Tsushima, Yaku-shima and Tanega-shima islets populations (the Tsushima and Osumi straits are mild barriers; Fig. 1), and a sister group with the other Japanese populations (Fig. 3), but future extensive study with a larger number of collection sites and specimens is needed to clarify the evolution of these species in Japan.
The Bayesian inference tree for C. chinensis has step-like topology (Fig. 3), and it may suggest dispersion from China–Korea to Okinawa-jima, and then Japan. Migration from Okinawa-jima to Japan may be possible by rafting on the Kuroshio Current (Osozawa et al. 2016), but crossing the East China Sea (Okinawa trough area) from China to Okinawa-jima would not be possible in such a manner because of the lack of favorable currents.
The COI haplotype network within Japan (Fig. 4) may also infer dispersion rather than vicariance. The expected migration routes inferred from Figure 4 are shown in Figure 1. In Kyushu, from Nagasaki (root haplotype), a southward route is separated into three independent routes: (i) route 1, Kagoshima; (ii) route 2, Yaku-shima; and (iii) route 3, Tanega-shima (not via Kagoshima); the northward route is route 4 toward Tsushima. Dispersion from Nagasaki, Kyushu, to Honshu and Shikoku appears both backward and forward: (i) route 1, Nishinomiya-Minobu; (ii) route 2-1, Nishinomiya-Yokote-Sendai; (iii) route 2-2, Nishinomiya-Yokote-Yoro; and (iv) route 2-3, Nishinomiya-Yokote-Hidaka (Shikoku). These are clearly not exact dispersion routes, and the network reflects vicariance.
Vicariance at about 1.7 Ma is also apparent for C. batesi and C. aurulenta + C. virgula (Fig. 3), although it is possible to input the date of 1.55 Ma for the node of the MRCA of C. batesi and C. aurulenta + C. virgula in BEAUti. Cicindela aurulenta and C. virgula are judged to have sympatrically speciated within China after the above vicariance.
Cicindela ferriei belongs to a different lineage from C. chinensis morphologically (Fig. 2) and genetically (Figs 3, 4). Speciation of C. ferriei was unrelated to the 1.55 Ma isolation of the Amami islands from China. It apparently differentiated much earlier at 2.8 Ma (Fig. 3). The Amami-Oshima and Tokuno-shima populations are not deeply genetically differentiated, which indicates imperfect isolation between each island for C. ferriei.
Introduced Cicindela in Yaeyama islands
It is evident that C. c. okinawana recently colonized Ishigaki-jima from Okinawa-jima, and C. batesi also recently colonized Iriomote-jima from Taiwan. The former immigration might have been related to the construction of the Nagura Dam in Ishigaki-jima, which continued until 1999. For the latter case, because of the inflow of the Kuroshio Current between Taiwan and Iriomote-jima and the distance of 200 km, such natural dispersion is considered to be very difficult (cf., Osozawa et al. 2016). However, no record of human introduction has been shown to date.
Conclusion
We show evidence for vicariant speciation of C. chinensis subspecies, and C. batesi vs C. aurulenta + C. virgula. We assigned a 1.55 Ma date to the multifurcation and basal node in a Bayesian inference tree, and obtained reliable base substitution rates both for mitochondrial COI and nuclear 28SrDNA genes of Cicindela. Cicindela ferriei belongs to a different lineage apparently diverged earlier than 1.55 Ma. Our phylogenetic analyses also appear to delimit the original habitat of C. c. okinawana in Ishigaki-jima and C. batesi in Iriomote-jima.
Acknowledgments
We thank Shinya Oba, Kimiko Kobayashi, Hajime Sugiyama, Yasuto Horinouchi and Shusuke Osozawa for making available Japanese samples, and Akira Mishima for making available Chinese samples. Bor-ming Jahn and Chin-Ho Tsai supported sample collections in Taiwan. This project was partly financed through the Osozawa Fund, Tohoku University. We thank Shuichi Ogino, Kyoji Osozawa, Hachijojima Tourist Association, CTI Engineering Co., Ltd., and NEWJEC, Inc. for contributing to this fund.




