Detection of antimicrobial substances from larvae of the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae)
Abstract
Maggots have become highly successful in the treatment of non-healing wounds and multidrug-resistant pathogen infections. The main objective of this study was to extract antibacterial substances from larvae of the black soldier fly, Hermetia illucens. To induce immune responses, we septically injured the larvae with a contaminated needle. Lyophilized H. illucens larvae were homogenized and extracted with acidic methanol. We examined the antifungal and antibacterial effects of the low molecular weight antimicrobial factors within the larval extract on the growth of a broad range of microorganisms, including Gram-positive Staphylococcus aureus, methicillin resistant Staphylococcus aureus (MRSA), and Gram-negative Pseudomonas aeruginosa. Furthermore, we isolated the anti-MRSA substances from the larval extract using high performance liquid chromatography. These investigations revealed that the larval extract possessed a broad-spectrum of antibacterial activity, demonstrating that secretions of H. illucens larvae prove useful in the fight against MRSA and can potentially be a source of novel antibiotic-like compounds for infection control.
Introduction
Maggot therapy is highly successful in cleansing infected and necrotic wounds (Sherman & Hall 2000) and has been practiced since the 1930s when William Baer's observations confirmed the reduction of bacterial load at the wound site by the use of maggots (Baer 1931). The main actions of fly maggots on wounds may be categorized into three modes: debridement, disinfection and bacterial death, and stimulation of tissue granulation and repair (Richardson 2004). However, the use of maggot therapy declined as antibiotics were developed and surgical techniques improved. Nevertheless, new strains of pathogenic fungi and bacteria developed primarily in response to overdose usage of antibiotics and thus were found commonly in hospitals and the wider community (Kerridge et al. 2005), renewing our interest in maggot therapy in the search for effective methods to treat non-healing wounds and control the evolution of resistance (Bexfield et al. 2004). Recently, several studies reporting antimicrobial activity of the hemolymph and maggot extract, either in the whole body or in the excreta/secreta, have provided impetus for the development of alternative antimicrobial products into therapeutically valuable anti-infective agents (Dang et al. 2006). It is well known that insects have a well-developed innate immune system, subdivided into cellular and humoral defense responses, the latter of which involve production of antimicrobial peptides (AMPs) that are synthesized in the fat body and subsequently secreted into the hemolymph (Bulet & Hetru 1999; Hoffmann & Reichhart 2002).
The larvae of the black soldier fly, Hermetia illucens, are scavengers that can live in extremely harsh environments, such as manures and compost, inhabited by bacteria and fungi. Animal wastes and rotten plants can be decomposed and recycled by larvae. Additionally, the black soldier fly is sometimes found in carrion. These biological characteristics suggest that the soldier fly may be rich in generations of AMPs and other substances possessing activity against drug-resistant “superbugs”. Recently, the antibacterial effect of H. illucens larval extract against Gram-negative bacteria was evaluated (Choi et al. 2012). However, insects infected with the microorganism could contain more biologically and pharmacologically active chemicals and evidence has been reported that antibacterial activity of the extract from induced larvae was much stronger than that of native larvae (Hou et al. 2007). The aim of the present study is to improve antimicrobial substances by inducing immunoreaction in H. illucens larvae and testing the antifungal and antibacterial activity of its extract against a range of Gram-positive and Gram-negative microorganisms, including the clinically important strains of methicillin-sensitive (MSSA) and methicillin-resistant Staphylococcus aureus (MRSA), and also to isolate anti-MRSA substances form the larval extract.
Materials and methods
Larvae
Fifth instar larvae of the black soldier fly (Hermetia illucens) were obtained from Nuree Inc., Baekgok, Korea, and reared at 32°C, 62% humidity with 24 h dark cycle.
Microorganisms
The following bacterial species were used: Escherichia coli (KCCM 11234), Enterobacter aerogenes (KCCM 12177), Psuedomonas aeruginosa (KCCM 11328), MRSA (methicillin-resistant Staphylococcus aureus), Staphylococcus aureus (KCCM 40881), Bacillus subtilis (KCCM 11316), Kocuria rhizophila (KCCM 11236), Micrococcus luteus (KCCM 11326), Staphylococcus epidermidis (KCCM 35494). The following fungus was used: Candida albicans (KCCM 11282). Escherichia coli and Staphylococcus were grown in tryptic soy broth, while other bacteria were grown in nutrient broth, and C. albicans in yeast malt broth.
Preparation of the extract of the black soldier fly larvae
The larvae were dried after washing with water containing disinfectant and rinsing with sterile water. Subsequently, they were individually pricked deeply with a fine needle dipped in S. aureus (OD600 = 2.4). The lyophylized larvae were thoroughly ground and extracted with acidified methanol (methanol/water/acetic acid; 90/9/1; v/v/v). The extract was centrifuged at 1600 × g for 10 min at 4°C and taken to dryness in a rotary evaporator under reduced pressure. Proteins and lipids were removed from the sample by sequential extraction with chloroform and ethyl acetate. All the fractions were lyophilized and stored in a refrigerator at −20°C until use.
Inhibition zone assay
Antimicrobial activity of Sep-Pak C18 (Waters) and high performance liquid chromatography (HPLC) eluent components were measured by inhibition zone assay as described previously (Park et al. 2013). Thin plates (1 mm) of 1% agarose containing 6 × 104 cells/mL were prepared and wells of 3 mm diameter were punched out of the plates. Sep-Pak C18 and HPLC eluants were lyophilized and dissolved in distilled water at a final concentration of 1 μg/μL and 5 μL of sample was loaded into each well into appropriate wells. After overnight incubation at 37°C, the diameters of clear zones were measured.
Minimal inhibitory concentration
Minimal inhibitory concentrations (MICs) were performed by sequential dilution in sterile 96-well cell culture plates. The microorganisms were diluted in proper medium at a starting OD600 = 0.001 (approximately 5 × 105 CFU/mL) and 90 μL dispensed to the plates. Serial dilutions of the larval extract dissolved in distilled water were loaded with 10 μL to the plate and incubated for 16 h (M. luteus was incubated for 42 h) at 37°C (B. subtilis, E. aerogenes, K. rhizophila, M. luteus were incubated at 30°C). The MIC was determined at 600 nm using microplate reader (EL-800, Bio-Tek Instruments, Winooski, VT, USA). All experiments were performed in quintuplicate.
Purification of antimicrobial substances
The water-soluble extract was applied to Sep-Pak C18 and washed with distilled water (20 mL) twice and then eluted with each 20 mL of 10%, 20%, 30%, 50%, and 80% acetonitrile (ACN). The anti-MRSA fraction (10% ACN eluant) of preparative purification using Sep-Pak C18 was further purified by HPLC on a 4.6 × 250 mm Sim-pack VP-ODS (Shimadzu) connected to Futecs HPLC system with a simple linear gradient from 0.1% (v/v) trifluoroacetic acid (TFA) to 25% (v/v) ACN (0.1% (v/v) TFA) at a flow rate of 1 mL/min at room temperature. The elution pattern was monitored at 214 nm and chromatographic fractions were tested exhibiting activity against MRSA by inhibition zone assay.
Results
Antimicrobial activities of the larval extracts
The water-soluble extract had antibacterial activity against all the Gram-positive and Gram-negative bacteria except S. aureus and S. epidermidis; MRSA (MIC = 25 mg/mL), S. epidermidis (MIC = 50 mg/mL), K. rhizophila (MIC = 25 mg/mL), M. luteus (MIC = 25 mg/mL), B. subtilis (MIC = 12.5 mg/mL), E. coli (MIC = 12.5 mg/mL), E. aerogenes (MIC = 25 mg/mL), P. aeruginosa (MIC = 12.5 mg/mL). The water-soluble extract also had antifungal activity; C. albicans (MIC = 25 mg/mL). The MIC against S. aureus, however, could not be determined even at 100 mg/mL. MIC values of the ethyl acetate extract were a similar to that of the water-soluble extract, but the MIC against S. aureus 12256 and E. coli 11234 could not be determined even at 100 mg/mL. Meanwhile, the chloroform extract had no activity against all the microorganisms (Table 1). The water-soluble extract showed a broad-spectrum antimicrobial activity against a range of Gram-positive and Gram-negative bacteria and yeast in a concentration-dependent growth manner. The water-soluble extract exhibited dramatic microbial growth at specific concentrations, but the extract exhibited concentration-dependent bacterial killing on the growth of S. aureus and S. epidermdis. Enhanced bacterial growth was observed with the water-soluble extract in low concentration (about 3 mg/mL) (Fig. 1).
| Microorganisms | Strain | MIC of the extract fractions (mg/mL) | MIC of antibiotics (μg/mL) | |||
|---|---|---|---|---|---|---|
| Water | Ethyl acetate | Chloroform | Methicillin | Ampicillin | ||
| Gram-positive bacteria | ||||||
| MRSAa (clinically isolated) | 25 | NTb | >100 | >80 | >80 | |
| Staphylococcus aureus | KCCM 40881 | >100 | >100 | >100 | 80 | 2.5 |
| S. aureus | KCCM 12256 | 100 | NT | >100 | 2.5 | 2 |
| S. epidermidis | KCCM 35494 | 50 | 25 | >100 | >20 | 10 |
| Kocuria rhizophila | KCCM 11236 | 25 | NT | >100 | <0.3125 | <0.3125 |
| Micrococcus luteus | KCCM 11326 | 25 | NT | >100 | NT | NT |
| Bacillus subtilis | KCCM 11316 | 12.5 | 25 | >100 | 0.078125 | 0.078125 |
| Gram-negative bacteria | ||||||
| Escherichia coli | KCCM 11234 | 12.5 | >100 | >100 | >80 | 20 |
| Enterobacter aerogenes | KCCM 12177 | 25 | NT | >100 | >20 | >20 |
| Pseudomonas aeruginosa | KCCM 11328 | 12.5 | 25 | >100 | NT | NT |
| Yeast | ||||||
| Candida albicans | KCCM 11282 | 25 | 50 | >100 | >20 | >20 |
- a MRSA, methicillin-resistant Staphylococcus aureus.
- b NT, not tested.
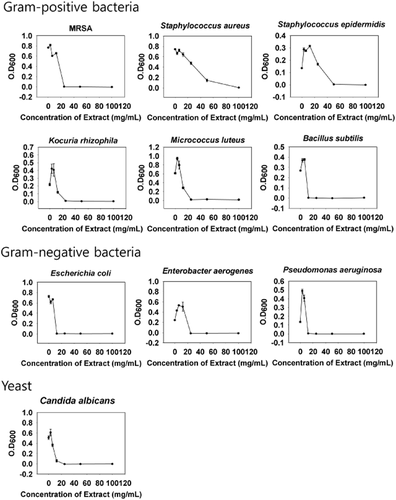
Antimicrobial activity of water-soluble extract from Hermetia illucens on the growth of microorganisms at different extract concentrations. Each value is expressed as mean ± standard error. Microorganisms were incubated in the presence of different water-soluble extract concentrations (0–100 mg/mL) for 16 h (except for Micrococcus luteus, which was incubated for 42 h) at 37°C (except for Bacillus subtilis, Enterobacter aerogenes, Kocuria rhizophila, and M. luteus, which were incubated at 30°C).
Purification of antimicrobial substances
To isolate anti-MRSA substances, the water-soluble fraction of the larval extract was applied to Sep-Pak C18 cartridges, and eluted with each 20 mL of 10%, 20%, 30%, 50%, and 80% ACN. Antibacterial activities of the Sep-Pak C18 eluants were measured by inhibition zone assay against MRSA, E. coli, and B. subtilis. Significant anti-MRSA activity was observed at the fraction eluted with 10% ACN. While the eluants did not show any antibacterial activity against E. coli, they exhibited a strong activity against B. subtilis, with the strongest inhibitory zone shown in 30% CAN. Wash1 fraction had antibacterial activity against all the tested bacteria, while wash2 fraction did not have the same activity (Fig. 2). The second step of purification was performed using HPLC with a Shim-Pack VP-ODS column (Shimadzu, Kyoto, Japan), which revealed multiple peaks, indicating the presence of various small compounds. Furthermore, large inhibitory zones against MRSA were detected from fractions 48, 61, and 78, while smaller zones were observed from fractions 50–74 including dark-brown zones (Fig. 3).
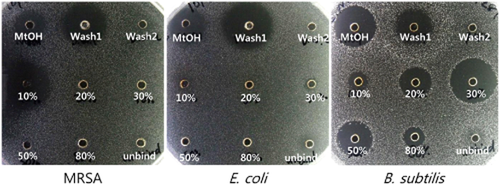
Inhibition zone assay of preparative purification against methicillin resistant Staphylococcus aureus (MRSA), Escherichia coli, and Bacillus subtilis. MtOH, aqueous fraction of acidic methanol extract; Wash1, 2, washing fractions of preparative purification using Sep-Pak C18; 10% –80%, 10% –80% acetonitrile (ACN) eluent; unbind, unbinding fraction.
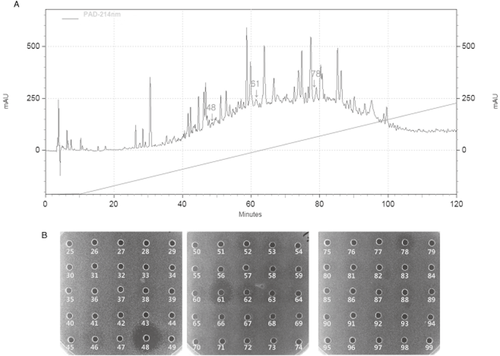
High performance liquid chromatography (HPLC) purification of the 10% acetonitrile (ACN) solid phase fraction, as prepared from aqueous extract of Hermetia illucens. (A) Chromatogram of HPLC purification using Sim-pack VP-ODS. The fractions exhibiting activity potent against methicillin resistant Staphylococcus aureus (MRSA) were represented by arrows. (B) Inhibition zone assay analysis of the fractions against MRSA.
Discussion
It is well known that the larvae of black soldier fly, Hermetia illucens, live in extremely harsh environments, suggesting that the soldier fly may be rich in generations of AMPs and other substances possessing activity against microorganisms, including MRSA. Previously, it has been reported that a methanol extract of H. illucens larvae indicated antibacterial effects against Gram-negative bacteria (Choi et al. 2012). However, according to a study of housefly (Musca domestica), antibacterial activities of the extract of inoculated larvae were two times stronger and broader than that of the native larvae (Hou et al. 2007). Consistent with this, our pre-experimental result also showed that the aqueous extract of H. illucens larvae with an immune response induced by septic needle, demonstrate stronger antibacterial activity against MRSA than that of the native larvae (data not shown).
The major goals of this study were: (i) to improve the expression of antimicrobial substances of H. illucens larvae inducing immune response; (ii) to extract the immunized larvae; and (iii) to test and determine their antibacterial activities. The acidic methanol extraction method was employed for the extraction of antimicrobial substances from the larvae, because the acidic methanol could denature and precipitate large proteins and polypeptides, while efficiently extracting small molecules (Meylaers et al. 2002). The acidic methanol extract of the larvae was sequentially extracted with chloroform and ethyl acetate to remove lipids. One of the most commonly used broth dilution techniques was employed to determine the minimal inhibitory concentration (MIC) of the extract under defined conditions. All the MIC values of the aqueous fraction of the extract were in a similar range from 12.5 to 25 mg/mL, while Staphylococcus, including S. aureus and S. epidermidis, demonstrated relatively lower MIC values. In particular, the MIC value of MRSA was stronger than that of S. aureus and S. epidermidis, suggesting that species-specific antibacterial substances may be expressed in H. illucens larvae and extracted in aqueous fraction. The chloroform fraction of the extract did not show antimicrobial activities against all tested bacteria with the result that no antimicrobial substances were extracted in this fraction. According to a previous report, the methanol extract of native H. illucens larvae had Gram-negative specific antibacterial activities including Klebsiella pneumonia, Neisseria gonorrhoeae, and Shigella sonnei (Choi et al. 2012). However, the extract of acidic methanol of immunized larva used in this study demonstrated broader antimicrobial activities against all the bacteria and yeast except S. aureus. This appears to provide strong evidence that expression of antibacterial substances was improved and diversified with the induced immune response. The soluble fraction of the extract was tested against 10 strains of Gram-positive and Gram-negative bacteria and a fungus in a concentration-dependent manner, revealing its broad-spectrum antimicrobial activity as shown in Figure 1. The enhanced bacterial growth observed with larval extract in concentration dependent growth assay using low concentration may also be due to the high nutritional value of the extract (Bexfield et al. 2004).
To isolate anti-MRSA substances, we focused on a specific fraction H. illucens extract, eluted at 10% ACN with Sep-Pak C18 cartridges, which showed a significant activity against MRSA. The eluants of Sep-Pak C18 did not show antibacterial activities against E. coli, while the soluble-extract did. This result revealed that the antibacterial substances exhibiting activity against E. coli passed through the column, meaning that the anti-E. coli substances were strongly hydrophilic in character (Fig. 2). The second step of purification was performed using HPLC with a Shim-Pack VP-ODS column, which revealed multiple peaks, indicating the presence of various small compounds. Large inhibitory zones were detected from fractions 48, 61, and 78, while smaller zones were observed from fractions 50–74 (Fig. 3). The fractions displaying strong activity must be pooled for further purification and study of structural properties of the pure substance. It should be noted that H. illucens larvae secrete dark-brown colored substances due to melanization, or biosynthesis of melanin, a phenolic biopolymer involved in the insect immunity. The cytotoxic phenols have already been investigated extensively (Sugumaran 2002) and are well established as compounds displaying broad-spectrum antibacterial effect and, therefore, the presence of these antibacterial compounds in aqueous extract of H. illucens is possible (fractions 50–74). Our results show that the water-soluble fraction of the whole-body extract possessed significantly stronger antibacterial activity against MRSA, suggesting the presence of more than one “antibacterial” substance acting in synergy to increase the effect. Additionally, the aqueous extract of H. illucens has proven to be highly robust, capable of withstanding several freeze–thaw cycles and lyophilization, and is stable as a freeze-dried preparation, all of which are important properties in terms of development of a product for pharmaceutical purposes. It has been recently reported that the presence and production of a new class of small, perhaps semi-peptidergic antimicrobial substances may not be restricted to the hemolymph or fat body and therefore, when attempting to isolate those compounds, preparation of whole-body extract could be more efficient (Meylaers et al. 2002). A number of such small, low molecular weight antimicrobial dipeptides have been purified from Dipteran species. For example, the inducible antibacterial compound N-b-alanyl-5-S-glutathionyl-3,4-dihydroxyphenylalanine (5-S-GAD, 573 Da) was isolated from the adult fleshfly, Sarcophaga peregrine (Leem et al. 1996), and two small substances, β-alanyl-tyrosine (252 Da) and 3-hydroxykynurenine (224 Da), were isolated from larvae of the grey fleshfly, Neobellieria bullata (Meylaers et al. 2003). Other low molecular weight antimicrobial compounds reported from insects include p-hydroxycinnamaldehyde (148 Da), isolated from induced larvae of the sawfly, Acantholyda parki (Leem et al. 1999), and 1-lysophosphatidylethanolamine (451.2 Da) from native larvae of the housefly, Musca domestica (Meylaers et al. 2004).
In conclusion, all these features provide important evidence that larval secretions of H. illucens are a very rich source of substances with novel antimicrobial properties that could be highly useful in the fight against MRSA and control of other nosocomial infections. Further research on the characterization and purification of the extract may contribute considerably to the development of new antibiotics.
Acknowledgments
The present research was conducted by the research fund of Dankook University in 2012.




