Compositional Analysis of Coronary Artery Calcification in Dialysis Patients in vivo by Dual-Energy Computed Tomography Angiography
Abstract
While vascular calcification is an important factor regulating prognosis in dialysis patients, its components have not been adequately studied. We analyzed in vivo components of calcification in the coronary arteries of dialysis patients using the effective atomic number from dual-energy computed tomography. In dialysis patients (hemodialysis, N = 10; peritoneal dialysis, N = 12), average of median effective atomic number was 13.8 in the hemodialysis group, and 13.7 in the peritoneal dialysis group. No significant differences were seen between groups, with calcium oxalate monohydrate identified as the most common component in each. To confirm the accuracy of this method, we investigated the composition of surgically removed calcified tissues using already established methods. Comparison with the effective atomic number from dual-energy computed tomography showed that the results of calcification analysis were the same. We concluded that calcium oxalate monohydrate might be one of the major components of coronary artery calcification in dialysis patients.
Dialysis patients show accelerated calcification of both the lamina intima and lamina media of blood vessels. Dysregulation of calcium and phosphate homeostasis promotes vascular calcification 1. Understanding the mechanisms and components of calcification is crucial for designing novel therapeutic approaches to treat and prevent calcification-associated vascular disease. Previous ex vivo analyses have reported calcium carbonate (CC), calcium oxalate monohydrate (COM), dicalcium phosphate dehydrate (DCPD), octacalcium phosphate (OCP), and hydroxyapatite (HA) as components of calcified vascular tissues 2, 3. An in vivo study using a single-source dual-energy computed tomography (DECT) technique in non-dialysis patients 4 suggested that coronary artery calcifications (CACs) might consist of COM. However, little information has been reported regarding the components of calcified vascular tissue in those undergoing dialysis. In dialysis patients, various ex vivo studies 5, 6 have investigated components of arterial calcifications, suggesting HA as a major component. Cardiovascular disorders are a major cause of death in dialysis patients, and calcification of the coronary arteries is important. The objective of our in vivo study was therefore to determine which components contribute to CAC in dialysis patients using single-source DECT.
MATERIALS AND METHODS
Study 1: Analysis of coronary artery calcification in dialysis patients
Dual-energy computed tomography
In vivo analysis of calcified coronary arteries in chronic dialysis patients was performed using dual-energy computed tomography (DECT) coronary angiography. Patients underwent electrocardiogram-triggered single-source DECT. This modality provides numerous high and low kilovolt-peak projections in a single rotation with fast kilovolt-peak switching. Scanning by DECT enables analysis of the effective atomic number (EAN) of materials in vivo. EANs can be calculated using the following equation when the chemical formula of a compound or mixture of materials is known (http://www.ndt.net/article/ ndtce03/ papers /p037/p037.htm):
Theoretical EAN = √ ∑ f i z i (m = 2.94: by Mayneord method, f i: number of electrons in each element, z i: atomic number of each element).
We used this EAN method to evaluate the components of calcified coronary arteries in vivo in our study.
Details of the CT scanning technique were as follows. First, 90 min before examination, patients with baseline heart rate > 60 beats/min were orally administered a beta-blocker (Seloken; Astra Zeneca, Tokyo, Japan, dose: 1 mg/kg of body weight) to lower the heart rate. Second, patients received 0.3 mg of nitroglycerin (Nitropen; Nippon Kayaku, Tokyo, Japan) just before localization-image acquisition in each coronary CT angiography examination. Third, contrast medium (Iopamiron; Bayer HealthCare, Osaka, Japan) was injected using an automatic double-head power injector (DualShot GXV; Nemoto Kyorindo, Tokyo, Japan), as contrast medium at 25.0 mg I/kg per s and saline for 14 s, following test injection (contrast medium 10 mL and saline 15 mL). Fourth, CT was performed using a Discovery CT750 HD system (GE Healthcare, Chicago, IL, USA) at a tube voltage of 80/140 kVp, tube current of 600 mA, and rotation time of 0.35 ms.
In image processing, two experienced observers of coronary CT angiography processed the collected data using Advantage Workstation version 4.5 and GSI Viewer (GE Healthcare). The detailed image-processing technique was as follows. First, we reconstructed axial images at 65 keV with slice thickness of 0.625 mm in a field of view of 5 × 5 cm2 in the best cardiac phase during the diastolic phase. Second, we placed a region of interest (ROI) with a circular area measuring 0.5 mm in diameter centered around each CAC, and margins of ≥10 mm from other ROIs (Fig. 1). In this process, observers carefully excluded insufficient ROIs that were not suitable for analysis due to partial volume effects or non-calcified structures on contiguous slices used for CAC component analysis by EAN. Third, ROIs continued to be placed in this manner. Finally, the last ROI was placed with a margin ≥0.5 mm from the most distal edge of the CAC. Fourth, to analyze EAN for each ROI, we made histograms of EANs, calculated median values and compared the results to the theoretical EANs of potential components of CAC such as HA (16.1), COM (13.8), DCPD (11.2), and OCP (10.0). These theoretical values were obtained from the website of the National Institute of Standards and Technology website (http://physics.nist.gov/PhysRefData/Xcom/html/xcom1.html).
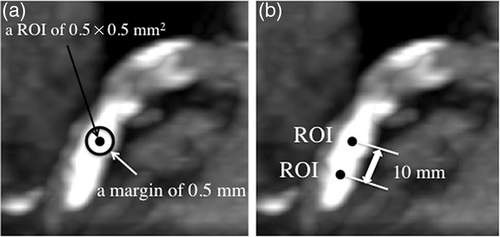
Patients
Potential subjects were 39 patients with chronic kidney disease receiving either hemodialysis (HD) (N = 17) or peritoneal dialysis (PD) (N = 22) at a tertiary medical care center.
All patients had undergone DECT coronary angiography, but no analyzable CACs were identified in 10 HD patients and 12 PD patients. As a result, a final total of seven HD patients and 10 PD patients were enrolled in this study.
Study 2: Comparison between measurement of calcified lesion by DECT and other measurement methods
To confirm the accuracy of EANs determined using the DECT method, measurement of the EAN by the DECT method and measurement by an already established measurement method were carried out using known substances or calcified biological samples. The compared substances were an HA pellet, a ureter stone, calcified arteries and calcified aortic valves. The HA pellet used was a 10 × 10 × 2 mm3 square-shaped sample, comprising ≥99% HA (APP-100; HOYA Technosurgical, Tokyo, Japan). The ureter stone was studied preoperatively using in vivo DECT, and component analysis of the sample was performed by infrared spectrophotometry after ureterolithotripsy. The brachiocephalic artery and aortic valve were obtained after endarterectomy and aortic valve replacement surgery, and calcified parts of each tissue were used for in vitro DECT measurement and X-ray diffraction analysis.
Statistical analysis
Results for HD and PD patients were statistically compared used unpaired t-testing.
Ethics
All study participants provided informed consent, and the study design was approved by the Institutional Committee on Human Research at Tokyo Women’s Medical University.
RESULTS
Study 1
Clinical background data such as age, sex, original disease, duration of dialysis, and laboratory data are shown in Table 1. The only significant difference between HD and PD groups was for serum albumin.
| HD (N = 7) | PD (N = 10) | P-value | |
|---|---|---|---|
| Age (years: mean ± SD) | 65 ± 15 | 67 ± 11 | 0.81 |
| Male (N) | 7 | 9 | 0.39 |
| Primary disease | |||
| Diabetes mellitus (N) | 1 | 4 | 0.25 |
| Nephrosclerosis (N) | 2 | 1 | 0.32 |
| Chronic glomerulonephritis (N) | 2 | 3 | 0.94 |
| Other (N) | 2 | 2 | 0.68 |
| Smoking (N) | 6 | 6 | 0.25 |
| Duration of dialysis (months; mean ± SD) | 74.0 ± 2.5 | 55.6 ± 1.9 | 0.44 |
| Body mass index (kg/m2; mean ± SD) | 23.1 ± 3.4 | 22.5 ± 1.7 | 0.64 |
| Laboratory data | |||
| Hemoglobin (g/dL: mean ± SD) | 11.2 ± 0.8 | 10.9 ± 1.3 | 0.68 |
| Serum albumin (g/dL: mean ± SD) | 3.9 ± 0.2 | 3.4 ± 0.4 | < 0.01 |
| Uric acid (mg/dL: mean ± SD) | 7.5 ± 1.6 | 6.3 ± 0.7 | 0.07 |
| Serum calcium (mg/dL: mean ± SD) | 9.0 ± 0.6 | 8.6 ± 0.5 | 0.25 |
| Phosphate (mg/dL; mean ± SD) | 5.8 ± 1.6 | 5.4 ± 1.0 | 0.52 |
| Calcium × Phosphate (mg2/dL2; mean ± SD) | 52.6 ± 15.4 | 46.6 ± 9.5 | 0.36 |
| Intact parathyroid hormone (pg/mL; mean ± SD) | 154 + 51 | 177 ± 204 | 0.52 |
| Non-HDL cholesterol (mg/dL; mean ± SD) | 112 ± 107 | 124 ± 36 | 0.80 |
| Pulse wave velocity (mean ± SD) | 1912 ± 601 | 1718 ± 245 | 0.42 |
| Ankle brachial index (mean ± SD) | 1.16 ± 0.17 | 1.2 ± 0.16 | 0.95 |
| Left ventricular mass index (g/m2; mean ± SD) | 1.38 ± 28 | 132 ± 26 | 0.25 |
| Intima-media thickness (mean ± SD) | 1.38 ± 0.48 | 1.3 ± 0.72 | 0.89 |
- HD, hemodialysis; HDL, high-density lipoprotein; PD, peritoneal dialysis.
Median EANs of CACs in dialysis patients are shown in Figure 2. Totals of 74 ROIs and 56 ROIs were measured in HD and PD patients, respectively. Average of median EAN was 13.8 ± 0.6 in the HD group, and 13.7 ± 0.5 in the PD group. No significant differences were seen between the two groups. The histogram showed both groups to be near the theoretical EAN for COM.
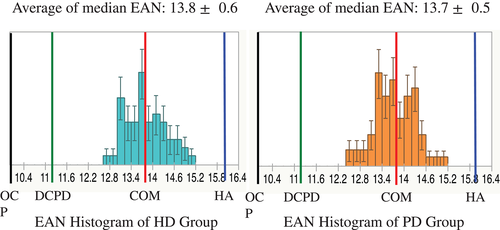
Study 2
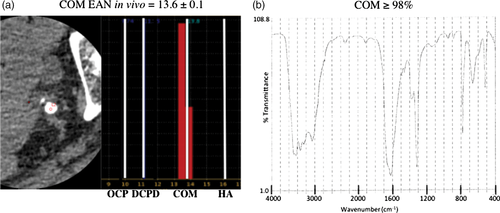
The EAN for the HA pellet was 16.5, close to the theoretical EAN for HA of 16.1. In an additional study, we scanned a urinary stone in vivo before surgery, showing an EAN of 13.16, similar to the theoretical EAN for COM. After surgery, we performed infrared spectrophotometry in vitro and revealed the ureter stone as comprising >98% COM (Fig. 3). In another study, the EAN of a calcified brachiocephalic artery after endarterectomy was 14.37, between the theoretical EANs of COM and HA, and the artery comprised >99% HA according to X-ray diffraction analysis. In another study using aortic valve, the EANs of calcified aortic valves after aortic valve replacement were 14.66, 13.25, and 15.46, and these valves comprised HA, calcium sulfate, and HA, respectively, according to X-ray diffraction analysis (Table 2). These results show that the EAN and results of measurement from established methods were almost the same.
| HA pellet | Ureteral stone | Brachiocephalic artery | Aortic valve | ||||
|---|---|---|---|---|---|---|---|
| DECT method | Effective atomic number | 16.5 | 13.16 | 14.37 | 14.66 | 13.25 | 15.46 |
| Theoretical composition | COM | COM & HA | HA | COM | HA | ||
| Other methods | Results of composition | COM | HA | HA | Calcium sulfate | HA | |
| Method of measurement | Infared spectrophotometry | X-ray diffraction analysis | X-ray diffraction analysis | ||||
- COM, calcium oxalate monohydrate; DECT, dual-energy computed tomography; HA, hydroxyapatite.
DISCUSSION
One of the serious complications affecting cause of death among dialysis patients is cardiovascular disorder, for which coronary vascular calcification is a crucial problem. Suppression of calcification represents an important goal, and requires analysis of the mechanism of calcification, and analysis of the components of calcification is important. However, few reports have examined the composition of calcification in the coronary arteries of dialysis patients. Compared to HD patients, PD patients show differences in calcium, phosphorus, intact parathyroid hormone and lipid metabolism, but no reports have described differences in CAC components. We measured the CAC components in HD and PD patients and examined whether differences exist. In this study, average of median EAN was 13.8 in the HD group, and 13.7 in the PD group. No significant differences were apparent between groups (P = 0.45). The histogram showed both groups were near to the theoretical EAN for COM. Kulkarni et al. 7 reported an analysis of ureteral stones using in vivo DECT and reported EANs of COM ranging from 11.2 to 14.4. Using this classification, over 90% of ROIs in the HD group and 78% of ROIs in the PD group comprised COM. The remaining ROIs (9.5% in HD patients, 16.1% in PD patients) all showed EANs ≥14.4, close to HA. In Study 2, calcified parts were analyzed using established methods and the results compared with those of the DECT method. No differences were found between results, and we considered that DECT coronary angiography analysis of the CAC part in the present study offered suitably accurate results. From these findings, COM was found to be important as one of a component of CAC in dialysis patients. A previous study of non-dialysis patients 4 suggested that CACs might consist of COM using a single-source DECT technique. From these results, we considered that components of coronary calcification in patients on HD and PD might not differ from those in non-dialysis patients.
Several studies 2-6, 8-14 have reported the composition of vascular calcifications. Schmid, et al. 8 studied the mineral deposits excised from atherosclerotic thoracic and abdominal aortas at autopsy. The samples were treated by pronase, papain, 0.6–1.8 N HCl, perchloric acid, nitric acid and heated at 70–1000°C. After these preparations, they did X-ray diffraction pattern analysis, electron probe analysis and scanning electron microscopy and revealed the sample consisted mainly of calcium apatite, carbonate and protein. Boström, et al. 9 studied calcified carotid arteries obtained at carotid endarterectomy. The specimens were fixed by 2% glutaraldehyde in 0.2 M cacodylte and dehydrated with ethanol and then transmission electron microscopy was done. By this study, calcified nodules consisted of primarily calcium and phosphate in a ratio of 1.2:1.6, which is consistent with presence of HA. Fitzpatrick, et al. 10 investigated coronary arteries obtained at autopsy. Specimens were fixed in 4% paraformaldehyde, decalcified in formic acid, fixed in ethanol and dehydrated in ascending alcohols, embedded in glycolmethylmethacrylate, and analyzed by energy-dispersive X-ray microanalysis which revealed that calcified plaques contained a calcium to phosphate molar ratio of 1.55:1 to 1.70:1, which corresponds closely to the known ratio of 1.66:1 of HA.
For study on uremic or dialysis patients, Contiguglia, et al. 5 investigated calcified femoral artery, which was taken from an end-stage renal disease patient at autopsy. The specimen was dried at 130°C for 16 h and then investigated by chemical and X-ray diffraction analysis and revealed arterial calcification composed of calcium (4660 mmols/kg), magnesium (174 mmols/kg) and phosphate (3199 mmols/kg). Schwartz, et al. 6 reported morphology of coronary atherosclerotic lesions in end-stage renal disease patients. They studied 27 end-stage renal disease patients including 21 hemodialysis patients and coronary artery specimens were taken at autopsy. Calcified plaques were heated at 130–900°C and then investigated by X-ray diffraction analysis and revealed components of calcified coronary arteries consisting of HA and calcium phosphate, but not calcium oxalate crystals.
Schlieper, et al. 11 studied iliac, brachial and coronary artery segments from 30 dialysis patients by microcomputed tomography, transmission electron microscopy including electron energy loss spectrometry, energy dispersive spectroscopy and electron diffraction and revealed calcification consisted of HA and/ or whitlockite. For handling samples, they fixed the sample with glutaraldehyde and ethyl glycidyl ether. As most such reports have identified HA as a component of calcified tissues, and some investigation have reported whitlockite 11, 12, though in terms of the handling of samples, most earlier studies were ex vivo in design and were performed under non-physiological conditions, with specimens burned or decalcified or examined under acidic conditions. As HA is one of the thermodynamically most stable substances in the human body and can even be created from calcium carbonate and phosphoric acid under specific conditions 15, for example, acidic or heated conditions, HA could potentially have been accidentally created by handling errors in earlier studies.
Fishbein et al. 13 reported calcium oxalate crystal deposits in atherosclerotic plaques in coronary arteries among non-dialysis patients in a pathological study. Matsui et al. 4 analyzed the composition of coronary calcification using single-source DECT and also clarified the presence of COM in the calcified area. Furthermore, Bischetti et al. 14 recently characterized the calcification on carotid plaques using energy dispersive X-ray microanalysis. They identified two types of calcium salt in the atheromatous plaques, HA and calcium oxalate, with calcium oxalate calcifications mainly in stable plaque and HA present in both stable and unstable plaque. However, those studies included no patients on dialysis. The present report offers the first evidence that COM is deposited in coronary arteriosclerotic lesions in dialysis patients. We did not measure serum oxalate in this study, but Recht et al. 16 reported serum concentrations of oxalate as 20 to 100 times higher in dialysis patients compared to normal kidney function people and oxalate crystals can easily combine with calcium and form deposits in tissues. In addition, uremic levels of oxalate crystals severely inhibit proliferation and migration of human endothelial cells and enhance endothelial injury, and Recht et al. concluded that oxalate represents a key factor in the development of calcifications. We thus thought that dialysis patients might reasonably be expected to show COM in calcified lesions. However, our study only showed that the EAN for calcification was in the range of COM. We have to interpret this result carefully, as EAN is just a representative number for materials and further investigation is required.
CONCLUSIONS
Dual-energy computed tomography using an effective atomic number method enables compositional analysis of calcified lesions in vivo. Hydroxyapatite has been reported as a major component of coronary calcifications. However, our in vivo study found that the effective atomic number of coronary artery calcifications in dialysis patients was in the range of calcium oxalate monohydrate. Calcium oxalate monohydrate might thus represent a major component of coronary artery calcifications in dialysis patients.
Acknowledgments
We thank Dr. T. Machida and Dr. E. Ueno for instruction in DECT and EAN. We also thank radiologists I. Tanaka and R. Fukui for constructing the methods used for DECT and EAN.
Conflict of Interest
The authors declare no conflict of interest.




