Identification and expression profiles of putative cytochrome P450 monooxygenase genes from Cnaphalocrocis medinalis (Lepidoptera: Pyralidae)
Abstract
In insects, cytochrome P450 monooxygenases (P450s) have received considerable attention for their roles in the detoxification of harmful chemicals and insecticide resistance. The rice leaf-folder, Cnaphalocrocis medinalis, is a severe lepidopteran rice pest. In this research, 36 putative P450 genes were identified from the transcriptome dataset of C. medinalis. Phylogenetic analysis showed that these genes fell into four clans, including the mitochondrial clan (6 genes), CYP2 clan (5 genes), CYP3 clan (16 genes) and CYP4 clan (9 genes), and were classified into 19 families and 30 subfamilies. Since the CYP6 P450 family in insects is the major family related to the detoxification of xenobiotics, the expression profiles of five genes in the CYP6 family were determined by real-time quantitative PCR. These genes were differentially expressed in various larval tissues, including the midgut, Malpighian tubules and fat body. Of these, CYP6AW1 and CYP6CV1 were mainly expressed in the midgut, while CYP6AE76 was highly expressed in the fat body. CYP6AB62 and CYP6AE28 were ubiquitously expressed. The transcription of CYP6AE28 was significantly induced by abamectin at LD20 dose. Taken together, the results provided useful information for the characteristics of P450s in C. medinalis and for future functional studies.
Introduction
All insects living in their environment are challenged by exposure to many different harmful chemicals, natural or synthetic. To fight against the toxins they encounter, insects have evolved multiple defense mechanisms involving a variety of detoxificative enzymes, and one of those is cytochrome P450 monooxygenases (P450s) (Li et al. 2007). P450s are a diverse superfamily of haem-thiolate enzymes playing crucial roles in the metabolism of a great range of xenobiotic compounds, including insecticides, plant secondary metabolites and toxic pollutants (Schuler 2011; Feyereisen 2012). Furthermore, the overexpression of specific P450 genes and elevated P450 enzyme activities are often observed in insects and appear to be associated with insecticide resistance (Li et al. 2007; Bass & Field 2011). Insect P450s are also involved in other essential physiological processes such as biosynthesis and catabolism pathways of juvenile hormone, ecdysone and cuticular hydrocarbon (Guittard et al. 2011; Feyereisen 2012; Qiu et al. 2012), and thus contribute to the regulation of insect growth, development and reproduction. In addition, antennae-specific P450s play a key role in protecting the insect's olfactory system by rapidly inactivating the stray odorant molecules surrounding the olfactory receptor neurons (Keeling et al. 2013; Leal 2013).
Cnaphalocrocis medinalis Guenée (Lepidoptera: Pyralidae), commonly known as the rice leaf-folder, is a severe rice insect pest. Outbreaks occur frequently throughout eastern Asia (Gurr et al. 2012). For a long time, the pest has been controlled mainly through spraying of insecticides. However, extensive application of insecticides resulted in reduced insecticide susceptibility for this insect species (Zheng et al. 2011; Zhang et al. 2014). P450s play essential roles in the metabolism of insecticides and development of insecticide resistance; however, to date, only a limited number of P450 genes have been identified in C. medinalis (GenBank data) and two of them were characterized as candidates involved in the metabolism of rice allelochemicals (CYP6AE28 and CYP6AE30) (Liu et al. 2010). In this work, we identified 36 putative P450s genes, including 32 novel genes, by searching the C. medinalis transcriptome dataset. The phylogenetic relationships of these P450s with their orthologs in other insect species were analyzed, and the expression profiles of genes belonging to CYP6 family were also determined. The results provide valuable information to facilitate further studies for understanding the functions of these C. medinalis P450s.
Materials and methods
Insects
Fourth-instar larvae of C. medinalis were collected from an experimental field of Anhui Agricultural University, Hefei, Anhui, China. The field studies did not involve endangered or protected species. No specific permits were required for the described field collections. Cnaphalocrocis medinalis is a common agricultural pest and is not included in the “List of Protected Animals in China”. Individuals were anesthetized on ice and dissected into different tissues (integument, midgut, Malpighian tubules and fat body). Each sample was immediately frozen in liquid nitrogen and stored at −80°C until use.
Insecticide treatment
Abamectin (purity >90%) was purchased from Veyong Bio-Chemical Co., Ltd, Shijiazhuang, Hebei, China, and diluted with analytical-grade acetone. The fourth-instar larvae of C. medinalis were exposed to the chemical by topical application, as described previously (Huang et al. 2011). Briefly, a sub-lethal concentration (LD20, 0.12 ng per larva) of abamectin was applied topically on the dorsal part of the larval middle abdomen by using a capillary microapplicator. Control insects were treated with acetone only. Abamectin-treated and control insects were collected after 24 h, frozen in liquid nitrogen immediately, and stored at −80°C before RNA extraction. Each treatment was biologically replicated three times and in each replicate fifteen larvae were collected.
RNA isolation and cDNA synthesis
Total RNA was isolated from different tissues of the C. medinalis larvae, as well as the abamectin-treated and control larvae, using Trizol reagent (Life Technologies, Carlsbad, CA, USA) following the manufacturer's protocol. The concentration of each RNA sample was determined using a Nanodrop spectrophotometer (Thermo Scientific, Wilmington, DE, USA). Total RNA (1 μg) from each sample was reverse-transcribed using the PrimeScript RT reagent Kit with gDNA Eraser (Takara, Dalian, China), and then diluted to 10 ng/μL with nuclease-free water.
Transcriptome searching
Previously, a transcriptome dataset of C. medinalis was released (NCBI SRA Acc. no. SRA058899, Li et al. 2012). We searched the dataset using the Basic Local Alignment Search Tool (BLAST, Altschul et al. 1997) to obtain putative P450 genes. The process could be divided into three stages. First, we performed a TBLASTN search to identify all P450 sequences that have full-length or partial open reading frames (ORFs), by using the annotated P450s from other insect species as references. Second, these partial P450 sequences were used as queries to re-screen the dataset; some of them could be assembled and extended to full-length or near full-length genes. Last, the BLASTX best matches of these partial P450s also were used as templates to predict potential targets, and the outputs were examined manually to determine the closest relative to the partial gene. As a result, 36 putative P450 sequences were identified. These putative P450 genes were amplified from whole larva cDNA and were confirmed to be the target genes by direct DNA sequencing (primers were listed in File S1). Furthermore, these sequences were compared by searching against the NCBI non-redundant protein database using BLASTX (cut-off e-value of 10–5).
Bioinformatic analyses
The functional domains were predicted by searching the NCBI's conserved domain database (http://www.ncbi.nlm.nih.gov/structure/cdd/cdd.shtml). The transmembrane anchors were determined with TMHMM (http://www.cbs.dtu.dk/services/tmhmm). The amino acid sequences of C. medinalis P450s together with their orthologs were aligned using Clustal Omega (http://www.ebi.ac.uk/tools/msa/clustalo). A phylogenetic tree was constructed in MEGA5.05 software using the neighbor-joining method with 1000-fold bootstrap resampling (Tamura et al. 2011). P450 sequences used are listed in File S2.
Real-time quantitative PCR
Real-time quantitative polymerase chain reaction (qPCR) was run on a StepOne Plus Real-time PCR system (Applied Biosystems, Foster City, CA, USA) using SYBR Premix ExTaq II (Tli RNaseH Plus) (Takara, Dalian, China). Each reaction (20 μL) contained a SYBR Premix ExTaq (10 μL), a cDNA template (1 μL), a sense primer and an anti-sense primer (0.2 μM each) and nuclease-free water. The thermal conditions were one cycle of 95°C for 30 s, 40 cycles of 95°C for 5 s and 60°C for 25 s. A heat-dissociation protocol was added at the end of the thermal cycle to verify that only one PCR product was detected by the fluorescence dye. A pre-run test confirmed the constant expression of the house-keeping gene (β-actin; GenBank Acc. no. JN029806) in each sample, and a no template control (NTC) was also included in the experiment to detect potential contamination. Each sample was run in triplicate from three biological replicates. Quantification of gene expression levels was carried out according to the 2–ΔΔCt method (Livak & Schmittgen 2001). Primer pairs for qPCR were list in File S1.
Data analyses
The qPCR results were analyzed using the Data Processing System (DPS) software v9.5 (Tang & Zhang 2013). The paired Student's t-test and one-way analysis of variance (anova) with the least significant difference (LSD) test were applied, respectively, for comparing the difference between two samples and the differences among multiple samples. The level of significance was set at P < 0.05.
Results
Identification of P450 genes in C. medinalis
Thirty-six putative P450 genes were identified from the C. medinalis transcriptome dataset. Of these, 29 genes have completed ORFs while 7 genes are incomplete cDNAs, lacking 5′- and/or 3′-termini (Table 1). These sequences were verified by cDNA amplification and direct sequencing. The specific names for these P450 genes were assigned by the Cytochrome P450 Nomenclature Committee (D Nelson, University of Tennessee, Memphis, TN, USA) and were classified into 19 families and 30 subfamilies (Table 1). A new P450 subfamily, CYP321F, which was recently discovered in the rice striped stem borer, Chilo suppressalis (Lepidoptera: Pyralidae) (Wang et al. 2014), was also found in C. medinalis (Table 1).
| Gene name | Acc. no. | Length (aa) | Anchor | BLASTX best hit | |||
|---|---|---|---|---|---|---|---|
| Species | Gene name | Acc. no. | Identity (%)/e-value | ||||
| Mitochondrial clan | |||||||
| CYP301A1 | KP001112 | 527 | No | Bombyx mori | CYP301A1 | BAM73846 | 90/0.0 |
| CYP314A1 | KP001113 | >470 | – | Microplitis demolitor | CYP314A1 | XP_008543674 | 88/0.0 |
| CYP333A13 | KP001114 | 497 | No | Danaus plexippus | CYP333A3 | EHJ68052 | 58/0.0 |
| CYP333B27 | KP001115 | 508 | No | Helicoverpa armigera | CYP333B3 | AID54862 | 62/0.0 |
| CYP333B28 | KP001116 | 504 | No | Helicoverpa armigera | CYP333B3 | AID54862 | 63/0.0 |
| CYP339A1 | KP001117 | 580 | No | Bombyx mori | CYP339A1 | NP_001121192 | 70/0.0 |
| CYP2 clan | |||||||
| CYP18A1 | KP001118 | 539 | Yes | Bombyx mori | CYP18A1 | NP_001077078 | 81/0.0 |
| CYP304F17 | KP001119 | >509 | Yes | Chilo suppressalis | CYP304F13 | AHW57295 | 69/0.0 |
| CYP305B1 | KP001120 | 485 | No | Bombyx mori | CYP305A1 | BAM73852 | 64/0.0 |
| CYP306A1 | KP001121 | >507 | – | Helicoverpa armigera | CYP306A1 | AID54855 | 82/0.0 |
| CYP307A2 | KP001122 | 530 | No | Chilo suppressalis | CYP307A2 | AHW57298 | 84/0.0 |
| CYP3 clan | |||||||
| CYP6AB62 | KP001124 | >347 | – | Chilo suppressalis | CYP6AB46 | AHW57300 | 75/2e-170 |
| CYP6AE28 | KP001126 | 522 | Yes | Cnaphalocrocis medinalis | CYP6AE28 | CAX94849 | 99/0.0 |
| CYP6AE76 | KP001127 | 523 | Yes | Cnaphalocrocis medinalis | CYP6AE30 | CBB07053 | 72/0.0 |
| CYP6AW1 | KP001123 | 498 | Yes | Chilo suppressalis | CYP6AW1 | AHW57309 | 79/0.0 |
| CYP6CV1 | KP001125 | 500 | Yes | Cnaphalocrocis medinalis | CYP6CV1 | CAZ65618 | 98/0.0 |
| CYP9A78 | KP001130 | 527 | No | Mamestra brassicae | CYP9 | AAR26518 | 58/0.0 |
| CYP9A79 | KP001131 | 531 | Yes | Cnaphalocrocis medinalis | CYP9A38 | CAZ65619 | 61/0.0 |
| CYP9A80 | KP001132 | 532 | Yes | Cnaphalocrocis medinalis | CYP9A38 | CAZ65619 | 65/0.0 |
| CYP9G18 | KP001128 | 524 | No | Bombyx mori | CYP9G3 | NP_001108456 | 63/0.0 |
| CYP9G19 | KP001129 | 524 | Yes | Bombyx mori | CYP9G3 | NP_001108456 | 60/0.0 |
| CYP321C7 | KP001134 | 496 | Yes | Spodoptera littoralis | CYP321A11 | AFP20596 | 55/0.0 |
| CYP321F5 | KP001133 | 498 | Yes | Chilo suppressalis | CYP321F3 | AHW57324 | 66/0.0 |
| CYP324A19 | KP001135 | 498 | No | Helicoverpa armigera | CYP324A1 | AID54859 | 54/0.0 |
| CYP337B12 | KP001136 | 493 | Yes | Helicoverpa armigera | CYP337B1 | AFO72901 | 52/0.0 |
| CYP338A1 | KP001137 | 460 | Yes | Spodoptera littoralis | CYP338A2 | AFP20586 | 61/0.0 |
| CYP345A4 | KP001138 | 522 | Yes | Bombyx mori | CYP354A4 | BAM73820 | 68/0.0 |
| CYP4 clan | |||||||
| CYP4G111 | KP001141 | 559 | Yes | Microplitis demolitor | CYP4G15 | XP_008546138 | 95/0.0 |
| CYP4G112 | KP001142 | 556 | Yes | Chilo suppressalis | CYP4G90 | AHW57329 | 91/0.0 |
| CYP4G113 | KP001144 | 565 | Yes | Manduca sexta | CYP4G49 | ADE05583 | 73/0.0 |
| CYP4L18 | FN356973 | >492 | – | Cnaphalocrocis medinalis | CYP4L18 | CAX94851 | 100/0.0 |
| CYP4M25 | KP001139 | 502 | Yes | Cnaphalocrocis medinalis | CYP4M25 | CAX94850 | 99/0.0 |
| CYP4AU19 | KP001140 | >374 | – | Helicoverpa armigera | CYP4AU1 | AID54874 | 74/0.0 |
| CYP4CG17 | KP001143 | 502 | Yes | Manduca sexta | CYP4CG1 | ADE05577 | 57/0.0 |
| CYP341A17 | KP001145 | >362 | – | Spodoptera frugiperda | CYP341A11 | AGO62011 | 62/2e-160 |
| CYP367B12 | KP001146 | 506 | Yes | Spodoptera littoralis | CYP367B6 | AFP20603 | 63/0.0 |
- aa, amino acid residues; –, not determined because of sequence truncation.
TMHMM prediction showed that transmembrane regions composed of hydrophobic residues were found in the N-termini of the 20 P450s (Table 1). The region was known to anchor P450s in the endoplasmic reticulum membrane (Feyereisen 2012).
Almost all the P450s showed highly identities to those of other lepidopteran species, with more than 52% identity at the amino acid level (Table 1). The exceptions were CYP314A1 and CYP4G111, which showed 88% and 95% identities, respectively, to their orthologs from the parasitoid wasp Microplitis demolitor (Hymenoptera: Braconidae). Additionally, CYP4L18 identified in this study is a partial fragment of the previously isolated C. medinalis CYP4L18 (GenBank Acc. no. CAX94851). Also, CYP6AE28, CYP6CV1 and CYP4M25 have extremely high identities (>98%) with the previously isolated C. medinalis CYP6AE28 (CAX94849), CYP6CV1 (CAZ65618) and CYP4M25 (CAX94850), respectively, with some small changes in their amino acid residues (Table 1).
A multiple alignment analysis was performed for the deduced protein sequences of these P450s, and the structural characteristics were highlighted (Fig. 1). Among these sequences, the helix-C (WxxxR), helix-K (ExxR) and haem-binding domain (PFxxGxRxCxG) were well conserved, while the PERF motif (PxxFxPE/DRF) and helix-I (GxE/DTT/S) were more variable. One microsomal P450, CYP307A2, adds three residues to the helix-C motif (Fig. 1).
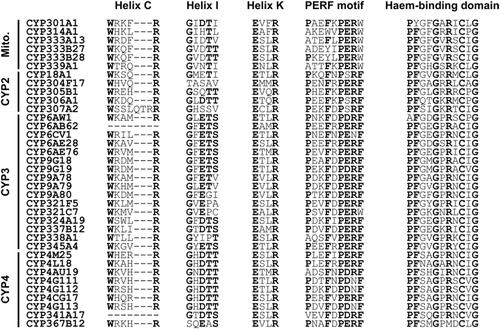
Alignment of functional domains of the 36 Cnaphalocrocis medinalis P450s. Conserved residues are indicated in bold.
Phylogenetic analysis
In order to better understand the relationships of insect P450s, the C. medinalis P450s together with previously annotated P450s from Drosophila melanogaster (Tijet et al. 2001), Apis mellifera (Claudianos et al. 2006), Bombyx mori (Ai et al. 2011) and Ch. suppressalis (Wang et al. 2014) were included in a phylogenetic analysis. In the neighbor-joining tree (Fig. 2), the 36 C. medinalis P450s fell into four major clades: (i) the mitochondrial clan; (ii) CYP2 clan; (iii) CYP3 clan; and (iv) CYP4 clan (Feyereisen 2012). Most of the C. medinalis P450s (16 genes) were clustered within the CYP3 clan, followed by the CYP4 clan (9 genes). The C. medinalis P450s from the CYP3 clan were clustered into divergent families, such as the CYP6, CYP9, CYP321, CYP324, CYP337, CYP338, CYP345 and other families, whereas P450s from the CYP4 clan were mainly clustered into the CYP4 family (Fig. 2). The mitochondrial and CYP2 clans included six and five C. medinalis P450 genes, respectively. In addition, all of the C. medinalis P450s have close relationships with orthologs from lepidopteran species B. mori and Ch. suppressalis (Fig. 2).
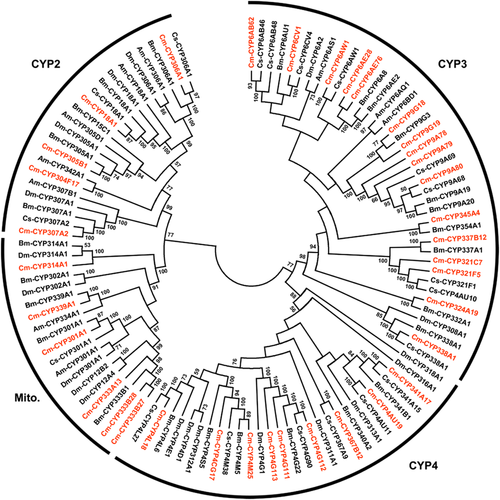
Phylogenetic relationships of P450s from Cnaphalocrocis medinalis (Cm-prefix), Apis mellifera (Am-), Bombyx mori (Bm-), Chilo suppressalis (Cs-) and Drosophila melanogaster (Dm-). The neighbor-joining tree was constructed using MEGA5.05 software (Tamura et al. 2011); bootstrap values above 50% are shown. The C. medinalis P450s are highlighted in red.
Transcriptional profiles of P450 genes in the CYP6 family
Because the CYP6 P450 family in insects is the major family related to the detoxification of xenobiotics, the expression profiles of five C. medinalis P450 genes (CYP6AW1, CYP6AB62, CYP6CV1, CYP6AE28 and CYP6AE76) in the CYP6 family were investigated by qPCR assays. The results showed that these genes were differentially expressed in various larval tissues, including integument, Malpighian tubules, midgut and fat body (Fig. 3). Midgut expressed significantly higher levels of CYP6AW1 and CYP6CV1 than other tissues. CYP6AE76 was predominantly expressed in the fat body (Fig. 3). The remaining two genes, CYP6AB62 and CYP6AE28, showed no significant difference between Malpighian tubules, midgut and fat body (Fig. 3).
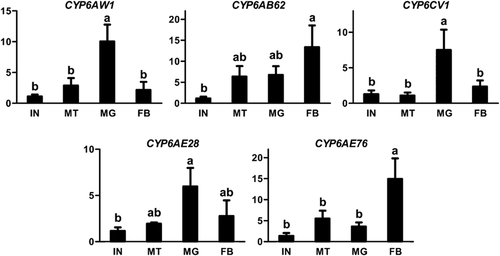
Relative expression levels of five CYP6 genes in various larval tissues possibly related to detoxification, including integument (IN), Malpighian tubules (MT), midgut (MG) and fat body (FB). Gene expression levels in different tissues were normalized relative to that in integument. Different lower-case letters indicate significant difference (one-way anova, P < 0.05).
Further, the transcriptional changes of these five genes in larvae exposed to abamectin were determined by qPCR. The transcriptional levels of three genes, CYP6AB62, CYP6CV1 and CYP6AE28, were increased after exposure, but only CYP6AE28 showed significant difference between treated and control individuals (Fig. 4). On the contrary, CYP6AW1 and CYP6AE76 were slightly down-regulated following insecticide exposure, but their differences between abamectin-treated insects and controls were not significant (Fig. 4).
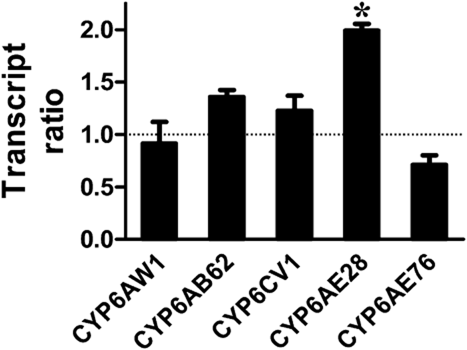
Transcript ratios of P450 genes in abamectin-treated larvae versus control larvae. *, significant difference in transcription levels between treated insects and controls (paired Student's t-test, P < 0.05). Error bars represent standard error (SE) of the mean (n = 3).
Discussion
Thirty-six putative P450 genes were identified by searching the C. medinalis transcriptome dataset. The number of P450 genes in C. medinalis is relatively low, especially when compared to the total number of P450s identified from other model insect species. For instance, bioinformatic analysis revealed 90, 111, 48 and 143 P450 genes, respectively, in D. melanogaster (Tijet et al. 2001), Anopheles gambiae (Ranson et al. 2002), A. mellifera (Claudianos et al. 2006) and Tribolium castaneum (Zhu et al. 2013). Within the order Lepidoptera, 84, 90 and 77 P450s were identified in B. mori (Ai et al. 2011), Plutella xylostella (You et al. 2013) and Ch. suppressalis (Wang et al. 2014), respectively. Obviously, the number of C. medinalis P450 genes identified in this study is less than expected and could just represent one-half of the whole repertoire of the gene family. This phenomenon possibly occurs because the samples collected for transcriptome sequencing are limited; another possibility is that the Illumina sequencing technology is not powerful enough to exhaustively obtain all P450s, especially those genes with extremely low expression level. A full exploration of the P450 gene family in C. medinalis is needed in future studies.
CYP314A1 and CYP4G111 from C. medinalis showed high identities to their respective orthologs from the parasitoid wasp M. demolitor. To determine whether the two genes belong to C. medinalis or its parasite M. demolitor, we performed reverse transcription PCR (RT-PCR) using cDNA samples from non-parasitized C. medinalis larvae and found the two genes can be amplified (data not shown), confirming that CYP314A1 and CYP4G111 belong to C. medinalis. The high identities of the two pairs of CYPs from C. medinalis and M. demolitor indicated a functional conservatism within these genes.
The phylogenetic analysis showed that these P450s are widely distributed in various clans, including the mitochondrial, CYP2, CYP3 and CYP4 clans, with high bootstrap support. Mitochondrial and CYP2 clans include plenty of members associated with the ecdysteroid metabolism pathway, such as the D. melanogaster mitochondrial P450s CYP301A1, CYP302A1 and CYP314A1, as well as the CYP2 P450s CYP18A1, CYP306A1 and CYP307A1 (Niwa et al. 2004; Guittard et al. 2011; Feyereisen 2012; Sztal et al. 2012). In contrast, some members from the CYP3 and CYP4 clans are involved in the detoxification of synthetic insecticides and plant natural compounds (Li et al. 2007; Feyereisen 2012). The variation in sequences across the C. medinalis P450 genes suggests a potential diversity at the functional level, and the functional diversification is likely lead to a better adaptation for insects to ecological environments (Schuler 2011; Feyereisen 2012). Indeed, in C. medinalis, two previously reported P450 genes, CYP6AE28 and CYP6AE30, are inducible by allelochemicals in rice; thus they may be involved in the adaptation to the host-plant (Liu et al. 2010).
The CYP6 family is a major P450 family implicated in the detoxification of xenobiotics in various insect species. For example, CYP6D1 is overexpressed in pyrethroid-resistant strain of the housefly, Musca domestica, and can metabolize pyrethroid insecticides and organophosphate insecticide chlorpyrifos (Hatano & Scott 1993). Also, the D. melanogaster CYP6G1 and Nilaparvata lugens CYP6AY1 have been found to metabolize imidacloprid (Joußen et al. 2008; Ding et al. 2013). In addition, the Helicoverpa armigera CYP6AE14 and Amyelois transitella CYP6AB11 are responsible for the detoxification of gossypol and furanocoumarin, respectively (Mao et al. 2007; Niu et al. 2011). In this case, investigation of members belonging to this family may identify novel genes involved in xenobiotic detoxification. We found that five CYP6 genes were expressed in different larval tissues including midgut, Malpighian tubules and fat body. The distribution of P450 genes in these tissues has also been reported in insect species such as D. melanoganster (Chung et al. 2009), Locusta migratoria (Guo et al. 2012) and Bactrocera dorsalis (Huang et al. 2013). CYP6AW1 and CYP6CV1 were more highly expressed in the midgut; the tissue is well known for its fundamental function in metabolism of xenobiotics including insecticides and plant toxins (Després et al. 2007). CYP6AE76 was more abundantly transcribed in the fat body. The insect fat body has multiple metabolic functions such as storing and releasing energy, and were recognized as a major site of P450-mediated detoxification (Arrese & Soulages 2010). P450s expressed in these tissues were expected to be closely related to the metabolism of xenobiotics.
Abamectin is a natural fermentation product of the bacterium Streptomyces avermitilis. The chemical has been widely used to control C. medinalis (Zheng et al. 2011; Zhang et al. 2014), as well as other insect and mite pests. Pharmacological experiments indicated that glutamate-gated chloride channels are the primary targets of the chemical (Bloomquist 2003). P450 genes in response to abamectin have been reported in other insect species, including B. mori (Xuan et al. 2015), P. xylostella (Hu et al. 2014) and B. dorsalis (Huang et al. 2013). In this study, we found one CYP6 gene (CYP6AE28) of C. medinalis was induced after application of abamectin and expected as a candidate possibly involved in the detoxification of abamectin. On the other hand, considering that CYP6AE28 can be induced in response to a resistant variety of rice (Liu et al. 2010), the gene may play a dual role in the adaptation to both insecticides and host-plants. However, functional studies are required to test this hypothesis.
In conclusion, 36 putative P450 genes were identified from the transcriptome dataset of C. medinalis. These P450s fell into four clans, the mitochondrial clan, CYP2 clan, CYP3 clan and CYP4 clan, and were classified into 19 families and 30 subfamilies. Expression profiles of five CYP6 P450 genes were investigated. These genes were differentially expressed in various tissues, indicating a functional diversification among them. CYP6AE28 was upregulated following exposure to abamectin, suggested a possibility that the gene may be involved in the detoxification of the chemical. Further studies are required to fully appreciate the roles of P450 genes in C. medinalis, especially the function involved in insecticide detoxification and resistance.
Acknowledgments
We thank Dr David Nelson (University of Tennessee, Memphis, TN, USA) for his help in P450 nomenclature. This work was supported by the National Natural Science Foundation of China (31401734, 31402017).




