Efficacy and Safety of Adsorptive Granulocyte and Monocyte Apheresis in Elderly and Pregnant Patients With Ulcerative Colitis
Abstract
In patients with active ulcerative colitis (UC), adsorptive granulocyte/monocyte apheresis (GMA) is expected to promote remission. We conducted a retrospective cohort study to evaluate the efficacy and safety of GMA in patients with active UC. Twenty-one UC patients including five pregnant or lactating mothers and four elderly patients (aged >60 years) received up to 10 GMA sessions. UC severity was evaluated at baseline and after GMA therapy according to Lichtiger's Clinical Activity Index (CAI). We defined clinical remission as CAI ≤4. Overall, the median CAI score after GMA therapy had decreased from 9 to 4 (P < 0.001). The clinical remission rate was 62%, but in the elderly and pregnant or lactating mothers, the remission rates were 100% and 60%, respectively. No severe adverse effects were seen in this study. Our results may support GMA as an effective and safe treatment for active UC patients, including elderly patients and pregnant cases.
Ulcerative colitis (UC) is one of the two major phenotypes of the chronic inflammatory bowel disease (IBD) defined as an idiopathic, nonspecific inflammatory disorder involving primarily the mucosa and the submucosa of the large intestine 1. Currently, the etiology of UC is not fully understood; it afflicts millions of individuals throughout the world with debilitating symptoms, impairing function and quality of life 1. For example, at the end of 2016, the number of UC patients in Japan had increased to almost 170 000, reflecting a gradual increase with time. Although it has been reported that most affected individuals are in their twenties to forties, the number of elderly onset UC is increasing 2. Moreover, in female patients, a higher disease prevalence and activity are observed in their twenties or thirties, which are the childbearing ages.
Granulocyte/monocyte apheresis (GMA) is one of the treatment options as induction therapy for patients with UC 3. Nowadays, in Japan as well as in the European countries, GMA is favored by patients for its safety profile as well as for being a nonpharmacologic strategy for treating patients with active UC. The primary action of GMA for inducing anti-inflammatory effect is to selectively deplete elevated granulocytes and monocyte as blood passes through the column 4, 5. GMA initiates generation of regulatory B and T cells 5, which are the key requirements for a normal immune function. Further, unadsorbed leukocytes which pass through the column show downregulation of adhesion molecules and reduction of inflammatory cytokine production 4, 6, 7. These are thought to be the main mechanisms of GMA induced anti-inflammatory effect and most likely support its clinical efficacy in patients with active UC 7, 8. Moreover, recent large-scale cohort studies reported its efficacy and safety in elderly patients 9-11. However, little is known about the efficacy and safety of GMA among pregnant and lactating patients with UC. The purpose of the present study was to evaluate the efficacy and safety of GMA in active UC patients, including pregnant cases and lactating mothers who often have to avoid common pharmacologic treatment 8, 12, 13.
PATIENTS AND METHODS
Study design, setting, and patients
We conducted a retrospective cohort study involving patients with UC at Saku Central Hospital Advanced Care Center from June 2014 to October 2018. All patients aged >18 years with active UC who had been treated with GMA were entered into this study. We defined elderly patients as those who were more than 60 years of age based on a previous study 10. Our investigation protocol was reviewed and approved by the Ethical Committee of Saku Central Hospital Advanced Care Center. Further, the study was undertaken in accordance with the Principles of Good Clinical Practice with diligence and adherence to the Helsinki Declaration at all times.
Granulocyte and monocyte adsorptive apheresis
GMA was performed with the Adacolumn (JIMRO Co., Ltd., Takasaki City, Japan) which contains cellulose acetate beads as leukocytapheresis carriers for myeloid lineage leukocytes 5, 8. Each patient received up to 10 GMA sessions, at one, two, or three sessions per week. The duration of each session was 60 min at a flow rate of 30 mL/min. A total of 1800 mL of blood was processed during each GMA session. Either heparin, including low-molecular-weight heparin (LMH), or nafamostat mesilate (NM) was used as an anticoagulant during the 60 min GMA time.
Assessment of clinical efficacy and safety
The primary endpoint of interest was the clinical activity index (CAI) score. The score was defined according to the Lichtiger et al. to indicate clinical disease activity in patients with UC 14. The CAI scores were compared at baseline and after GMA therapy. We also assessed the rate of clinical remission and clinical response. The former was defined as an estimated CAI score of 4 or less, and the latter was defined as a reduction in CAI score by more than 2 points, but still CAI remaining above 4. We also assessed the adverse events related to the treatment. A serious adverse event was defined as that which caused withdrawal of the GMA therapy. We evaluated the proportion of adverse effects as a fraction of total number of GMA sessions.
Statistical analysis
The baseline characteristics of the patients were expressed as frequencies for categorical variables and median (interquartile range [IQR]) for continuous variables. We used the Wilcoxon signed-rank test to determine the significance of differences before and after GMA therapy. We conducted a total of three subgroup analyses. We described the changes in CAI score, clinical remission, and clinical response rates as well as the proportion of adverse effects among elderly patients group (aged >60 years), pregnant woman or lactating mothers group, and the group with UC refractory to steroids or anti-TNF-α antibodies. P value less than 0.05 was considered statistically significant. Statistical analyses were performed with the JMP 11 software (SAS Institute Inc., Cary, NC, USA).
RESULTS
Characteristics of the study population
The patients’ main demographic variables are presented in Table 1. The median age (IQR) was 37 years (27–46), with 14 (67%) female. There was one patient with mild, 19 patients with moderate, and one patient with severe UC according to Lichtiger et al. 14. Regarding response to conventional corticosteroids, four patients had steroid-refractory UC and eight had steroid-dependent UC. One case was complicated by pyoderma gangrenosum (PG). Among the 21 patients, there were cases with particular background 13; four elderly (aged >60 years), four lactating mothers, and one pregnant woman.
| Characteristics | Patients (N = 21) | Measurement range |
|---|---|---|
| Age (years) | 37 (27–46) | 19–72 |
| Gender (male/female) | 7/14 | |
| Pregnant | 1 | |
| Lactating | 4 | |
| Duration of UC (years) | 10 (7–10) | 0.1–26 |
| Disease location (total/left) | 17/2 | |
| Severity of UC (mild/moderate/severe) | 1/19/1 | |
| Response to prednisolone (responder/dependent/refractory) | 9/8/4 | |
| Extraintestinal complications | 1 | |
| Clinical activity index | 9 (7–9) | 5–11 |
| Stool frequency (/day) | 10 (7–10) | 1–15 |
| Bloody stool score | 2 (1–3) | 0–3 |
| Abdominal pains | 20 | |
| Anorexia | 13 | |
| Nausea | 7 | |
| Body temperature | 37.2 (35.9–38.4) | 35.9–38.4 |
| Heart rate (/min) | 87 (75–100) | 65–111 |
| Laboratory data | ||
| Hb (g/dL) | 12.7 (11.7–13.3) | 10.1–16.9 |
| WBCs (×103/μL) | 9.1 (6.7–10.7) | 3.4–15.2 |
| Neutrophils (×103/μL) | 5.9 (4.6–8.4) | 1.6–12.7 |
| Platelets (×104/μL) | 33.4 (30.4–37.2) | 23.8–63.4 |
| Albumin (g/dL) | 3.7 (3.2–3.9) | 2.4–4.7 |
| Creatinine (mg/dL) | 0.6 (0.51–0.7) | 0.42–0.85 |
| BUN (mg/dL) | 11 (8–12) | 7–18 |
| Erythrocyte sedimentation rate (mm/h) | 16 (12–20) | 4–35 |
| CRP (mg/dL) | 0.8 (0.2–4.1) | 0.03–11.4 |
| PSL (mg/kg/day) | 0.8 (0.6–1.0) | 0.4–1.2 |
| Concomitant drugs | ||
| PSL | 1 | |
| 5-ASA | 6 | |
| 5-ASA + PSL | 5 | |
| 5-ASA + TNF-α inhibitor | 1 | |
| 5-ASA + IM + PSL | 1 | |
| 5-ASA + IM + PSL enema | 1 | |
| 5-ASA + PSL enema | 1 | |
| PSL + TNF-α inhibitor | 1 | |
- 5-ASA, 5-aminosalicylate; IM, immunomodulator (thiopurine); TNFα, tumor necrosis factor-alpha. Data are presented as the median (IQR) values or as the number of patients.
GMA treatment strategy and the outcomes
At our institute, GMA is regularly being applied to treat IBD patients. Regarding the 21 patients of this study, in line with our routine practice, GMA treatment frequency was initially set for 67% of the patients to receive two GMA sessions/week, 19% to receive three sessions/week, and 14% to receive one session/week. Then, depending on the conditions of the patients, they could be treated in an outpatient setting, which led to the initial two sessions/week and three sessions/week groups decreasing to 38% and 14%, respectively, while the one session/week group increased to 48%. In total, the 21 patients received 176 GMA sessions, the median number (IQR) of GMA sessions was 10 (8–10). The median duration of GMA treatment course was 29 (21–43) days.
Anticoagulant
Selection of anticoagulant, unfractionated heparin, LMH, or NM was based on the knowledge of whether or not the patients had bloody stool, or showing heparin allergy. Up to 68% of our patients were to receive NM as anticoagulant, 21% LMH, and 11% unfractionated heparin. However, patients were allowed to switch from one anticoagulant to another and at the end of the GMA treatment course, 38% of patients received NM, 33% received LMH and 29% received unfractionated heparin.
Vascular access
GMA therapy was undertaken at our dialysis center. After receiving a treatment request from gastroenterologists, the nephrologists explained the specific treatment method to the patient. Then, nephrologists determined the site of venipuncture based on physical examination, usually the antecubital vein in one arm, while the contralateral antecubital vein was accessed for blood return to the patient from the column outflow using a 17-G needle. Basically, treatment involves the peripheral veins of both arms. When two peripheral veins were difficult to be accessed, noncuffed catheters or single needle methods were selected (13% and 7%, respectively). In single needle methods, the average blood flow rate was adjusted to 30 mL/min, and the upper limit of monitor pressure was set at 150–180 mmHg and the lower limit of monitor pressure was set at 10–30 mmHg if necessary.
The overall clinical efficacy of GMA therapy
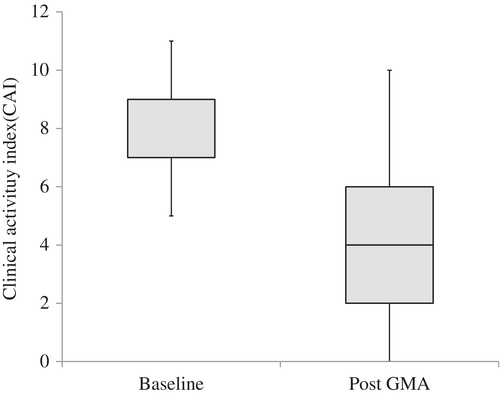
The changes in the CAI score after GMA therapy relative to baseline are shown in Figure 1. The median CAI score (IQR) decreased from 9 (7–9) at baseline to 4 (2–6) after treatment. The overall clinical remission and clinical response rates were 62% and 76%, respectively. Among a total of seven UC patients who did not achieve clinical remission, some received additional pharmacologics or a change in the type of drug they were receiving. All these patients had achieved clinical remission within almost 2 months after GMA therapy. No patients required surgery.
The overall treatment safety
In Table 2, adverse events observed during the course of GMA therapy are presented. The overall adverse events rate was 4.5% (eight of 176 GMA sessions). Adverse events included headache (N = 2), fever (N = 2), chest discomfort (N = 2), nausea (N = 1), and systemic erythema (N = 1). Headache and fever improved with oral administration of acetaminophen. Regarding chest discomfort and nausea, these disappeared by decreasing the dosage of NM or changing to LMH. Other adverse events, which were observed only during the first GMA treatment session receded spontaneously. However, none of these events led to interruption of GMA therapy, and all patients completed their scheduled GMA therapy. Further, there was no coagulation in the GMA blood lines, which may cause treatment interruption.
| Adverse events | Number of GMA sessions | ||
|---|---|---|---|
| Total (N = 176) | Elderly patients (N = 40) | Pregnant or lactating women (N = 43) | |
| Headache | 2 (1.1%) | 0 | 0 |
| Fever | 2 (1.1%) | 0 | 1 (2.3%) |
| Chest discomfort | 2 (1.1%) | 0 | 2 (4.6%) |
| Systemic erythema | 1 (0.6%) | 0 | 0 |
| Nausea | 1 (0.6%) | 0 | 0 |
Efficacy and safety of GMA therapy in the elderly UC patients
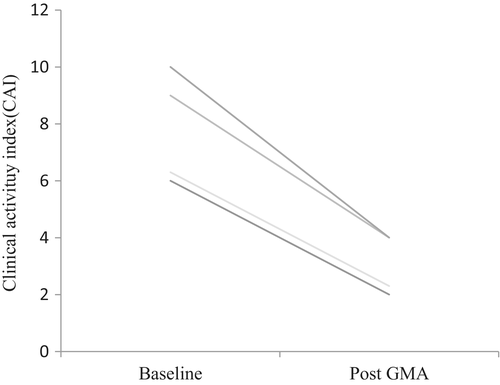
Our cohort included four elderly patients with UC, two male and two female, with the median age (IQR) of 65 years (63–67). At baseline, two patients who had steroid-refractory UC, one was treated with 5-aminosalicylate (5-ASA) alone and one with prednisolone alone. Two steroid responder patients were treated with 5-ASA alone or a combination of immunomodulator, prednisolone, and 5-ASA. The clinical remission rate in these four elderly UC patients was 100%, median CAI score (IQR) at baseline 8 (6–9) decreased to 3 (2–4) after treatment (Fig. 2). Further, no adverse event was reported, and each elderly UC patient completed all 10 GMA sessions.
Efficacy and safety of GMA therapy in pregnant or lactating mothers with UC
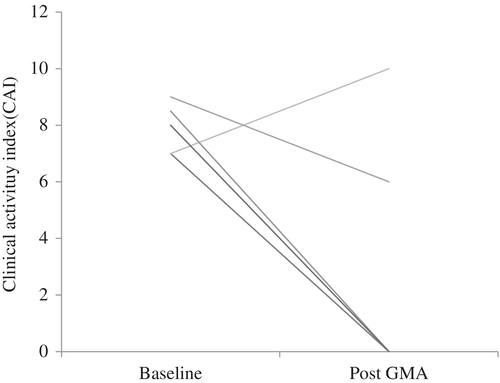
The clinical remission rate was an 80% in pregnant or lactating mothers who had a median UC disease duration (IQR) of 7 years (2–10). The median CAI score (IQR) of 8 (7–8) at baseline decreased to 0 (0–6) at post-treatment (Fig. 3). One pregnant female had steroid-dependent UC, relapsed with moderate activity, but her symptoms improved by increasing the dosage of 5-ASA in combination with GMA. This patient had bloody stool, so NM was used as an anticoagulant instead of heparin. For four lactating mothers, one case was postpartum onset and others were relapsed cases (one steroid refractory, three steroid dependent). The reported adverse events were transient fever and chest discomfort.
Efficacy and safety of GMA therapy in patients with drug refractory UC
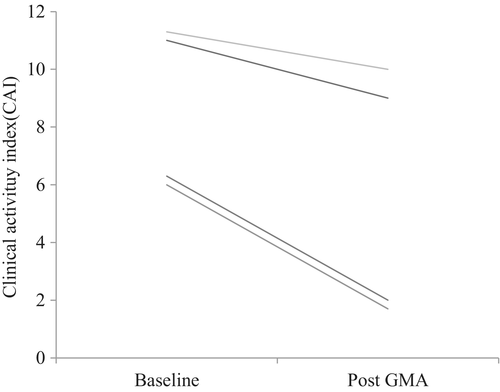
Four patients with drug refractory UC were included in our study. All four patients were refractory to steroid treatment. Both clinical remission and response rates were 50%, median CAI score (IQR) at baseline 9 (6–11) decreased to 6 (2–9) after GMA therapy (Fig. 4). There was no report of adverse events in this subgroup.
Efficacy of GMA in the UC case complicated by PG
One female patient in her fifties had been diagnosed with UC 16 years before initiating this GMA therapy. A year before coming to our hospital, her UC had relapsed with moderate activity and was complicated by PG. At one time point, her UC reached a remission level, but subsequently relapsed (CAI score 10) together with PG. Intensive GMA (two sessions per week) for her UC and steroid ointment for her PG lesions (on her right thigh) was started. Improvement of UC symptoms was observed 3 weeks after initiating the GMA therapy. Treatment of her PG was switched from steroid ointment to petrolatum. Upon completion of 10 GMA sessions, her CAI score decreased from 10 to 2 and her PG lesions also had markedly improved.
DISCUSSION
In our retrospective cohort study involving 21 patients with active UC, the CAI score after GMA had significantly decreased. The overall clinical remission rate was 62% and no severe adverse effects were observed. Although the number of elderly patients and pregnant or lactating mothers were small in the present study, the results were consistent with those in other UC patients in this study.
GMA is the treatment to deplete the extra load of myeloid lineage leukocytes present in patients with active IBD, and are thought to exacerbate and perpetuate the disease 8. However, at present, IBD, which includes UC and Crohn's diseases are lifelong chronic conditions of unknown etiology and there is no complete cure for IBD. Therefore, the aim of the GMA therapy is to suppress symptoms and sustain remission. Efforts are also needed to control or prevent treatment-related side effects. For the treatment of UC, in addition to the first-line medication with 5-ASA, there are multiple other treatment options such as corticosteroids, anti-TNF-α antibodies, and calcineurin inhibitors. In fact, the options are increasing, and the choice and the order of use of different drugs are based on the mechanisms of action of any given medication. Corticosteroids are the most common drugs used to induce remission for UC patients who do not achieve stable remission with the first-line 5-ASA. However, when corticosteroids are administered, over 20% of patients have a transition to steroid refractory or steroid dependent UC 15. Accordingly, in patients receiving GMA at our hospital, 62% were steroid refractory or steroid dependent. The patients we selected for GMA therapy did not achieve remission with conventional drugs. In fact, for UC patients, apheresis therapy is one of the recommended treatment options in the clinical practice guidelines for IBD set by the Japanese Society of Gastroenterology 16.
One of the main aspects of our study is that we suggested the efficacy and safety of GMA therapy among pregnant or lactating mothers. There are many cases of IBD onset in the young, which often overlaps with the time of pregnancy, and in some cases it is difficult to achieve a favorable pregnancy outcome. To our knowledge, there are a few case reports from Europe and Japan on GMA treatment in IBD patients during pregnancy or lactation period 17, 18. Those reports indicated the efficacy and safety of GMA, while the number of UC patients was smaller than that of our study. Given that GMA is recommended as one treatment option among pregnant and lactating mothers with pustular pregnancy in “Pustular psoriasis (generalized) clinical practice guidelines 2014 edition,” GMA treatment may have the potential to become favorable in pregnant and lactating mothers with UC 19, 20.
Recently, there is increasing evidence for GMA therapy in elderly patients with UC. Our study found that all elderly patients (aged >60 years) with moderate UC achieved clinical remission and no adverse event was reported. A retrospective study involving 96 UC patients reported that the clinical remission rate was 71% in the elderly (aged ≥65 years), which is not significantly different from that in nonelderly patients 11. A multicenter retrospective study showed that only 37% of elderly patients (aged >60 years) achieved clinical remission. However, 19% of all patients had severely active UC 10. Although clinical remission rate varied among studies, in the elderly UC patients, the course of the disease, the efficacy of therapeutic interventions, the treatment related adverse events, and the impact on the quality of life are very different from those of younger patients. Because the prevalence and severity of comorbidities is high in the elderly, many elderly patients are taking multiple drugs for such comorbidities. Moreover, the elderly patients treated with corticosteroids or TNF-α inhibitors were reported to have a high rate of serious infections and mortality 21, 22. Therefore, GMA seemed to be the treatment of choice in the elderly, especially in nonsevere cases for its high efficacy and favorable safety profile 11.
There were some case reports to suggest that GMA seemed to be effective not only for patients with IBD, but also for IBD patients complicated by PG. Since IBD is a systemic inflammatory disease, there are patients with extraintestinal manifestations complicating the IBD per se. Additionally, patients with PG sometimes have serious psychological morbidity. For example, it has been reported that GMA therapy was successful in a case with very severe Crohn's disease complicated by PG and cases with drug refractory neutrophilic skin lesions like PG 23, 24. These reports may indicate an important role for neutrophils in PG-like skin lesions 25.
Several limitations should be taken into account before a further interpretation of our findings. First, we could not evaluate long-term therapeutic efficacy of GMA therapy in the present cohort of UC patients. Second, because there was no control group, it was difficult to determine whether GMA was effective or not. Third, the sample size was small. Fourth, since the endoscopic examination could not be done before and after the GMA therapy in all patients, we could not evaluate the Mayo score. Finally, another limitation is related to external validity, in particular, the single-center design. Further studies, with larger sample sizes in multicenter settings are needed to confirm our findings.
CONCLUSION
Among active ulcerative colitis patients including pregnant or lactating mothers and elderly patients, the clinical activity index score after granulocyte/monocyte apheresis therapy had significantly decreased. The overall clinical remission rate was 62% and no severe adverse effects were observed. Our results may support GMA as an effective and safe nonpharmacologic treatment option for active UC patients, including elderly and pregnant cases.
Acknowledgments
We thank the staff at the Department of Clinical Engineering, Nephrology and Gastroenterology, Saku Central Hospital Advanced Care Center for their valued support.
Conflict of Interest
None.




