Convective Dialysis Reduces Mortality Risk: Results From a Large Observational, Population-Based Analysis
Abstracts
According to many studies, extracorporeal dialysis with convective methods is associated with better clinical outcomes and a survival benefit compared to diffusive techniques. However, there is no full agreement on the actual superiority of this kind of renal replacement therapy on hard end-points such as mortality. We performed a retrospective epidemiological cohort study to provide “real-world” evidence on the impact of convective and non-convective dialysis techniques on all-cause and cardiac mortality and biochemical outcomes among dialysis patients in Sicily, the southernmost region of Italy. Data of all incident adult patients (N = 6529) who have started chronic extracorporeal dialysis over the period 2009–2015 were retrieved from the Sicilian Registry of Nephrology, Dialysis and Transplantation. There were 1558 patients receiving convective techniques (23.86%). Overall mortality rate was 45.21% with a significant difference between convective (31.39%) and non-convective (49.55%) groups (P < 0.0001). After adjustment for potential confounders in multiple Cox regression models of increasing complexity, the mortality risk remained significantly lower for patients treated with convective methods (HR, 0.581; 95%CI, 0.525 to 0.643; P < 0.0001). Moreover, the convective group had a better blood chemistry profile, improved dialysis efficacy, and reduced mortality rate from cardiac diseases compared to the non-convective group. As a sensitivity analysis, patients were categorized according to propensity score quartiles and the hazard ratio for both all-cause and cardiac mortality was significantly lower for the convective group in each quartile. In conclusion, despite the observational and retrospective design, the results of the present study further support the use of convective therapies for the treatment of end-stage renal disease.
Chronic kidney disease (CKD) is increasing worldwide primarily due to the growing incidence of diabetes mellitus and cardiovascular diseases. It follows that the number of patients requiring renal replacement therapy (extracorporeal dialysis, peritoneal dialysis or kidney transplantation) is expected to rise progressively in the coming years 1.
Thanks to scientific advances, nephrologists have available several extracorporeal dialysis techniques for the treatment of patients with end-stage renal disease. Convective methods (acetate-free biofiltration [AFB], hemofiltration [HF], hemodiafiltration [HDF], hemodiafiltration with endogenous reinfusion [HFR]) are more effective than diffusive modalities (bicarbonate hemodialysis [HD]) in removing fluids and higher-molecular weight solutes 2. It has been suggested that this greater efficiency may be associated with a reduction in the incidence and severity of symptoms during the dialysis session and with a clinical improvement of patients with end-stage renal disease (ESRD). Observational studies have reported a lower mortality rate among patients treated with convective techniques compared to those undergoing HD. This probably occurs at least in part because convective treatments more effectively remove solutes such as beta-2-microglobulin, phosphorus, various cytokines and homocysteine 3-7.
Despite such evidence, the most common technique used is still HD (standard or with biocompatible membranes). The most likely reason is economic, given the different costs between the two types of treatment. A role in determining the widespread adoption of HD is also played by the lack of conclusive answers on hard outcomes from randomized clinical trials. The last Cochrane meta-analysis on the topic, published in 2015, has revealed that there is no difference in all-cause mortality but convective techniques appear to be associated with lower cardiovascular mortality, reduced number of intradialytic hypotension episodes and higher dialysis dose than diffusive therapies; however, the authors state that the included trials showed several biases and, consequently, the obtained results cannot be considered fully informative and conclusive 8.
The aim of the present analytical epidemiological study has been therefore to provide “real-world” evidence on the impact of convective and non-convective dialysis techniques on all-cause and cardiac mortality rate and biochemical outcomes in all Sicilian incident adult chronic dialysis patients over the period 2009–2015, by retrieving the required demographic, clinical and dialytic data from the Sicilian Registry of Nephrology, Dialysis and Transplantation (Registro Siciliano di Nefrologia, Dialisi e Trapianto, RSNDT).
PATIENTS AND METHODS
Study design and data collection
We performed a retrospective epidemiological analytical cohort study involving all incident adult patients with ESRD who have started chronic renal replacement therapy from 1 January 2009 to 31 December 2015 in Sicily, the southernmost region of Italy. From all patients initiating dialysis in the period indicated above (N = 7153), those who received continuous treatments for acute kidney injury or peritoneal dialysis and <18-year-old patients were excluded. According to these inclusion and exclusion criteria, we recruited 6529 patients (Fig. 1). All relevant latest available demographic, clinical, biochemical and dialytic data of the study cohort, summarized in Table 1, were extracted from the RSNDT in accordance with the ethical standards and in respect of privacy. Disaggregated data of all individuals enrolled were provided in tabular format and it was not possible to trace back to the identity of patients or dialysis centers. The Sicilian Registry was established in December 2008 (decree n. 03423/08 of the Sicilian Regional Department of Health) 9 to assemble, analyze and summarize data about the demographic and clinical characteristics, dialysis details, comorbidities and mortality of patients undergoing chronic renal replacement therapy as well as information regarding renal transplantation in Sicily. Data collection is based on the REGDIAL web platform (Cooperativa EDP La Traccia, Matera, Italy). The degree of compliance of data considered in the present study with those requested every 6 months to all dialysis centers by the Registry Staff is reported in Table 1. It is important to emphasize that the Sicilian Registry includes 100% of the mandatory data.

| Parameter | All patients (N = 6529) |
Patients on dialysis with convective techniques (N = 1558, 23.86%) |
Patients on dialysis with non-convective techniques (N = 4971, 76.14%) |
P-value (convective vs. non-convective techniques) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Values | Completeness of data | Values | Completeness of data | Values | Completeness of data | ||||
| Gender | 100% | 100% | 100% | ||||||
| Male, N (%) | 4037 (61.83%) | 1024 (65.72%) | 3013 (60.61%) | <0.0001 | |||||
| Female, N (%) | 2492 (38.17%) | 534 (34.28%) | 1958 (39.39%) | <0.0001 | |||||
| Age, years | 76 (65–82) | 100% | 72 (60–80) | 100% | 76 (66–83) | 100% | <0.0001 | ||
| Patients >65 years old, N (%) | 4916 (75.29%) | 100% | 1050 (67.4%) | 100% | 3866 (77.8%) | 100% | <0.0001 | ||
| Ethnicity, N (%) | 100% | 100% | 100% | ||||||
| Caucasian | 6409 (98.16%) | 1536 (98.59%) | 4873 (98.03%) | <0.0001 | |||||
| Other | 120 (1.84%) | 22 (1.41%) | 98 (1.97%) | <0.0001 | |||||
| Period of observation, mo. | 19 (6–39) | 100% | 31 (13–50) | 100% | 16 (5–33) | 100% | <0.0001 | ||
| Mortality rate, N (%) | 2952 (45.21%) | 100% | 489 (31.39%) | 100% | 2463 (49.55%) | 100% | <0.0001 | ||
| Diabetes mellitus, N (%) | 2234 (34.22%) | 100% | 569 (36.5%) | 100% | 1665 (33.5%) | 100% | 0.028 | ||
| Arterial hypertension, N (%) | 3265 (50.01%) | 100% | 959 (61.55%) | 100% | 2306 (46.39%) | 100% | <0.0001 | ||
| Cardiac diseases, N (%) | 1691 (25.90%) | 100% | 479 (30.74%) | 100% | 1212 (24.38%) | 100% | <0.0001 | ||
| BMI, kg/m2 | 24.74 (21.64–28.09) | 58.40% | 25.78 (22.62 to 29.06) | 70.03 | 24.43 (21.33 to 27.68) | 54.76% | <0.0001 | ||
| iPTH, pg/mL | 218.70 (122.00–367.45) | 34.31% | 230.00 (138.25–367.75) | 45.38% | 214.00 (116.30–367.32) | 30.84% | 0.0618 | ||
| Albumin, g/dl | 3.52 (3.10–3.90) | 40.47% | 3.70 (3.30–4.00) | 48.71% | 3.50 (3.00–3.89) | 37.88% | <0.0001 | ||
| Total cholesterol, mg/dL | 150.00 (128.00–175.00) | 22.21% | 150.00 (128.00–175.00) | 27.53% | 149.00 (128.00–174.25) | 20.53% | 0.2545 | ||
| Triglycerides, mg/dL | 119.00 (90.00–163.00) | 31.89% | 124.00 (91.00–165.00) | 38.90% | 117.00 (89.00–161.00) | 29.70% | 0.0754 | ||
| Hematocrit, % | 32.28 ± 4.56 | 45.37% | 32.92 ± 4.32 | 54.17% | 32.03 ± 4.63 | 42.61% | <0.0001 | ||
| Hemoglobin, g/dL | 10.50 (9.60–11.20) | 69.01% | 10.70 (10.00–11.40) | 78.24% | 10.40 (9.40–11.01) | 66.12% | <0.0001 | ||
| Calcium, mg/dL | 8.70 (8.20–9.30) | 56.67% | 8.80 (8.30–9.30) | 66.82% | 8.70 (8.10–9.30) | 53.49% | <0.0001 | ||
| Phosphorus, mg/dL | 4.30 (3.60–5.20) | 65.49% | 4.33 (3.65–5.10) | 75.22% | 4.30 (3.60–5.20) | 62.44% | 0.9246 | ||
| Ca × P product, mg2/dL2 | 37.80 (30.94–45.59) | 55.32% | 38.21 (31.47–45.00) | 65.21% | 37.44 (30.67–45.84) | 52.22% | 0.3296 | ||
| Potassium, mEq/L | 4.98 ± 0.82 | 45.34% | 4.97 ± 0.80) | 54.56% | 4.98 ± 0.83 | 42.45% | 0.9204 | ||
| Ferritin, μg/L | 209.60 (76.00–447.50) | 34.11% | 223.00 (78.00–453.00) | 42.23% | 203.00 (75.84–444.25) | 31.56% | 0.4112 | ||
| C-reactive protein, mg/dL | 3.44 (1.10–10.41) | 23.66% | 4.00 (1.30–11.25 | 29.85% | 3.15 (1.00–10.00) | 21.73% | 0.0520 | ||
| Alkaline phosphatase, U/L | 107.00 (77.00–174.00) | 30.12% | 109.00 (78.00–159.00) | 34.34% | 107.00 (77.00–177.00) | 28.81% | 0.6435 | ||
| Iron, μg/mL | 51.00 (35.00–71.00) | 35.90% | 53.00 (38.00–73.00) | 48.84 | 50.00 (34.08–71.00) | 31.84% | 0.0052 | ||
| Transferrin, mg/dL | 179.00 (146.50–214.50) | 31.18% | 183.00 (150.00–216.00) | 41.21% | 177.00 (144.00–213.00) | 28.04% | 0.0347 | ||
| No. dialysis sessions per week | 3 (3–3) | 98.74% | 3 (3 to 3) | 99.23% | 3 (3 to 3) | 98.59% | <0.0001 | ||
| Duration of dialysis session, min | 240 (210–240) | 67.36% | 240 (210–240) | 82.67% | 210 (180–240) | 62.56% | <0.0001 | ||
| Dialyzer surface area, m2 | 1.70 (1.50–1.80) | 39.96% | 1.80 (1.60–2.00) | 46.85% | 1.70 (1.40–1.80) | 37.80% | <0.0001 | ||
| Blood flow, mL/min | 300.00 (250.00–300.00) | 70.62% | 300.00 (300.00–320.00) | 86.91% | 300.00 (250.00–300.00) | 65.52% | <0.0001 | ||
| Ultrafiltration, L | 2.00 (1.50–3.00) | 65.80% | 2.50 (1.50–3.00) | 78.75% | 2.00 (1.20–3.00) | 61.74% | <0.0001 | ||
| Vascular access, N (%) | 68.22% | 82.93% | 63.61% | ||||||
| Permanent or temporary CVC | 1546 (34.71%) | 328 (25.39%) | 1218 (38.52%) | <0.0001 | |||||
| AVF or AVG | 2908 (65.29%) | 964 (74.61%) | 1944 (61.48%) | <0.0001 | |||||
| Kt/V | 1.37 (1.20–1.58) | 61.04% | 1.45 (1.26–1.68) | 72.46% |
1.34 (1.19–1.54) | 57.45% | <0.0001 | ||
| Kt/V, N (%) | 61.04% | 72.46% | 57.45% | ||||||
| <1.2 | 913 (22.91%) | 196 (17.36%) | 717 (25.10%) | <0.0001 | |||||
| ≥1.2 | 3072 (77.09%) | 933 (82.64%) | 2139 (74.90%) | <0.0001 | |||||
| URR, % | 69.64 (65.04–75.00) | 40.74% |
71.30 (66.38–77.14) | 53.91% | 69.02 (64.66–74.08) | 36.61% | <0.0001 | ||
| URR, N (%) | 40.74% | 53.91% | 36.61% | ||||||
| <65% | 648 (24.36%) | 163 (19.40%) | 485 (26.65%) | <0.0001 | |||||
| ≥65% | 2012 (75.64%) | 677 (80.60%) | 1335 (73.35%) | <0.0001 | |||||
| Mean pre-D systolic BP, mmHg | 135.00 (126.25–144.29) | 72.11% | 136.56 (126.77–145.85) | 83.70% | 134.50 (126.00 to 143.43) | 68.48% | 0.0005 | ||
| Mean pre-D diastolic BP, mmHg | 70.00 (64.62–76.77) | 72.11% | 70.45 (64.99–77.20) | 83.70% | 70.00 (64.49 to 76.6) | 68.48% | 0.2188 | ||
| Mean post-D systolic BP, mmHg | 130.00 (121.13–140.00) | 61.51% | 130.00 (121.26–140.00) | 75.42% | 130.00 (121.00–139.68) | 57.15% | 0.2961 | ||
| Mean post-D diastolic BP, mmHg | 69.29 (62.71–74.69) | 61.51% | 69.00 (62.61–75.00) | 75.42% | 69.35 (62.75–74.15) | 57.15% | 0.5301 | ||
- Data are expressed as mean ± standard deviation, median (interquartile range) or percentage as appropriate.
- AVF, native arteriovenous fistula; AVG prosthetic arteriovenous graft; BMI, body mass index; BP, blood pressure; Ca × P, calcium × phosphorus product; Cr, creatinine; CVC, central venous catheter; iPTH, intact parathyroid hormone; post-D, post-dialysis; pre-D, pre-dialysis; URR, urea reduction rate.
Distribution of patients according to the dialysis techniques, in turn divided into convective and non-convective modalities, is reported in Table 2. Patients were defined as part of one group or the other based on the last treatment indicated. The number of patients who changed dialysis technique was small enough to be ignored.
| Convective treatments (N = 1558, 23.86%) | Non-convective treatments (N = 4971, 76.14%) | ||
|---|---|---|---|
| Technique | N (%) of patients | Technique | N (%) of patients |
| Online-HDF | 1018 (65.34%) | Bicarbonate HD with synthetic low-flux and Kuf < 40 membranes | 1962 (39.47%) |
| AFB | 336 (21.57%) | Bicarbonate HD with synthetic high-flux and Kuf > 40 membranes | 1761 (35.43%) |
| HF | 94 (6.03%) | Bicarbonate HD with biocompatible membranes | 870 (17.50%) |
| HDF | 89 (5.71%) | Other/unknown HD | 157 (3.16%) |
| Online-HFR | 21 (1.35%) | Standard bicarbonate HD | 129 (2.59%) |
| Bicarbonate HD | 89 (1.79%) | ||
| Home bicarbonate HD | 3 (0.06%) | ||
- AFB, acetate-free biofiltration; HD, hemodialysis; HDF, hemodiafiltration; HF, hemofiltration; HFR, hemodiafiltration with on-line endogenous reinfusion; Kuf, ultrafiltration coefficient.
The period of observation was calculated for each patient between initiation of renal replacement therapy and last observation, which corresponded to the end of the study (31 December 2015) or time of death, renal transplantation, functional recovery or other exit from dialysis therapy. Patients who exited from dialysis therapy were kept under observation and included in the performed statistical analyses.
Causes of death suggested by the Registry Platform follow the 1995 ERA-EDTA codification 10. Nephrologists must define the cause of death as uncertain/not determined if this event does not occur at the dialysis center. For the purposes of the present study, the recorded causes of mortality were divided into cardiac (myocardial ischemia or infarction, cardiac arrest/sudden death, hyperkalemia, hypertensive heart failure, other causes of heart failure, cardiac arrest due to undetermined causes) and non-cardiac (vascular diseases, malignancies, infections, gastrointestinal disorders, cachexia, social causes, other) (Table 3).
| Causes of death | No. (%) patients |
|---|---|
| Cardiac diseases | 1042 (35.30%) |
| Non-cardiac diseases | 1092 (36.99%) |
| Vascular diseases | 243 (8.23%) |
| Malignancies | 257 (8.71%) |
| Infections | 92 (3.12%) |
| Gastrointestinal disorders | 51 (1.73%) |
| Cachexia | 298 (10.09%) |
| Social causes | 6 (0.20%) |
| Other | 145 (4.91%) |
| Uncertain/not determined | 818 (27.71%) |
- Overall mortality rate was 45.21% (N = 2952) among the study population.
Study end-points
The primary end-point of our study was to evaluate any difference in all-cause mortality in Sicilian hemodialysis patients by comparing convective and non-convective techniques.
The secondary end-point was to assess potential differences in biochemical variables, dialysis parameters and cardiac mortality rate between the two groups.
Statistical analysis
Normally distributed continuous variables were expressed as mean ± standard deviation (SD), non-normal variables as median and interquartile range (IQR) and categorical data as percentage frequency. Data distribution was evaluated by the D'Agostino-Pearson test for normal distribution. To test differences between groups, Student's unpaired t-test was used for normally distributed values, Mann–Whitney test for non-normal variables and χ2 test for categorical data. Pearson's correlation coefficient was calculated to evaluate correlations between normally distributed variables. Rank correlation and Spearman's rho coefficient were used to assess correlations between non-normal variables. The incidence rate for each cause of death in the two groups (convective and non-convective) and the incidence rate ratio (IRR) were calculated to assess the effects of dialysis technique on the considered causes of death.
Survival analyses were performed by the Kaplan–Meier method with log-rank test for comparison of survival curves. In order to analyze the effect of different risk factors for all-cause and cardiac mortality, correlation analyses were carried out to search for potential confounders (Tables S1 and S2) in accordance with the definition of “confounder” proposed by Jager et al. (variable associated with both exposure and outcome, which is not an effect of the exposure and is not part of the pathogenetic pathway between exposure and outcome) 11; variables whose data were available for less than 30% of patients were not tested as possible confounding factors. Missing values were replaced by multiple imputation method. Univariate Cox regression models followed by multiple regression analyses based on models of increasing complexity (i.e. including all significant univariate correlates as covariates) were used to adjust the relationships between dialysis technique and all-cause and cardiac mortality. To further control for confounding by indication, a sensitivity analysis was performed by calculating the propensity score (PS) through logistic regression. The propensity score was calculated by using the following risk factors: age, gender, ethnicity, arterial hypertension, diabetes mellitus, and cardiac diseases. Then, patients were stratified into PS quartiles in order to calculate hazard ratios for all-cause and cardiac mortality in each quartile; the obtained results were reported in forest plot graphs. In each quartile, patients are deemed to be similar for factors used to derive the PS thus accounting for confounding by indication. P-values <0.05 were considered statistically significant for all analyses. Statistical analysis was performed using MedCalc (version 12.7.0.0; MedCalc Software bvba, Belgium), R (version 3.3.2, The R Foundation for Statistical Computing) and SPSS (version 22.0.0.0; IBM Corporation, Armonk, NY, USA) software.
RESULTS
Patients’ baseline characteristics
Baseline characteristics of the study cohort are described in Table 1. The recruited 6529 dialysis patients were 76 (IQR 65–82) years old; 75.29% of them (N = 4916) were over 65 years old. More than half of patients were male (61.83%). Diabetes mellitus was present in 2234 subjects (34.22%), arterial hypertension in 3265 (50.01%), and cardiac diseases in 1691 (25.90%). The period of observation was 19 (IQR 6–39) months.
There were 1558 patients on dialysis with convective techniques (23.86%), whereas there were 4971 patients receiving non-convective treatments (76.14%). Convective and non-convective groups were different for some variables including gender (male 65.72% vs. 60.61%; P < 0.0001), age (72 [60–80] vs. 76 [66–83]; P < 0.0001), percentage of >65-year-old patients (67.4% vs. 77.8%; P < 0.0001), percentage of patients with diabetes mellitus (36.5% vs. 33.5%; P = 0.028), arterial hypertension (61.55% vs. 46.39%; P < 0.0001) and cardiac diseases (30.74% vs. 24.38%; P < 0.0001). In regard to vascular access for dialysis, the percentage of patients with permanent or temporary central venous catheters (CVCs) was 25.39% in the convective group and 38.52% in the non-convective group (P < 0.0001), whereas the percentage of patients with native arteriovenous fistula (AVF) or prosthetic arteriovenous graft (AVG) was 74.61% in the convective group and 61.48% in the non-convective group (P < 0.0001).
Kaplan–Meier method, Cox regression and propensity score analyses for all-cause mortality
Overall mortality rate was 45.21% (N = 2952) with a statistically significant difference between convective (N = 489 [31.39%]) and non-convective (N = 2463 [49.55%]) groups (P < 0.0001). By employing the Kaplan–Meier method with log-rank test, the significant difference in cumulative survival probability found between the two groups (P < 0.0001) (Fig. 2) was confirmed also stratifying patients according to presence or absence of diabetes mellitus, age over or under 65 years and all possible combinations of these two variables (P < 0.0001 in all survival analyses performed).
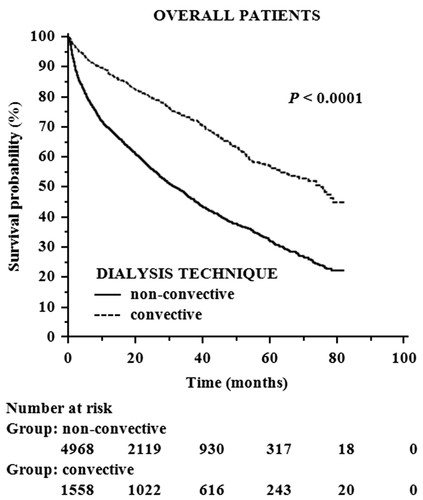
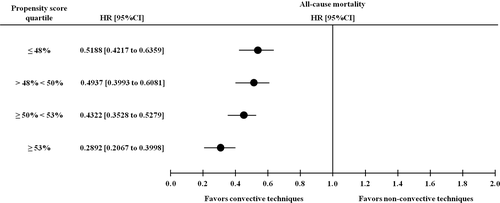
At univariate Cox regression analysis, convective treatments were associated with lower risk of all-cause mortality (hazard ratio [HR], 0.441; 95% confidence interval [CI], 0.400 to 0.486; P < 0.0001). Of note, the mortality risk remained significantly lower for patients treated with convective methods compared to the non-convective group in Cox models of increasing complexity adjusting for demographic data (Table 4 - model 1: HR, 0.552; 95% CI, 0.499 to 0.609; P < 0.0001), blood test results and comorbidities (Table 4 - model 2: HR, 0.579; 95% CI, 0.522 to 0.641; P < 0.0001) and dialysis-related factors (Table 4 - model 3: HR, 0.581; 95% CI, 0.525 to 0.643; P < 0.0001). The risk was lower also for patients with higher levels of albumin, hematocrit or potassium, AVF or AVG as vascular access, history of arterial hypertension, greater duration of dialysis session, and lower age (Table 4). By stratifying patients into PS quartiles, the hazard ratio was significantly and consistently lower in the convective group throughout all strata (Fig. 3) confirming that the protective effect of convective treatments is independent of potential confounders.
| Variable | Crude HR (95% CI) | P-value | Model 1 HR (95% CI) | P-value | Model 2 HR (95% CI) | P-value | Model 3 HR (95% CI) | P-value |
|---|---|---|---|---|---|---|---|---|
| Convective (0 = no; 1 = yes) | 0.441 (0.400 to 0.486) | <0.0001 | 0.552 (0.499 to 0.609) | <0.0001 | 0.579 (0.522 to 0.641) |
<0.0001 | 0.581 (0.525 to 0.643) | <0.0001 |
| Age, years | 1.030 (1.026 to 1.033) | <0.0001 | 1.028 (1.024 to 1.031) | <0.0001 | 1.026 (1.023 to 1.030) | <0.0001 | ||
| Gender (0 = M; 1 = F) | 1.051 (0.976 to 1.132) | 0.185 | 1.032 (0.955 to 1.115) | 0.424 | 1.026 (0.951 to 1.108) | 0.506 | ||
| BMI, kg/m2 | 0.991 (0.981 to 1.002) | 0.099 | 1.000 (0.988 to 1.012) | 0.949 | 1.001 (0.988 to 1.014) | 0.897 | ||
| Vascular access (0 = CVC; 1 = AVF or AVG) | 0.736 (0.715 to 0.757) | <0.0001 | 0.800 (0.775 to 0.826) |
<0.0001 | 0.812 (0.786 to 0.839) | <0.0001 | ||
| Albumin, g/dL | 0.732 (0.666 to 0.805) | <0.0001 | 0.779 (0.699 to 0.868) | <0.0001 | ||||
| Ca × P, mg2/dL2 | 1.003 (0.999 to 1.007) | 0.136 | 1.002 (0.999 to 1.006) | 0.211 | ||||
| Hematocrit, % | 0.972 (0.961 to 0.983) | <0.0001 | 0.973 (0.963 to 0.984) | <0.0001 | ||||
| Ferritin, μg/L | 1.000 (1.000 to 1.000) | 0.800 | 1.000 (1.000 to 1.000) | 0.811 | ||||
| Arterial hypertension (0 = no; 1 = yes) | 0.625 (0.576 to 0.678) | <0.0001 | 0.651 (0.598 to 0.708) | <0.0001 | ||||
| Potassium, mEq/L | 0.896 (0.854 to 0.939) | <0.0001 | 0.910 (0.868 to 0.954) | <0.0001 | ||||
| iPTH, pg/mL | 1.000 (1.000 to 1.000) | 0.376 | 1.000 (1.000 to 1.000) | 0.331 | ||||
| Duration of dialysis session, m’ | 0.996 (0.995 to 0.997) | <0.0001 | ||||||
| Mean pre-D systolic BP, mmHg | 0.998 (0.995 to 1.001) | 0.187 |
- Model 1: dialysis technique + demographic data; Model 2: Model 1 + blood test results and comorbidities; Model 3: Model 2 + dialysis-related factors.
- AVF, native arteriovenous fistula; AVG, prosthetic arteriovenous graft; BMI, body mass index; BP, blood pressure; Ca × P, calcium × phosphorus product; CI, confidence interval; CVC, central venous catheter; HR, hazard ratio; iPTH, intact parathyroid hormone; pre-D, pre-dialysis.
Cox regression and propensity score analyses for cardiac mortality
Convective dialysis was associated with reduced mortality rate due to all considered causes: cardiac diseases (IRR, 0.525; 95%CI, 0.4492 to 0.6113; P < 0.0001), vascular diseases (IRR, 0.5222; 95%CI, 0.3736 to 0.7176; P < 0.0001), malignancies (IRR, 0.3699; 95%CI, 0.256 to 0.5219; P < 0.0001), infections (IRR, 0.4353; 95%CI, 0.2369 to 0.7531; P = 0.0019), gastrointestinal disorders (IRR, 0.3847; 95%CI, 0.1562 to 0.8277; P = 0.01), cachexia (IRR, 0.3392; 95%CI, 0.2387 to 0.4715; P < 0.0001), social causes (IRR, 0); other (IRR, 0.293; 95%CI, 0.1683 to 0.4821; P < 0.0001), uncertain/not determined (IRR, 0.3078; 95%CI, 0.2486 to 0.3779; P < 0.0001).
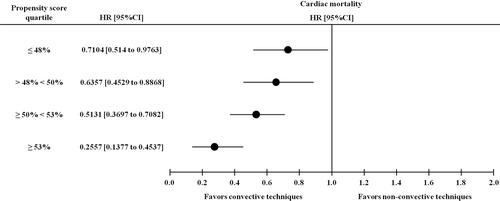
Focusing on cardiac mortality, Cox regression models are reported in Table 5. The risk for cardiac mortality resulted as lower in patients receiving convective therapies when compared with those treated with non-convective methods at the crude Cox analysis (HR, 0.694; 95% CI, 0.561 to 0.858; P = 0.001). In multiple Cox models of increasing complexity, convective treatment remained as an independent protective factor after adjustment for BMI (Table 5 - model 1: HR, 0.687; 95% CI, 0.554 to 0.850; P = 0.001) and blood test results and comorbidities (Table 5 - model 2: HR, 0.690; 95% CI, 0.556 to 0.856; P = 0.001). Also for cardiac mortality, stratification by PS quartiles revealed that the hazard ratios were significantly lower in patients receiving convective therapies in all PS strata (Fig. 4).
| Variable | Crude HR (95% CI) | P-value | Model 1 HR (95% CI) | P-value | Model 2 HR (95% CI) | P-value |
|---|---|---|---|---|---|---|
| Convective (0 = no; 1 = yes) | 0.694 (0.561 to 0.858) | 0.001 | 0.687 (0.554 to 0.850) | 0.001 | 0.690 (0.556 to 0.856) | 0.001 |
| BMI, kg/m2 | 1.028 (1.010 to 1.045) | 0.002 | 1.027 (1.008 to 1.046) | 0.006 | ||
| Albumin, g/dL | 0.945 (0.793 to 1.127) | 0.528 | ||||
| Serum calcium, mg/dL | 0.973 (0.884 to 1.071) | 0.579 | ||||
| Cardiac diseases (0 = no; 1 = yes) | 1.038 (0.861 to 1.251) | 0.696 | ||||
| Diabetes mellitus (0 = no; 1 = yes) | 0.977 (0.810 to 1.179) | 0.808 | ||||
| Hematocrit, % | 0.988 (0.966 to 1.010) | 0.269 | ||||
| Potassium, mEq/L | 0.959 (0.829 to 1.109) | 0.560 | ||||
| iPTH, pg/mL | 1.000 (1.000 to 1.001) | 0.077 |
- Model 1: dialysis technique + demographic data (only BMI resulted to be significantly correlated with cardiac mortality among baseline and demographic characteristics); Model 2: Model 1 + blood test results and comorbidities
- BMI, body mass index; CI, confidence interval; HR, hazard ratio; iPTH, intact parathyroid hormone.
Differences in biochemical variables
Convective and non-convective groups significantly differed for some biochemical parameters. In particular, patients receiving convective treatments had higher values of albumin (3.70 [3.30–4.00] vs. 3.50 [3.00–3.89] g/dL; P < 0.0001), hematocrit (32.92 ± 4.32 vs. 32.03 ± 4.63%; P < 0.0001), hemoglobin (10.70 [10.00–11.40] vs. 10.40 [9.40–11.01] g/dL; P < 0.0001), calcium (8.80 [8.30–9.30] vs. 8.70 [8.10–9.30] mg/dL; P < 0.0001), iron (53.00 [38.00–73.00] vs. 50.00 [34.08–71.00] μg/mL; P = 0.0052), and transferrin (183.00 [150.00–216.00] vs. 177.00 [144.00–213.00] mg/dL; P = 0.0347) than patients undergoing non-convective therapies.
Differences in dialysis parameters
Patients belonging to the convective group were characterized by greater duration of dialysis sessions (240.00 [210.00–240.00] vs. 210.00 [180.00–240.00] m’; P < 0.0001), dialyzer surface area (1.80 [1.60–2.00] vs. 1.70 [1.40–1.80] m2; P < 0.0001), blood flow (300.00 [300.00–320.00] vs. 300.00 [250.00–300.00] mL/min; P < 0.0001) and ultrafiltration (2.50 [1.50–3.00] vs. 2.00 [1.20–3.00] l; P < 0.0001) compared to the non-convective group. Moreover, convective techniques were associated with higher Kt/V (1.45 [1.26–1.68] vs. 1.34 [1.19–1.54]; P < 0.0001) and urea reduction rate (URR) values (71.30 [66.38–77.14] vs. 69.02 [64.66–74.08] %; P < 0.0001) than non-convective therapies. In particular, the percentage of patients with Kt/V values ≥1.2 was 82.64% in the convective group and 74.90% in the non-convective group (P < 0.0001), whereas the percentage of patients achieving URR values ≥65% was 80.60% in the convective group and 73.35% in the non-convective group (P < 0.0001).
DISCUSSION
In the present retrospective epidemiological cohort study, we observed that the enrolled Sicilian ESRD patients receiving chronic renal replacement therapy with convective techniques showed a lower overall and cardiac mortality rate than patients treated with non-convective methods. After adjustment for potential confounders in multiple Cox models of increasing complexity, dialysis technique (convective versus non-convective) remained significantly associated with all-cause and cardiac mortality and this was also true in the propensity score analysis (Table 4). Moreover, the convective group had a better blood chemistry profile as regards albumin, hematocrit, hemoglobin, calcium, iron and transferrin values, and an improved dialysis efficacy in terms of Kt/V and URR (Table 1).
The key physical principles underlying extracorporeal renal replacement therapy are ultrafiltration, diffusion and convection. Ultrafiltration allows fluid removal through a semipermeable membrane by the creation of a different hydrostatic pressure between blood and dialysate compartments. Diffusion and convection are responsible for solute movement across the dialysis membrane. The former consists of the movement of solutes driven by a concentration gradient. In the latter, molecules are removed because they are dragged by the fluid movement; water and electrolytes that need to be replaced are then added to the blood before (pre-dilution) or after (post-dilution) the filter. Thanks to this process, uremic toxins with higher molecular weight can be effectively cleared. Some peptides and proteins can also be eliminated by adsorption on the dialysis membrane 12.
Conventional HD, which is based on diffusive solute transport, adequately removes fluids and small molecules such as creatinine, urea or phosphate, and it is able to correct acid-base and electrolyte imbalance. Middle uremic toxins (molecular weight ranging from 500 Da to 60 kDa) 13 and protein-bound molecules are not effectively cleared by diffusive techniques and tend to accumulate with potential worsening of clinical outcomes 14. Conversely, the combination of diffusion and convection (HDF) enhances blood purification by removing both small and middle-sized substances 15.
The greater survival we found in the cohort of patients receiving convective therapies can be explained by the ability of convective transport to remove uremic toxins more effectively than mere diffusion, as revealed by several studies. For example beta-2-microglobulin, considered as a risk factor for cardiovascular events and all-cause mortality in CKD patients 16-18, is removed by online-HDF to a greater extent compared to high-flux HD 19, 20 and pre-dialysis serum levels of this molecule as well as those of C-reactive protein seem to decrease with increase of convection volumes 21. High-volume online-HDF is also able to reduce both free and total serum concentrations of the uremic toxins indoxyl-sulfate and p-cresyl-sulfate better than HD 22. This may be relevant because these protein-bound molecules seem to be associated with high risk of morbidity and mortality 23-25.
Acetate-free biofiltration, a low-volume diffusive-convective dialysis technique, increases the clearance of middle molecules such as FGF23 26 or sclerostin 27, which are emerging markers of bone and vascular disease in CKD 28, 29, and seems to be associated with reduced synthesis or release of pro-inflammatory and pro-apoptotic factors by vascular cells 30 and with no activation of polymorphonuclear neutrophils and monocytes 31 compared to HD. AFB also improves control of pre-dialysis mean arterial blood pressure, lowers the risk of intradialytic hypotension 32 and reduces left ventricular mass index 33. Another example is the ability of HFR, which combines convection, diffusion and adsorption, to allow a greater decrease in the serum levels of hepcidin-25, a hormone regulating iron metabolism and linked with cardiovascular events 34, compared to low-flux and high-flux HD, probably through increased removal and decreased inflammation-induced production of this molecule 35.
The relationship between convective methods and reduced levels of systemic inflammation could account for the better biochemical profile we detected in patients receiving this kind of replacement therapy, primarily in regard to biomarkers of anemia and iron metabolism.
Based on the data from the literature shown above, it should be granted that the improved removal of uremic toxins with convective treatments is associated with lower morbidity and mortality. Nevertheless, various randomized controlled clinical trials performed up to now failed to prove unequivocally the superiority of this type of extracorporeal dialysis on merely diffusive techniques regarding hard end-points such as mortality.
A Cochrane meta-analysis published in 2006 (20 trials, 657 participants) and aimed at evaluating the differences in mortality and clinical outcomes between convective methods and HD had already highlighted the scarcity of evidence in this topic due to quality concerns and low power of included trials 36.
In later years, other clinical studies involving ESRD patients treated with different dialysis techniques were carried out. The Convective Transport Study (CONTRAST) 37 evaluated all-cause mortality and a composite end-point of fatal and nonfatal cardiovascular events in 714 patients randomly assigned to receive online-HDF or low-flux HD for a mean follow-up of 3 years. No differences were found between the two groups, but a subgroup analysis suggested lower all-cause mortality among patients treated with high-volume HDF. The Turkish OL-HDF Study enrolled 782 patients to compare post-dilution online-HDF and high-flux HD with a mean follow-up of 22.7 ± 10.9 months 38. The authors did not find any statistically significant difference for the composite outcome of all-cause death and nonfatal cardiovascular events as well as for cardiovascular and overall mortality, hospitalization rate and occurrence of intradialytic hypotension. However, a post hoc analysis revealed that patients receiving high-volume (>17.4 L) online-HDF had a lower risk for overall and cardiovascular mortality than those treated with high-flux HD. Limitations of the study included the inadequate statistical power and the lower age and the better health status of participants compared to the current European dialysis patients. The survival benefit that seemed to be associated with high-volume online-HDF was confirmed by the On-Line Hemodiafiltration Survival Study (or Estudio de Supervivencia de Hemodiafiltración OnLine [ESHOL]) 39. In this trial, 906 HD patients were randomly assigned to continue HD or to move to high-efficiency post-dilution online-HDF, with a mean follow-up of 1.91 ± 1.10 years. Patients receiving online-HDF showed a lower risk of all-cause, cardiovascular and infection-related mortality compared to the HD group.
The most recent Cochrane meta-analysis 8, performed in 2015 as an update of the one published in 2006 36 and including 40 randomized controlled trials (3483 patients), demonstrated that convective techniques may decrease cardiovascular but not all-cause mortality and have unclear effects on other outcomes such as nonfatal cardiovascular events and hospitalization. Nevertheless, a high risk of bias was identified in many of the included studies.
Once again, the question on whether convective treatments are clinically better than diffusive therapies remains unresolved.
With respect to the causes of death, we decided to investigate the relationship between dialysis technique and cardiac mortality. Convective treatments were associated with a lower risk for death due to cardiac diseases in the univariate analysis. This finding remained statistically significant even adjusting for potential confounders in Cox models of increasing complexity (Table 5). Such results confirm the reduced mortality rate from cardiac events already reported among patients receiving convection-based therapies compared to those treated with non-convective methods. 8
The strengths of the present work are: the large sample size, the geographical homogeneity of the population, the completeness of mandatory data, the possibility to adjust for several risk factors in Cox multiple regression models, and the implementation of the propensity score analysis as a sensitivity analysis. Moreover, though it was a retrospective research, this observational study provides “real-world” evidence unlike randomized control trials. This study has also some limitations. Firstly, the observational design does not allow drawing final conclusions on the better survival probability we found to be associated with convective therapies when compared with non-convective ones. Secondly, the cause of death was reported as unknown for a high number of patients and this has impaired the ability to accurately analyze the differences in mortality causes between the two study groups. Lastly, it is possible that the lower all-cause mortality we found among patients receiving convective dialysis could partly depend on high convection volumes but data on convective volumes were not available.
CONCLUSIONS
The improvement in dialysis adequacy, clinical outcomes and survival among end-stage renal disease patients treated with convective techniques is supported by much, although not conclusive, scientific evidence. Despite the limitations typical of a retrospective observational study, our findings can further support the use of renal replacement therapies based on convection with a good degree of confidence, given the high number of patients recruited.
If future large and well-designed randomized controlled clinical trials overcoming the limitations of the already performed studies will confirm these results, public health policies should implement and extend the use of convective treatments to all categories of uremic patients, as suggested by our stratified survival analyses. This also would entail a modulation of prices, because the main reason for the still wide use of HD is precisely the lower cost of this therapy if compared with convective treatments.
Acknowledgments
Data have been kindly provided by the Staff of the Sicilian Registry of Nephrology, Dialysis and Transplantation (Registro Siciliano di Nefrologia, Dialisi e Trapianto, RSNDT): B. Piazza, V. Agnello, P. Di Gaetano, R. Alberti.
RSNDT workgroup members: A. Alagna, R. Aliffi, C. Altieri, M. Arnone, V. Barraco, F. Barresi, C. Bartoli, G.G. Battaglia, E. Battiati, A. Bauro, L. Bellissimo, R. Bevelacqua, M. Buemi, G. Buscaino f.f, F. Caputo, S. Caruselli, C. Cassetti, P. Castellino, S. Castellino, A. Caviglia, S. Cesare, S. Chiarenza, A. Ciancio, M.A. Cinardo, G. Ciurcina, S. Costa, L.A. Cottone, I. Cutaja, C. D'Amico, A.V. D'Anca, G. Daidone, A. De Gregorio, D. Di Benedetto, M.R. Di Francesca, P. Di Gregorio, V. Di Marca f.f., A. Di Martino, A. Di Mauro, E. Di Natale, A. Failla, E. Farinella, C. Fede, R. Fichera, M. Fici, C. Gerbino, M. Giandalia, A. Granata, F. Grippaldi, A. Gurrieri, A. Iacono, E. Iannetti, C.M. Incardona, R. La Barbera, F. La Bella, A. La Corte, S.A.A. La Rosa, G. Latassa, M. Li Vecchi, A. Liardo, A. Lo Cascio, C. Lo Dico, F. Lo Faro, M.A. Lo Piano, G. Locascio, N. Longo, G. Lupini, M. Mancusi, S. Maringhini, M.T. Masuzzo, F. Messina, P. Monardo, G. Montalto, F. Mucaria, A. Murgo, S. Musso, A. Nardo, G. Nicolosi, A. Ocello, G. Oddo, R. Parsi, A. Pisacane, A. Pitti, F. Purrello, C. Quari, L. Racco, D. Rallo, F. Randazzo, A. Re, A. Reina, B. Ricciardi, G. Rizzari, M. Roccaro, M. Romè, U. Rotolo, G. Sallemi, G. Sciacca, R. Scurria, G. Seminara f.f., C. Sessa, C. Todaro, F. Tornese, D. Trimboli, O. Trovato, G. Tumino, P. Veroux, S. Vinciguerra, S. Vittoria f.f, L.M. Zanoli, A.M. Zoccolo.
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Financial Support
The study was performed thanks to a grant of the Italian Society of Nephrology (Società Italiana di Nefrologia, SIN).




