Clinical effects of combination therapy with continuous renal replacement therapy and continuous intravenous sodium infusion therapy
Abstract
Introduction
This single-center retrospective study investigated the clinical effects of combination therapy involving continuous renal replacement therapy (CRRT) and continuous intravenous sodium infusion therapy (cIVNa) in critically ill patients with prerenal acute kidney injury (AKI) who were expected to experience insufficient plasma refilling.
Method
The clinical data of 92 patients were analyzed. Clinical data from the control (CRRT, n = 49) and intervention (CRRT + cIVNa, n = 43) groups were compared statistically.
Results
Combination therapy increased blood pressure and urine volume, while reducing hypotension events, indicating hemodynamic stabilization. Furthermore, it significantly improved the 90-day survival rate (61.9% vs. 38.8%, p < 0.05), 60-day and 90-day survival rates without RRT (59.5% vs. 28.6%, p < 0.01; 54.8% vs. 26.5%, p < 0.01, respectively), survival discharge rate from intensive care unit, CRRT withdrawal rate, and renal replacement therapy withdrawal rate.
Conclusion
Combination therapy with continuous renal replacement therapy and continuous intravenous sodium infusion therapy may be a useful treatment option for critically ill patients with prerenal acute kidney injury who require continuous renal replacement therapy.
1 INTRODUCTION
The mortality rate of patients with acute kidney injury (AKI) requiring renal replacement therapy (RRT) is high, ranging from 44% to 69% in multi-center randomized controlled trials (RCTs) [1-8]. In critically ill patients with AKI, RRT-associated hypotension is a major problem that has been reported to aggravate in-hospital mortality [9] and impair renal recovery [10]. Continuous renal replacement therapy (CRRT), which causes fewer adverse hemodynamic events than intermittent hemodialysis therapy (IHD), is the gold standard treatment for critically ill patients with AKI who require RRT. However, even with CRRT, blood pressure fall during RRT occurs in 19%–43% of patients [11]. The clinical benefits of IHD, when combined with various dialysis techniques, including the use of high-sodium dialysate, may be equivalent to those of CRRT for hemodynamic stabilization and prognosis [1].
Recently, high-sodium dialysate has been used to prevent hypotension and improve mechanical fluid removal during IHD and sustained low-efficiency dialysis (SLED) in critically ill patients with AKI [11-14]. High-sodium dialysate has a higher sodium concentration than the usual dialysate used in chronic hemodialysis patients (i.e., 138–140 mEq/L). When using high-sodium dialysate during IHD or SLED for critically ill patients, major reports recommend that the dialysate sodium concentration be above 145 mEq/L [11, 13, 15, 16]. High-sodium dialysate supplies sodium to the patient's blood via diffusion, increases intravascular and interstitial sodium concentrations, improves plasma refilling [11, 13, 17, 18], suppresses rapid decreases in extracellular fluid volume [13, 17, 18], and maintains hemodynamics during RRT.
To maintain hemodynamics during RRT in critically ill patients with AKI, the use of high-sodium dialysate, low-temperature dialysate, dialysate with bicarbonate buffer, and high-calcium dialysate is recommended, in addition to various RRT modalities such as prolonged or frequent IHD, SLED, and extracorporeal ultrafiltration methods [11, 13]. Among these, the use of a high-sodium dialysate has been reported as a strong intervention. A secondary analysis of RCTs reported that IHD using these hemodynamic maintenance techniques may improve prognosis compared with conventional CRRT [19]. However, there are no reports on the application of the principle of utilizing high-sodium dialysate in CRRT to improve hemodynamics and fluid management.
Since January 2019, we have implemented combination therapy of CRRT with continuous intravenous sodium infusion therapy (cIVNa) as an alternative to CRRT using high-sodium dialysate in our intensive care unit (ICU). We believe that cIVNa can increase the sodium concentration in the blood and interstitium; therefore, it is expected to exert the same effects as a high-sodium dialysate. Indeed, many cases of hemodynamic stabilization and improvement of fluid management were experienced with this combination therapy; however, its beneficial clinical effects remain unclear. In this study, we report, for the first time, the clinical effects of combination therapy with CRRT and cIVNa in critically ill patients with prerenal AKI, who were expected to have insufficient plasma refilling.
2 MATERIALS AND METHODS
2.1 Study design
- Patients aged <18 years;
- Patients with CKD G5 whose baseline estimated glomerular filtration rate was <15 mL/min/1.73 m2 before admission to the ICU;
- Patients who underwent CRRT before admission to the ICU;
- Patients who underwent IHD before the start of CRRT;
- Conditions other than prerenal AKI, including active nephritis, renovascular AKI, postrenal AKI, thrombotic microangiopathy, and rhabdomyolysis;
- Patients requiring special treatments, including immunosuppressive therapy (autoimmune diseases and graft-versus-host disease), chemotherapy (tumor lysis syndrome), plasma apheresis, and blood and organ transplantation (hepatic failure and liver transplantation-related events);
- Patients who exhibited sufficient refilling at CRRT initiation (systolic blood pressure > 140 mmHg without vasopressors or urine output >100 mL/h).
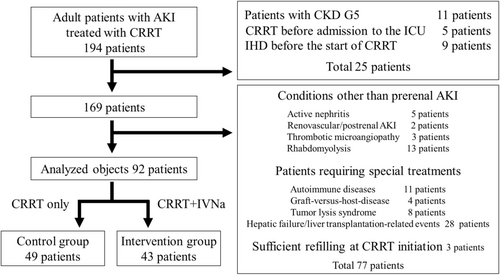
A total of 102 patients were excluded, and 92 patients were analyzed (Figure 1). Among them, 49 patients were treated with conventional CRRT (control group) and 43 patients were treated with a combination therapy of CRRT and cIVNa (intervention group). The clinical data between the groups were compared statistically. As the current study was not a prospective intervention study, all patients in the intervention group were treated after January 2019, whereas most patients in the control group were treated before that time.
2.2 Clinical data collection methods
Clinical data were collected from the medical records of patients. The following background characteristics were collected: sex, age, systolic blood pressure, serum level of creatinine, and urea nitrogen at CRRT initiation, event of decreased urine volume (<100 mL in the 6 h before CRRT initiation), background disease (post-operation, heart disease, infection, pancreatitis), characteristics of surgeries (use of cardio-pulmonary bypass, cardiac, vascular, digestive, other organs, accompanied with infectious disease, emergency, scheduled), APACHE II score at ICU admission, SOFA score at ICU admission and CRRT initiation, vasopressors and ventilator use at CRRT initiation, and the use of extracorporeal membrane oxygenation (ECMO) or ventricular assist device (VAD) at CRRT initiation and during CRRT, and treatment duration with CRRT.
The following clinical outcomes were collected: 28-, 60-, and 90-day survival; RRT, CRRT, and IHD dependence among 90-day survivors; 28-, 60-, and 90-day survival without RRT; survival discharge from ICU and hospital; “CRRT withdrawal” defined as survival for >7 days without CRRT after its discontinuation; “RRT withdrawal” defined as survival for >7 days without RRT after its discontinuation; and reintroduction of CRRT or RRT during hospitalization after their withdrawal. These outcomes were also collected for the subgroups with and without ECMO/VAD.
Additionally, the following CRRT-related adverse events within 14 days after CRRT initiation were collected: hypotension requiring rapid volume loading (crystalloid solution, blood transfusion, and albumin preparations), hypotension requiring increases in vasopressor doses, hypertension requiring antihypertensive drugs, arrhythmia requiring antiarrhythmic drugs and/or electrical defibrillation, bleeding events, and new-onset hypernatremia events. Hypernatremia was defined as a serum sodium level ≥ 150 mEq/L. An increase in the vasopressor dose and/or rapid fluid volume loading for hypotension was defined as “hypotension requiring intervention.” Data on hypernatremia events at the time of CRRT withdrawal and new-onset hypernatremia events within 14 days of CRRT withdrawal were also collected.
Furthermore, systolic blood pressure, in-out balance data (fluid management data), and serum sodium levels were collected daily during the first week after CRRT initiation. Systolic blood pressure was measured every 24 h after CRRT initiation. Fluid management data included the amount of urine volume, mechanical fluid removal with ultrafiltration, total administered fluid, total excreted fluid (including urine, drainage fluid, and bleeding), and total fluid balance. Due to challenges in calculating body fluid volume data owing to transfer from another ward and missing data after ICU discharge, fluid management data were only collected from the day after CRRT initiation in the ICU. Fluid management data were collected from 6 a.m. on the same day to 6 a.m. the next day, excluding data from the day of death or discharge. Serum sodium levels were evaluated during the ICU stay, including data on the day of death or discharge. The number of patients with serum sodium levels ≥145 was also assessed.
2.3 Statistical analysis
Categorical data are expressed as numbers (percentages) and were compared using Fisher's exact test. Normally and non-normally distributed data are expressed as means ± standard deviation and median (interquartile range [IQR]), respectively. Student's t-test and the Mann–Whitney U test were used to compare normally and non-normally distributed data, respectively. The results of systolic blood pressure and fluid management data are expressed as mean ± standard error, and serum sodium levels are expressed as mean ± standard deviation. All factors were compared using the Mann–Whitney U test. All statistical analyses were performed using EZR in R Commander (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [20]. All analyses were two-tailed, and statistical significance was set at p < 0.05.
2.4 Methods of cIVNa and its adjustment during CRRT in the ICU
Dialysates with sodium concentrations of ≥145 mEq/L are recommended for IHD or SLED in critically ill patients [11, 13, 15, 16]. Some studies have used dialysates with sodium concentrations of 150 mEq/L [1, 6, 14] or 160 mEq/L [21]. When patients undergo chronic IHD with a high-sodium dialysate, dialysate sodium concentrations are adjusted during IHD treatment according to the patient's hemodynamics, mechanical fluid removal rate, and other medical conditions [17, 18]. Typically, the sodium concentration in the dialysate is increased when blood pressure decreases via a decline in serum osmolality and/or circulating plasma volume, and the sodium concentration decreases after stabilization of blood pressure. Such adjustments can easily be made using special IHD consoles equipped with a sodium infuser. However, CRRT consoles that can adjust the sodium concentration in the treatment fluid are not commercially available. Although high-sodium CRRT treatment fluid can be prepared by the addition of a high-sodium solution, this preparation is often complicated, and adjusting the optimal sodium concentration in a timely manner during CRRT sessions can be difficult. Therefore, cIVNa was used during CRRT as an alternative.
In the ICU, a commercially available CRRT treatment fluid (Sublood BSG, Fuso Co., Ltd., Osaka, Japan: Na 140 mEq/L, K 2.0 mEq/L, Ca 3.5 mEq/L, HCO3− 35.0 mEq/L, acetic acid 0.5 mEq/L) was used. The standard anticoagulant was 30 mg/h of nafamostat mesylate, the standard quantity of blood flow was 100–150 mL/min (6–9 L/h), and the standard quantity of treatment fluid was 800 mL/h (0.8 L/h, consisting of 600 mL/h of dialysate and 200 mL/h of replacement fluid). Due to issues with the Japanese insurance system, the use of 600–800 mL/h of treatment fluid is standard for CRRT in Japan, which translates to 10.0–13.3 mL/kg/h for a 60 kg patient. Consequently, the efficiency of CRRT in Japan is lower than the international standard of 20–25 mL/kg/h [16, 22].
A 50 mEq/20 mL NaCl solution was used for cIVNa. Under the above CRRT condition of blood flow and treatment fluid, sodium infusion dosage for cIVNa was 2.5–10 mEq/h (1.0–4.0 mL/h, 60–240 mEq/day).
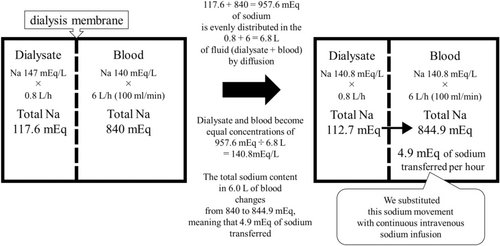
Figure 2 shows an ideal model for substituting cIVNa with a high-sodium dialysate. In CRRT, 0.8 L/h of dialysate with a sodium concentration of 147 mEq/L was in contact with 6.0 L/h of blood with a sodium concentration of 140 mEq/L through the CRRT dialysis membrane. Because sodium is a small molecule, it was distributed evenly in 6.8 L of fluid (dialysate + blood) by diffusion, resulting in equal sodium concentrations of 140.8 mEq/L in the dialysate and blood. At this point, the total sodium content in 6.0 L of blood changed from 840 to 844.9 mEq, meaning that 4.9 mEq of sodium was transferred from the dialysate to the blood per hour. Similarly, when CRRT was performed with 0.8 L/h of dialysate, 6–9 L/h of blood flow rate, and high-sodium dialysate (Na 144–154 mEq), approximately 2.5–10 mEq/h of sodium moved from the dialysate to the blood. This sodium movement was substituted with cIVNa. To be precise, various factors, such as the difference between hemodialysis and hemofiltration, sieving coefficient, and Gibbs–Donnan effect, must be considered; however, for simplicity, the above calculation was used.
In actual clinical practice, sodium dosage in cIVNa was initiated at approximately 5 mEq/h and adjusted by 1.25–2.5 mEq/h based on hemodynamics and the required mechanical fluid removal. The dosage was increased if blood pressure was low or if more mechanical fluid removal was required and decreased if blood pressure became high or mechanical fluid removal was not required.
In cases with rapid improvement in plasma refilling due to improvement in inflammation, severe congestion, or hyponatremia, sodium dosage in cIVNa was started carefully in small amounts or was not initiated at all. When there was a concern about acidosis progression due to chloride loading by cIVNa, sodium bicarbonate was used partially or completely instead of the NaCl solution.
Mechanical fluid removal by ultrafiltration of CRRT removes fluid with a sodium concentration of approximately 140 mEq/L [17], whereas commercially available intravenous infusion solutions and tube feedings have relatively low sodium concentrations, causing a sodium in–out minus balance. Therefore, the sodium dosage of cIVNa was adjusted to compensate for this sodium loss. For example, when 1 L/day of nutrition preparation with a sodium concentration of 50 mEq/L is administered, an additional 90 mEq/day sodium (3.75 mEq/h) is added to the cIVNa. This sodium supplementation strategy results in a total sodium supply of 140 mEq/day. When 1 L of mechanical fluid is removed with a sodium concentration of 140 mEq/L, the sodium in-out balance is theoretically expected not to be reduced by this adjustment, maintaining the extracellular fluid volume. Additionally, sodium is not included in certain intravenous medications, such as sedatives, antiarrhythmics, and antibiotics. These medicines may aggravate the sodium in–out balance, and the amount of sodium produced by cIVNa is adjusted upward when these medications are used.
After improving the urine volume and CRRT withdrawal, cIVNa was either continued at approximately 2.5 mEq/h or nutrition preparation adjusted to a high-sodium concentration (approximately 100 mEq/L) was used, depending on the patient's hemodynamic status.
In the ICU, nephrologists familiar with critical care and dialysis management made these adjustments. Since there are various precautions associated with the cIVNa method, it should be administered by such specialists.
3 RESULTS
3.1 Baseline characteristics of patients
There were no significant differences in the baseline characteristics of patients with prerenal AKI between the control (CRRT) and intervention (CRRT + cIVNa) groups (Table 1). Most patients were in the postoperative course and had heart disease, with relatively few having an infection. Additionally, the majority of patients underwent cardiac or vascular surgeries using cardio-pulmonary bypass. More than half of the surgeries were emergencies. There were no differences in the surgical characteristics between the control and intervention groups. There were no differences in the systolic blood pressure, serum levels of creatinine and urea nitrogen at CRRT initiation, percentage of patients with decreased urine volume (<100 mL of urine volume in the 6 h before CRRT initiation), severity scores such as APACHE II and SOFA scores, and the rate of using vasopressors and ventilators between the control and intervention groups. The use of ECMO or VAD was approximately 26% during CRRT in both groups, and no significant differences were detected. The median (IQR) treatment duration with CRRT was 5.5 (2.0–12.3) and 8 (4.0–15.5) days in the control and intervention groups, respectively, and no significant differences were detected.
| Control (CRRT) | Intervention (CRRT + cIVNa) | p-value | |
|---|---|---|---|
| Cases, n | 49 | 43 | |
| Gender (men), n (%) | 38 (77.6%) | 31 (72.1%) | 0.63 |
| Age [years] | 72 (64.0–78.0) | 71 (52.5–77.5) | 0.48 |
| Systolic blood pressure [mmHg] | 99.0 ± 26.3 | 95.0 ± 23.2 | 0.44 |
| Serum level of creatinine [mg/dL] | 3.2 (2.5–4.2) | 2.9 (2.3–4.1) | 0.42 |
| Serum level of urea nitrogen [mg/dL] | 60.3 (43.6–89.8) | 68.3 (41.1–100.2) | 0.86 |
| Patients with event of decreased urine volume < 100 mL in the 6 h before CRRT initiation, n (%) | 19/46 (41.3%) | 22 (51.2%) | 0.40 |
| Background disease: post-operation, n (%) | 39 (79.6%) | 34 (79.1%) | 1.00 |
| Background disease: heart disease, n (%) | 26 (53.1%) | 30 (69.8%) | 0.13 |
| Background disease: infection, n (%) | 13 (26.5%) | 15 (34.9%) | 0.50 |
| Background disease: pancreatitis, n (%) | 2 (4.1%) | 1 (2.3%) | 1.00 |
| Operation: use of cardio-pulmonary bypass, n (%) | 24/39 (61.5%) | 25/34 (73.5%) | 0.32 |
| Operation: cardiac, n (%) | 14/39 (35.9%) | 16/34 (47.1%) | 0.35 |
| Operation: vascular, n (%) | 24/39 (61.5%) | 21/34 (61.8%) | 1.00 |
| Operation: digestive, n (%) | 5/39 (12.8%) | 2/34 (5.9%) | 0.44 |
| Operation: other organs, n (%) | 3/39 (7.7%) | 4/34 (11.8%) | 0.70 |
| Operation: accompanied with infectious disease, n (%) | 4/39 (10.3%) | 6/34 (17.6%) | 0.50 |
| Operation: emergency, n (%) | 20/39 (51.3%) | 21/34 (61.8%) | 0.48 |
| Operation: scheduled, n (%) | 19/39 (48.7%) | 13/34 (38.2%) | 0.48 |
| APACHE II score at ICU admission | 30 (23–33.0) | 29 (23–31.5) | 0.42 |
| SOFA score at ICU admission | 13 (9–15) | 13 (10–15) | 0.64 |
| SOFA score at CRRT initiation | 14 (11–16) | 14 (10–16) | 0.49 |
| Vasopressors use, n (%) | 38 (77.6%) | 36 (83.7%) | 0.60 |
| Ventilator use, n (%) | 38 (77.6%) | 33 (76.7%) | 1.00 |
| ECMO/VAD at CRRT initiation, n (%) | 10 (20.4%) | 9 (20.9%) | 1.00 |
| ECMO/VAD during CRRT, n (%) | 13 (26.5%) | 11 (25.6%) | 1.00 |
| Treatment duration with CRRT [days] | 5.5 (2.0–12.3) | 8 (4.0–15.5) | 0.16 |
- Note: Categorical variables are presented as numbers (percentages) and were compared using the Fisher's exact test. Normally and non-normally distributed data are expressed as means ± standard deviation and median (interquartile range). The Student's t-test and Mann–Whitney U test were used to compare normally and non-normally distributed data, respectively.
- Abbreviations: cIVNa, continuous intravenous sodium infusion therapy; CRRT, continuous renal replacement therapy; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; IHD, intermittent hemodialysis therapy; VAD, ventricular assist device.
3.2 Main outcome
The survival rates at 90-day, 60-day without RRT, and 90-day without RRT were significantly higher in the intervention group than in the control group (61.9% vs. 38.8%, p < 0.05; 59.5% vs. 28.6%, p < 0.01; 54.8% vs. 26.5%, p < 0.01, respectively) (Figure 3). Furthermore, the survival discharge rate from the ICU, CRRT withdrawal rate, and RRT withdrawal rate were also significantly higher in the intervention group than in the control group (72.1% vs. 44.9%, p < 0.05; 81.4% vs. 49.0%, p < 0.01; 72.1% vs. 44.9%, p < 0.05, respectively). Regarding the modality of RRT, the utilization rate of IHD was significantly higher in the intervention group than in the control group (32.6% vs. 14.3%, p < 0.05), probably reflecting improved hemodynamics in the intervention group.
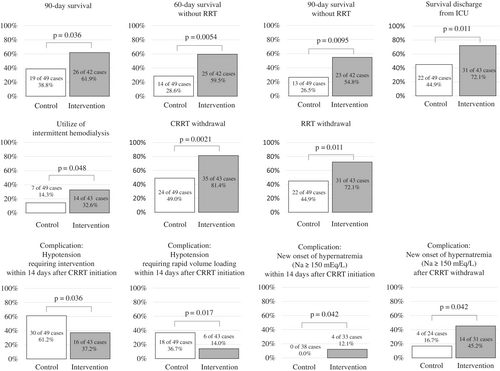
The survival rates at 28- and 60-day, and 28-day without RRT, as well as the survival discharge rate from the hospital were higher, whereas RRT, CRRT, and IHD dependence rates among 90-day survivors, CRRT reintroduction rate after CRRT withdrawal, and RRT reintroduction rate after RRT withdrawal were lower in the intervention group than in the control group; however, no significant differences were detected.
Patients with ECMO/VAD had markedly poorer prognosis than patients without ECMO/VAD; therefore, we analyzed the outcome results stratified by the use of ECMO/VAD during CRRT. Regardless of whether ECMO/VAD was performed, the CRRT withdrawal rate was significantly higher in the intervention group than in the control group (63.6% vs. 15.4%, p < 0.05; 87.5% vs. 61.1%, p < 0.05, respectively). In both subgroups with or without ECMO/VAD, the survival rates at 60- and 90-day, 90-day without RRT, and the RRT withdrawal rate were higher in the intervention group than in the control group; however, these differences were not significant (Table 2).
| Control (CRRT) | Intervention (CRRT + cIVNa) | p-value | |
|---|---|---|---|
| Cases, n | 49 | 43 | |
| 28-day survival, n (%) | 32 (65.3%) | 36 (83.7%) | 0.058 |
| 60-day survival, n (%) | 24 (49.0%) | 28/42 (66.7%) | 0.14 |
| 90-day survival, n (%) | 19 (38.8%) | 26/42 (61.9%) | 0.036* |
| RRT dependence rate among 90-day survivors, n (%) | 6/19 (31.6%) | 3/26 (11.5%) | 0.14 |
| CRRT dependence rate among 90-day survivors, n (%) | 3/19 (15.8%) | 1/26 (3.8%) | 0.30 |
| IHD dependence rate among 90-day survivors, n (%) | 3/19 (15.8%) | 2/26 (7.7%) | 0.64 |
| 28-day survival without RRT, n (%) | 19 (38.8%) | 25 (58.1%) | 0.09 |
| 60-day survival without RRT, n (%) | 14 (28.6%) | 25/42 (59.5%) | 0.0054** |
| 90-day survival without RRT, n (%) | 13 (26.5%) | 23/42 (54.8%) | 0.0095** |
| Survival discharge from the ICU, n (%) | 22 (44.9%) | 31 (72.1%) | 0.011* |
| Survival discharge from the hospital, n (%) | 18 (36.7%) | 24 (55.8%) | 0.09 |
| Modality of RRT:CRRT only, n (%) | 42 (85.7%) | 29 (67.4%) | 0.048* |
| Modality of RRT:CRRT + IHD, n (%) | 7 (14.3%) | 14 (32.6%) | 0.048* |
| CRRT withdrawal, n (%) | 24 (49.0%) | 35 (81.4%) | 0.0021** |
| CRRT reintroduction after CRRT withdrawal, n (%) | 5/24 (20.8%) | 5/35 (14.3%) | 0.73 |
| RRT withdrawal, n (%) | 22 (44.9%) | 31 (72.1%) | 0.011* |
| RRT reintroduction after RRT withdrawal, n (%) | 5/22 (22.7%) | 4/31 (12.9%) | 0.46 |
| Patients with ECMO/VAD during CRRT, n | 13 | 11 | |
| 60-day survival, n (%) | 2/13 (15.4%) | 6/11 (54.5%) | 0.08 |
| 90-day survival, n (%) | 2/13 (15.4%) | 6/11 (54.5%) | 0.08 |
| 90-day survival without RRT, n (%) | 1/13 (7.7%) | 5/11 (45.5%) | 0.06 |
| CRRT withdrawal, n (%) | 2/13 (15.4%) | 7/11 (63.6%) | 0.032* |
| RRT withdrawal, n (%) | 2/13 (15.4%) | 6/11 (54.5%) | 0.08 |
| Patients without ECMO/VAD during CRRT, n | 36 | 32 | |
| 60-day survival, n (%) | 22/36 (61.1%) | 22/31 (70.9%) | 0.44 |
| 90-day survival, n (%) | 17/36 (47.2%) | 20/31 (64.5%) | 0.14 |
| 90-day survival without RRT, n (%) | 12/36 (33.3%) | 18/31 (58.1%) | 0.051 |
| CRRT withdrawal, n (%) | 22/36 (61.1%) | 28/32 (87.5%) | 0.026* |
| RRT withdrawal, n (%) | 20/36 (55.6%) | 25/32 (78.1%) | 0.07 |
- Note: Variables are presented as numbers (percentages) and were compared using the Fisher's exact test. *p < 0.05, **p < 0.01, compared between variables in the intervention and control groups. “CRRT withdrawal” was defined as surviving for >7 days without CRRT after its discontinuation. “RRT withdrawal” was defined as surviving for >7 days without RRT after its discontinuation.
- Abbreviations: cIVNa, continuous intravenous sodium infusion therapy; CRRT, continuous renal replacement therapy; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; IHD, intermittent hemodialysis therapy; RRT, renal replacement therapy; VAD, ventricular assist device.
3.3 Complications
Among the complications that occurred within 14 days of CRRT initiation, hypotension events occurred less frequently in the intervention group than in the control group (Table 3). Especially, the incidence of hypotension requiring intervention or that requiring rapid volume loading was significantly lower (37.2% vs. 61.2%, p < 0.05; 14.0% vs. 36.7%, p < 0.05, respectively) (Figure 3). There were no significant differences in the incidences of hypertension requiring antihypertensive agents, arrhythmias, or bleeding (Table 3). The rate of new onset of hypernatremia (Na ≥150 mEq/L) within 14 days after CRRT initiation and within 14 days after CRRT withdrawal was significantly higher in the intervention group than in the control group (12.1% vs. 0%, p < 0.05; 45.2% vs. 16.7%, p < 0.05, respectively) (Figure 3). Hypernatremia (Na ≥150 mEq/L) at CRRT withdrawal was not observed in the control group but occurred more frequently in the intervention group (11.4%), although this difference was not statistically significant.
| Control (CRRT) | Intervention (CRRT + cIVNa) | p-value | |
|---|---|---|---|
| CRRT-related complications within 14 days after CRRT initiation | |||
| Cases, n | 49 | 43 | |
| Hypotension requiring intervention, n (%) | 30 (61.2%) | 16 (37.2%) | 0.036* |
| Hypotension requiring rapid volume loading, n (%) | 18 (36.7%) | 6 (14.0%) | 0.017* |
| Hypotension requiring increases in the vasopressor dose, n (%) | 27 (55.1%) | 15 (34.9%) | 0.06 |
| Hypertension requiring antihypertensive agents, n (%) | 11 (22.4%) | 14 (32.6%) | 0.35 |
| Arrhythmia requiring antiarrhythmic agents and/or electrical defibrillation, n (%) | 16 (32.7%) | 11 (25.6%) | 0.50 |
| Bleeding events, n (%) | 3 (6.1%) | 0 (0.0%) | 0.25 |
| New onset of hypernatremia (Na ≥150 mEq/L), n (%) | 0/38 (0.0%) | 4/33 (12.1%) | 0.042* |
| Complications at/after CRRT withdrawal | |||
| Patients who achieved CRRT withdrawal, n | 24 | 35 | |
| Hypernatremia(Na ≥150 mEq/L) at CRRT withdrawal, n (%) | 0 (0.0%) | 4 (11.4%) | 0.14 |
| New onset of hypernatremia (Na ≥150 mEq/L) within 14 days after CRRT withdrawal, n (%) | 4 (16.7%) | 14/31 (45.2%) | 0.042* |
- Note: Variables are presented as numbers (percentages) and were compared using the Fisher's exact test. *p < 0.05, **p < 0.01, compared between variables in the intervention and control groups. “Hypotension requiring intervention” were defined as hypotension requiring an increase in vasopressor dose and/or rapid volume loading.
- Abbreviations: cIVNa, continuous intravenous sodium infusion therapy; CRRT, continuous renal replacement therapy.
3.4 Changes in blood pressure, fluid management, and serum sodium levels
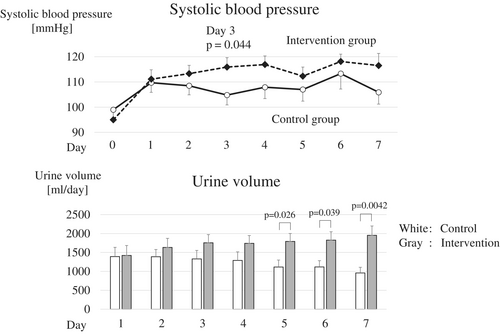
In the intervention group, the systolic blood pressure improved from the first day after CRRT initiation, and the mean systolic blood pressure was maintained at >110 mmHg (Figure 4 and Table 4). Notably, on day 3, systolic blood pressure was significantly higher in the intervention group than in the control group (115.9 ± 3.7 mmHg vs. 104.8 ± 3.9 mmHg, p < 0.05). After CRRT initiation, the urine volume gradually decreased in the control group, whereas it increased in the intervention group, with an average urine volume of >1500 mL from day 2. On days 5–7, urine volume was significantly higher in the intervention group than in the control group (p < 0.05, p < 0.05, and p < 0.01, respectively). Mechanical fluid removal in patients who continued RRT was significantly lower in the intervention group than in the control group on day 3 (p < 0.05), probably reflecting an increase in urine volume in the intervention group. There were no differences in the total volume of fluid administered between the two groups. The total excreted fluid in the intervention group was maintained at >2500 mL from day 2, and the total excreted fluid on days 6 and 7 was significantly higher in the intervention group than in the control group (p < 0.05 and p < 0.01, respectively). Regarding total fluid balance, the control group exhibited a positive balance from day 4, whereas the intervention group exhibited a negative balance from day 2, and the total fluid balance on day 7 was significantly less in the intervention group than in the control group (−300.8 ± 190.2 mL vs. 427.4 ± 193.1 mL, p < 0.05).
| Day after CRRT initiation | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|---|
| Cases in ICU, n | ||||||||
| Control | 49 | 44 | 42 | 40 | 37 | 31 | 31 | 31 |
| Intervention | 43 | 42 | 42 | 39 | 37 | 36 | 34 | 33 |
| Cases with RRT in ICU, n | ||||||||
| Control | 49 | 43 | 34 | 33 | 29 | 23 | 21 | 21 |
| Intervention | 43 | 41 | 39 | 33 | 28 | 25 | 21 | 20 |
| Systolic blood pressure (mmHg) | ||||||||
| Control | 99.0 ± 3.8 | 109.7 ± 3.8 | 108.5 ± 3.6 | 104.8 ± 3.9 | 107.9 ± 4.5 | 107.0 ± 4.6 | 113.3 ± 6.1 | 105.9 ± 4.7 |
| Intervention | 95.0 ± 3.5 | 111.1 ± 3.7 | 113.3 ± 3.3 | 115.9 ± 3.7 | 116.9 ± 3.4 | 112.3 ± 3.6 | 118.1 ± 2.9 | 116.5 ± 4.8 |
| p-value | 0.60 | 0.46 | 0.24 | 0.044* | 0.10 | 0.34 | 0.28 | 0.097 |
| Urine volume (mL) | ||||||||
| Control | 1390.6 ± 243.8 | 1385.6 ± 192.7 | 1328.5 ± 225.9 | 1288.7 ± 226.4 | 1115.1 ± 184.8 | 1117.4 ± 165.9 | 956.6 ± 150.9 | |
| Intervention | 1420.1 ± 263.6 | 1631.9 ± 239.9 | 1756.2 ± 215.4 | 1741.4 ± 205.4 | 1792.9 ± 208.8 | 1824.8 ± 222.8 | 1952.2 ± 246.5 | |
| p-value | 1.00 | 0.52 | 0.11 | 0.089 | 0.026* | 0.039* | 0.0042** | |
| Mechanical fluid removal volume in patients who continued RRT (mL) | ||||||||
| Control | 870.9 ± 154.3 | 1025.1 ± 194.7 | 960.1 ± 207.6 | 800.4 ± 184.8 | 1050.5 ± 217.3 | 1007.0 ± 212.1 | 917.7 ± 230.0 | |
| Intervention | 565.3 ± 116.2 | 620.8 ± 139.0 | 634.1 ± 161.2 | 766.4 ± 181.7 | 779.6 ± 201.0 | 983.2 ± 244.2 | 875.6 ± 199.5 | |
| p-value | 0.094 | 0.063 | 0.027* | 0.50 | 0.22 | 0.80 | 0.97 | |
| Mechanical fluid removal volume in patients who continued or discontinued RRT (mL) | ||||||||
| Control | 870.9 ± 154.3 | 893.6 ± 178.3 | 833.7 ± 187.7 | 663.2 ± 161.2 | 805.4 ± 185.1 | 755.2 ± 179.0 | 688.3 ± 187.7 | |
| Intervention | 565.3 ± 116.2 | 620.8 ± 139.0 | 581.3 ± 150.5 | 631.2 ± 157.6 | 609.1 ± 166.7 | 764.7 ± 205.2 | 673.5 ± 169.4 | |
| p-value | 0.094 | 0.25 | 0.047* | 0.47 | 0.28 | 0.81 | 0.88 | |
| Total administered fluid volume (mL) | ||||||||
| Control | 2707.1 ± 178.8 | 2592.6 ± 173.7 | 2382.7 ± 164.8 | 2581.8 ± 216.2 | 2626.9 ± 265.6 | 2333.8 ± 171.1 | 2215.9 ± 106.9 | |
| Intervention | 2524.0 ± 159.6 | 2383.2 ± 128.2 | 2590.4 ± 202.0 | 2331.3 ± 114.4 | 2542.6 ± 125.4 | 2458.9 ± 110.2 | 2395.4 ± 103.0 | |
| p-value | 0.39 | 0.28 | 0.78 | 0.45 | 0.73 | 0.74 | 0.29 | |
| Total excreted fluid volume (mL) | ||||||||
| Control | 2531.4 ± 263.6 | 2605.5 ± 201.3 | 2444.0 ± 230.2 | 2205.5 ± 213.7 | 2112.8 ± 201.9 | 2099.2 ± 187.2 | 1788.6 ± 167.7 | |
| Intervention | 2294.7 ± 249.5 | 2557.7 ± 197.8 | 2642.7 ± 161.7 | 2621.3 ± 145.0 | 2647.1 ± 151.5 | 2687.5 ± 156.0 | 2696.2 ± 195.1 | |
| p-value | 0.40 | 0.80 | 0.24 | 0.13 | 0.057 | 0.023* | 0.0019** | |
| Total fluid balance (mL) | ||||||||
| Control | +175.7 ± 347.2 | −12.9 ± 221.1 | −61.3 ± 256.4 | +376.3 ± 267.7 | +514.1 ± 316.8 | +234.6 ± 217.3 | +427.4 ± 193.1 | |
| Intervention | +229.3 ± 270.2 | −164.5 ± 197.5 | −52.3 ± 236.2 | −290.0 ± 141.3 | −101.7 ± 182.6 | −228.6 ± 175.6 | −300.8 ± 190.2 | |
| p-value | 0.59 | 0.64 | 0.99 | 0.061 | 0.13 | 0.068 | 0.034* | |
| Serum sodium level (mEq/L) | ||||||||
| Control | 143.1 ± 8.2 | 141.6 ± 5.3 | 140.4 ± 4.2 | 139.7 ± 4.4 | 139.3 ± 5.5 | 139.1 ± 6.0 | 138.8 ± 5.8 | 138.9 ± 6.1 |
| Intervention | 144.7 ± 7.9 | 144.1 ± 7.0 | 143.8 ± 5.9 | 143.9 ± 5.5 | 144.7 ± 6.0 | 144.4 ± 5.3 | 144.8 ± 5.6 | 145.9 ± 5.4 |
| p-value | 0.47 | 0.13 | 0.0030** | <0.001** | <0.001** | <0.001** | <0.001** | <0.001** |
| Serum Na ≥145 mEq/L, n (%) | ||||||||
| Control | 22/49 (44.9%) | 15/46 (32.6%) | 7/45 (15.6%) | 5/41 (12.2%) | 5/37 (13.5%) | 6/36 (16.7%) | 6/31 (19.4%) | 5/29 (17.2%) |
| Intervention | 22/43 (51.2%) | 18/42 (42.9%) | 15/42 (35.7%) | 15/41 (36.6%) | 21/39 (53.8%) | 18/37 (48.6%) | 18/36 (50.0%) | 17/33 (51.5%) |
| p-value | 0.68 | 0.038 | 0.047* | 0.019* | <0.001** | 0.0057** | 0.011* | 0.0074** |
- Note: Variables of systolic blood pressure and fluid management data are expressed as mean ± standard error, and serum sodium level are presented as mean ± standard deviation, and all of them were compared using the Mann–Whitney U test. Patients with serum Na ≥145 mEq/L are presented as numbers (percentages) and were compared using Fisher's exact test. *p < 0.05, **p < 0.01, compared between variables in the intervention and control groups.
- Abbreviations: CRRT, continuous renal replacement therapy; ICU, intensive care unit; RRT, renal replacement therapy.
In the control group, the serum sodium levels decreased from 143.1 ± 8.2 mEq/L at CRRT initiation to approximately 139 mEq/L in 4 days, and were maintained thereafter. In the intervention group, the serum sodium level was maintained at approximately 144 mEq/L, which was significantly higher than that in the control group from day 2. In the intervention group, the rates of patients with Na ≥145 mEq/L were approximately 30%–50% and were significantly higher than those in the control group from day 2.
4 DISCUSSION
The application of high-sodium dialysate during IHD or SLED to attenuate hemodynamic instability due to insufficient plasma refilling during RRT has been reported in the critical care field [12, 14, 21]. However, no prior studies have implemented this concept in CRRT. The current study, for the first time, successfully demonstrated that sodium supplementation with cIVNa, instead of high-sodium dialysate, can attenuate hemodynamic instability during CRRT. Systolic blood pressure and urine volume improved rapidly, and good hemodynamic conditions were maintained in the intervention group. The incidence of hypotension events significantly decreased in the intervention group. cIVNa may improve plasma refilling, maintain extracellular fluid volume, increase blood pressure and renal blood flow, and lead to hemodynamic stabilization. Furthermore, the intervention group (CRRT + cIVNa) exhibited significant improvements in the 90-day survival rate, 60-day and 90-day survival rates without RRT, survival discharge rate from ICU, CRRT withdrawal rate, and RRT withdrawal rate.
In recent RCTs, the 60- or 90-day survival rates of RRT-requiring AKI have been reported to be approximately 50% [5-8]. The prognosis in the control group of the present study was 49.0% for the 60-day survival rate and 38.8% for the 90-day survival rate, which was relatively poor. This may be due to the large number of patients treated with ECMO/VAD, which accounted for 26% of the study population. It has been reported that patients treated with ECMO/VAD and CRRT have a severely poor prognosis [23, 24]. Excluding patients with ECMO/VAD, the prognosis in the control group of the present study was 61.1% for the 60-day survival rate and 47.2% for the 90-day survival rate, which is close to recent reports. When patients were divided into subgroups with or without ECMO/VAD, CRRT withdrawal rates were significantly improved in the intervention group in both subgroups, suggesting that cIVNa had a positive effect on patient outcomes, irrespective of ECMO/VAD.
In recent years, IHD or SLED have often been used in combination with various dialysis techniques, including high-sodium dialysate, low-temperature dialysate, bicarbonate buffer dialysate, and high-calcium dialysate [11-14]. Douvris et al. reported a detailed review of countermeasures against hypotension during RRT, introducing the concept of using multiple dialysis techniques as a multimodal approach (MMA) [11]. We had partially applied the concept of MMA to CRRT before initiating the cIVNa intervention. In the ICU, we used a CRRT treatment fluid commercially available in Japan for all patients. This treatment fluid contains a bicarbonate buffer (35 mEq/L) with low acetate content (0.5 mEq/L) and a high calcium concentration (3.5 mEq/L). It was stored at room temperature (20–25°C) and used without warming, meaning that CRRT was conducted with low-temperature treatment fluid. All patients in the control group underwent CRRT with this partial MMA of low-temperature treatment fluid, bicarbonate dialysate, and high-calcium dialysate. In the intervention group, sodium supplementation with cIVNa was added as a substitute for the high-sodium dialysate, which led to significant hemodynamic stabilization. These findings suggest that sodium supplementation plays a key role in achieving hemodynamic stabilization effects among the MMA techniques. Sodium supplementation via cIVNa may exert strong effects not only by improving plasma refilling, but also by enhancing the sodium in-out balance. Mechanical fluid removal via ultrafiltration during the RRT typically removes fluid with a sodium concentration of approximately 140 mEq/L [17]. This substantial sodium loss may often be overlooked by physicians and could contribute to the poor prognosis of critically ill patients requiring RRT. Sodium supplementation via cIVNa can improve the sodium in-out balance in such patients; therefore, the cIVNa dose should be adjusted taking into account the quantity of mechanical fluid removed by RRT.
In contrast, the use of high-sodium dialysate is known to be associated with risks such as thirst, excessive plasma refilling, hypertension, and hypernatremia [17, 18]. In the current study, 45.2% of patients in the intervention group developed hypernatremia after CRRT withdrawal. This may be affected by the fact that the serum sodium level in the intervention group remained high at approximately 144 mEq/L after CRRT initiation. Furthermore, sodium management after CRRT withdrawal may also have affected the results. During the AKI recovery phase, urine with a sodium concentration close to 140 mEq/L is excreted because of insufficient tubular sodium reabsorption, often causing excess urine sodium excretion, decreased extracellular fluid, and hypotension. In our experience, discontinuing cIVNa immediately after discontinuing CRRT resulted in decreased blood pressure and urine output and early reintroduction of CRRT. Therefore, in the current study, the sodium supplementation dose of cIVNa was gradually tapered or nutrition preparation adjusted to be of a high-sodium concentration was used during AKI recovery. However, hypernatremia frequently occurred after CRRT withdrawal, highlighting the need to improve the tapering speed of sodium supplementation and timing of cIVNa discontinuation. During the AKI recovery phase, serum sodium levels and urinary sodium concentrations should be checked daily. When the serum sodium level increases or urinary sodium concentration decreases, which are signs of improved tubular sodium reabsorption function, sodium supplementation should be decreased or discontinued. When hypernatremia was thought to be induced by hypovolemia, excessive diuresis or mechanical fluid removal should also be tapered.
Given potential adverse events, cIVNa administration should be managed by physicians with sufficient knowledge and skills in high-sodium dialysate treatment. Considering these adverse events, cIVNa should be carefully indicated based on the patient's pathological condition, with careful consideration for timely reduction or discontinuation of cIVNa. cIVNa can be particularly harmful when rapid plasma refilling occurs during the recovery phase from sepsis, as it increases the risk of pulmonary congestion due to excessive plasma refilling and an elevated extracellular fluid volume. Therefore, reduction or discontinuation of cIVNa should be considered as soon as hemodynamic stability is achieved in such cases. In patients with heart failure, excessive plasma refilling and increased extracellular fluid volume via the cIVNa can lead to cardiac strain. Therefore, careful monitoring of the extracellular fluid volume and right heart strain, evaluated by physical examinations, chest X-ray photographs, and ultrasound cardiography, is needed. Furthermore, in patients with severe hyponatremia, cIVNa may rapidly increase serum sodium level [11], which may cause osmotic demyelination syndrome. In such cases, cIVNa should be avoided or initiated carefully with a small dose of sodium supplementation with frequent monitoring of serum sodium levels. In our ICU, nephrologists experienced in critical care and dialysis management were primarily responsible for managing CRRT and adjusting the cIVNa dosage under frequent and meticulous monitoring.
This study has several limitations. First, it was a small-scale retrospective study conducted at a single facility. Further prospective multi-center studies are warranted. Second, most patients in the control group were treated before 2018 and all patients in the intervention group were treated after 2019. Therefore, we were unable to adjust for or exclude the impact of differences in patient management methods due to advances in intensive care management (e.g., nutritional management, nursing, rehabilitation, early mobilization, examinations, and surgery). Hemodynamic stabilization via cIVNa may exert secondary effects, enabling aggressive nutritional intervention and rehabilitation for early mobilization. Third, to verify the effect of sodium supplementation via cIVNa, patients who required various special treatments, such as immunosuppressive therapy, chemotherapy, and organ transplants, were excluded from this study. However, sodium supplementation effect via cIVNa may also be effective in these patients, warranting further verification in these groups. Fourth, the utility of IHD and SLED with MMA, including a high-sodium dialysate, has been reported in recent years in critically ill patients [12, 14, 19]. This study could not determine whether “IHD or SLED using high sodium dialysate” or “CRRT using cIVNa” is the superior treatment for critically ill patients requiring RRT. IHD, SLED, and CRRT have their advantages and disadvantages [15], and the indications for each RRT may vary. Further studies are required to address this issue. Finally, the quantity of treatment fluid used for CRRT in Japan is less than the international standards. Therefore, the results of this study may not apply to patients who have undergone CRRT in other countries. Adjustments in treatment conditions may be necessary when using cIVNa in such patients.
In conclusion, compared with conventional CRRT, combination therapy of CRRT and cIVNa increased blood pressure, enhanced urinary volume, and reduced hypotension events, indicating hemodynamic stabilization. Furthermore, this intervention significantly improved clinical outcomes, including 90-day survival rate, 60-day and 90-day survival rate without RRT, survival discharge rate from ICU, CRRT withdrawal rate, and RRT withdrawal rate. These findings suggest that combination therapy may be a useful treatment option for hemodynamically unstable patients with AKI requiring CRRT, particularly those with expected insufficient plasma refilling.
ACKNOWLEDGMENTS
We would like to thank Editage (www.editage.com) for the English language proofreading.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflicts of interest or competing interests.
ETHICS STATEMENT
This study was conducted in accordance with the principles of the Declaration of Helsinki. The study protocol was approved by the Medical Ethics Committee of the Shinshu University School of Medicine (approval number: 5769).
Open Research
DATA AVAILABILITY STATEMENT
The data used in this study are available from the corresponding author upon request.




