The CABIM3, enriched space for the improvement of fruit flies used in the sterile insect technique, first approaches
Abstract
The High Biosecurity House of the Mediterranean Fly in Metapa (CABIM3, in its Spanish acronym) is a space within the new Moscamed Mexico facility designed to select favorable traits that mitigate the negative effects of mass production while enhancing male competitiveness, ultimately increasing the efficiency of the sterile insect technique (SIT). The CABIM3 serves as an environmentally enriched space where insects, whose offspring will initiate the mass production process, are confined. In this study, the sexual performance of males and oviposition behavior of females from mass-reared and wild strains of Anastrepha ludens (Loew) and A. obliqua (Macquart) were compared under field cage conditions, inside CABIM3 and in an orchard, to evaluate the suitability of CABIM3's environmental conditions, including light intensity, temperature, and relative humidity. The results revealed significant differences in male sexual performance and female oviposition behavior between the CABIM3 areas and the orchard. Despite these differences, our findings suggest that a breeding facility like CABIM3 could be a valuable tool for improving the attributes of insects used in SIT programs.
Introduction
The sterile insect technique (SIT) is a targeted pest control method employed as part of integrated pest management over wide areas. This technique is widely used to manage economically significant fruit fly species (Diptera: Tephritidae) in various regions worldwide (FAO/IAEA, 2017; Klassen & Vreysen, 2021). The SIT involves rearing large quantities of insects, sterilizing them, and releasing them into the field to induce sterility in pest populations (Knipling, 1955; Klassen et al., 2021). The technique's efficiency depends on releasing an adequate number of sterile males in a specific area to minimize mating opportunities between wild females and wild males. Sterile males must effectively locate, compete for, and mate with wild females, successfully transferring sperm carrying dominant lethal mutations and inducing prolonged refractory periods (Knipling, 1955; Hendrichs et al., 2002; Klassen et al., 2021).
However, several studies have shown that sterile males are at a disadvantage compared to wild males in sexual competition for mating with wild females (Cayol et al., 1999; Hendrichs et al., 2002). The low sexual competitiveness of sterile males has been mainly, but not exclusively, associated with artificial rearing processes that expose insects to conditions markedly different from their natural environments (McInnis et al., 1996; Cayol, 2000). Such conditions contribute to reduced longevity and diminished predator evasion abilities, which are important traits for surviving and finding and winning mating partners, since behavioral repertories changed during the rearing process (Leppla et al., 1983; Hendrichs et al., 2007; Dor et al., 2014; González-López et al., 2016; Dor & Liedo, 2019; Liedo et al., 2020b; Lance & McInnis, 2021), as well as decreased resilience to environmental stressors (Hoffmann et al., 2001; Weldon et al., 2013) and reduced overall activity patterns (Weldon et al., 2010).
The mass-rearing process plays a critical role in the SIT's success, with slight improvements in the daily survival rates or sexual competitiveness of sterile males having a greater impact on SIT efficiency than simply increasing the number of released insects (Liedo et al., 2020a; Barclay, 2021). Strategies to mitigate the adverse effects of mass-rearing include maintaining colonies under relaxed conditions to reduce selection for undesirable traits, regularly refreshing rearing strains, and actively selecting individuals with favorable traits such as mating compatibility with wild populations or survival in stressful environments (Díaz-Fleischer et al., 2009; Weldon et al., 2020; Lance & McInnis, 2021). Enhancing environmental conditions that support the maintenance of male behavioral polymorphisms and improve sexual competitiveness has also been proposed (Ochieng’-Odero, 1994; Cayol, 2000; Meza-Hernandez & Díaz-Fleischer, 2006).
In this context, the new Moscamed Mexico facility incorporates spaces designed to enhance male sexual competitiveness and select for favorable traits to improve SIT efficiency. The progeny produced in this environmentally enriched area will be used to initiate the mass-rearing process (mother colony). This area, designated as the High Biosecurity House of the Mediterranean Fly in Metapa (CABIM3, in its Spanish acronym), is a space of approximately 200 m2 (100 m2 of enriched areas and 100 m2 for operations, aisles and storage). The enriched areas are divided into three independent sections (areas 1, 2, and 3), each measuring around 6.0 × 5.3 m. A 1-m height difference separates contiguous areas, which are connected by access doors (Fig. 1). The facility is supported by metal columns and features a specialized double-layer glass coating that allows natural light to penetrate, simulating a natural outdoor environment. Inside, citrus and coffee plants will recreate field-like conditions in order to foster natural behaviors in flies. This environment is expected to favor the selection of traits that are critical to the success of the SIT, such as the ability to find resources (i.e., food and water), the ability to escape from predators, and sexual competitiveness.
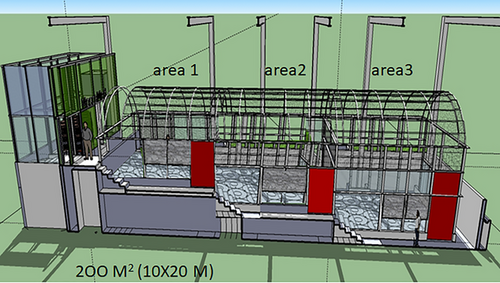
This study compared the sexual performance of mass-reared males and the oviposition behavior of laboratory and wild females of Anastrepha ludens (Loew) and A. obliqua (Macquart) under field cage conditions, inside the CABIM3 and in an orchard, to evaluate the suitability of CABIM3's environmental conditions.
Materials and methods
Study site
The study was conducted at the Department of Genetic Filtering of the Moscamed facility (SADER-SENASICA) in Metapa de Dominguez, Chiapas, Mexico. Laboratory conditions were kept at a photoperiod of 12 h : 12 h (light : dark), a temperature of 25 ± 2 °C, and relative humidity of 70% ± 10%.
Flies
Both male and female A. ludens and A. obliqua of wild and laboratory origin were evaluated. Wild flies were collected from infested sour orange (Citrus aurantium L.) and plum (Spondias mombin) fruits in the Soconusco region, Chiapas. Infested fruits were placed in 60 × 50 × 11.5 cm plastic trays with a 2-cm vermiculite layer (Strong lite, Products Corp., Seneca, IL) as a pupation substrate and transported to the laboratory. Third-instar larvae from the fruits were transferred to plastic containers with vermiculite (60% humidity) until adult emergence.
Laboratory flies were obtained from the mass production at the Moscafrut facility in Metapa de Dominguez (Orozco-Dávila et al., 2017). Adults from the genetic sexing strain Tapachula-7 of A. ludens and the standard bisexual strain of A. obliqua were evaluated. The Tapachula-7 strain is characterized by sexual differentiation in adults expressed through pupal color: brown for males and black for females. This trait is the result of a reciprocal translocation between a chromosomal locus and an autosome carrying the black pupa gene (bp) (Zepeda-Cisneros et al., 2014). Two days before emergence, male A. ludens and A. obliqua pupae were exposed to hypoxic conditions and subjected to a gamma irradiation dose of 80 Gy (60Co: GAMMABEAN—127; Nordion International Inc., Ottawa, Ontario, Canada).
Newly emerged adults were sex-separated and placed in 30 × 30 × 30 cm wooden cages covered with mosquito netting. Each cage contained 100 flies provided with water and a diet of hydrolyzed protein (MP Biomedicals, Santa Ana, CA, USA) mixed with standard sugar at a 1 : 3 ratio by weight. Flies were kept under laboratory conditions until evaluation. One day prior to the evaluations, the flies were marked with vinyl paint dots (Vinci, Vinci de México, S.A. de C.V.) on the thorax for strain identification (FAO/IAEA/USDA, 2019). Marking colors were alternated between strains in each replicate.
Given that age of sexual maturity of the flies may vary with origin, in this case laboratory-reared and wild, the tests were conducted with wild flies aged 16–20 d and laboratory-reared flies aged 10–14 d of A. ludens and wild flies aged 16–20 d and laboratory-reared flies aged 8–12 d of A. obliqua (sensu FAO/IAEA/USDA, 2019). For the oviposition behavior evaluations, 20 males and 20 females were confined in 30 × 30 × 30 cm cages 3 d prior to testing to allow mating. As oviposition sites, ten green spheres (4 cm in diameter) made of fucellerone (30 g/L; Tic Gums, Inc., Belcamp, MD) and dyed with 1 mL/L green vegetable dye (McCormick®) were wrapped in parafilm paper (American National Can Tm, Neenah, WI) to mimic natural oviposition substrates. The spheres were replaced daily to ensure consistent conditions.
Evaluations
The evaluations of male sexual performance and female oviposition behavior were carried out in field cages of 3 m in diameter × 2 m high made of white antiaphid mesh (50% shading capability) supported by a metal structure (Calkins & Webb, 1983; Chambers et al., 1983; FAO/IAEA/USDA, 2019). A cage was placed in the center of each area of CABIM3 (areas 1, 2, and 3, Fig. 1). As a control, a field cage was placed under the shade of a mango tree of the Ataulfo cultivar, approximately 15 years old, in a mixed orchard of mangoes and guavas of 1.3 ha at a distance of ≈ 75 m from CABIM3 (14°49ʹ33.8ʺN, 92°11ʹ46.1ʺW, 100 m above sea level). Three sour orange trees, approximately 3 years old and 180 cm high, were placed in the center of each field cage. The trees were planted in polypropylene pots of 50.8 cm in diameter × 52 cm high (60 L).
Male sexual performance
Thirty wild males, 30 sterile males, and 30 wild females were released inside each cage. Males were introduced 30 min before females to allow the establishment of territories, and matings were monitored continuously. Observations were conducted from 3:00 p.m. to 7:00 p.m. for A. ludens and from 7:30 a.m. to 12:00 p.m. for A. obliqua, which corresponds to the peak activity of each species. Each mating pair was captured in a test tube, and the male's strain was identified by paint color. Mating indexes were calculated using the FAO/IAEA/USDA quality control guidelines.
The Proportion of Matings (PM) measures the mating capacity of the flies and the suitability of the environment for mating, representing overall sexual activity. PM is calculated as the ratio of the number of collected pairs to the number of released females. The Relative Sterility Index (RSI) quantifies male sexual competitiveness by comparing the proportions of matings achieved by sterile and wild males. It is calculated using the formula RSI = SW/ (SW + WW), where SW is the number of matings by sterile males, and WW is the number of matings by wild males. RSI values range from 0 (all matings by wild males) to 1 (all matings by sterile males), with a value of 0.5 indicating equal competitiveness between the two groups.
Oviposition behavior
Bioassays were conducted separately for each fly species. In each field cage, 20 fucellerone spheres were hung among the branches of the orange trees, and 20 females from each fly strain were released. Continuous observations were made to record the arrival of females to the hosts (visits) and the dragging of the aculeus on the host, which was considered an indication of successful oviposition (Aluja et al., 2000). At the conclusion of the observations, oviposited hosts were examined under a stereoscope microscope to confirm oviposition. For A. ludens, which lays single eggs or clutches of up to 40 eggs (Aluja et al., 2000), hosts were dissected to determine clutch number and size (eggs per clutch). For A. obliqua, which only lays one egg in each oviposition event, the number of eggs per host was recorded, with each egg considered a separate oviposition (Aluja, 1994). The variables analyzed included the proportion of females visiting hosts, the proportion of females ovipositing per visit, the number of ovipositions per female, and, in the case of A. ludens, clutch size. All variables were compared between fly strains. Observations were conducted from 09:00 to 14:00 h for A. ludens and from 08:00 to 13:00 h for A. obliqua.
Environmental conditions
Temperature, relative humidity (HOBO Data Loggers, MX Temp/RH Logger, MX1101, USA, Bourne, MA), and luminosity (Extech Instruments Luxometer, HD 450, China) were recorded every 30 min in each experimental arena.
Data analysis
Proportions were analyzed using a generalized linear model (GLM) with a binomial distribution and a logit link function, and clutch size (A. ludens) was compared using a GLM with a Poisson distribution and a log link function (Crawley, 1993; Agresti, 2018). Pairwise comparisons of means were conducted when significant differences (α ≤ 0.05) were detected. Environmental variables (temperature, relative humidity, and light intensity) were analyzed using a repeated measures ANOVA (JMP v.11, SAS Institute, Cary, NC, USA).
Results
Male sexual performance
For A. ludens, the PM values significantly differed among experimental arenas (χ2 = 47.16, DF = 3, P = 0.0001). Higher PM values were observed in the orchard compared to the CABIM areas. Among CABIM3 areas, the area 1 show the highest PM values (Table 1). The RSI values did not differ significantly among arenas (χ2 = 3.64, DF = 3, P = 0.3027). Overall, the mean RSI value (mean ± SE) was 0.40 ± 0.04, indicating that sterile and wild males obtained similar proportions of matings across arenas, with a slight advantage for wild males (Fig. 2A).
| Proportion of Matings (PM) | ||
|---|---|---|
| Arena | A. ludens | A. obliqua |
| Orchard | 0.35 ± 0.033 a | 0.46 ± 0.048 a |
| Area 1 | 0.23 ± 0.050 b | 0.18 ± 0.037 b |
| Area 2 | 0.17 ± 0.038 c | 0.06 ± 0.019 c |
| Area 3 | 0.15 ± 0.054 c | 0.01 ± 0.008 d |
- Note: Values per fly species separated by different letters indicate statistical differences (χ2 contrast test).
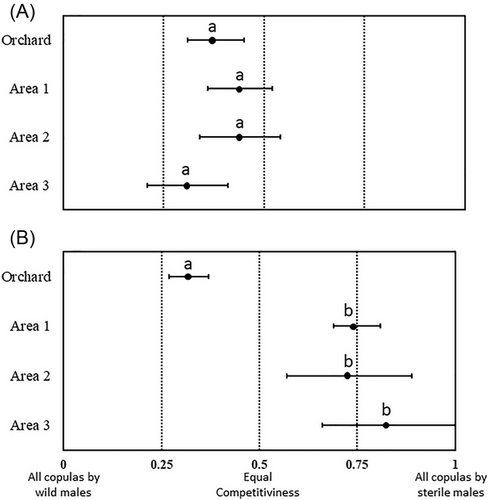
For A. obliqua, the PM values also varied significantly across arenas (χ2 = 301.27, DF = 3, P = 0.0001), with the highest values in the orchard, followed by area 1, area 2, and area 3 of CABIM3 (Table 1). The RSI values showed significant differences among arenas (χ2 = 49.59, DF = 3, P = 0.0001), with the orchard differing from the CABIM3 areas, which did not vary significantly among themselves. The lowest RSI values were recorded in the orchard, indicating that wild males achieved a higher proportion of matings compared to sterile males. On the other hand, high RSI values were observed in the different CABIM3 areas, indicating that sterile males achieved a higher proportion of matings relative to wild males (Fig. 2B).
Oviposition behavior of Anastrepha ludens
Proportion of visits
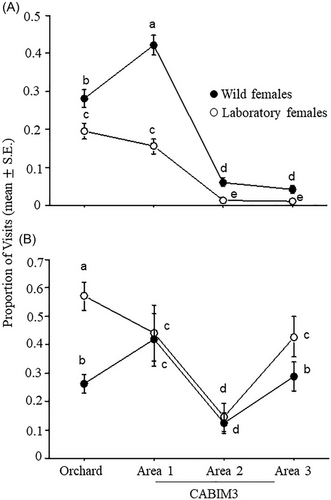
The proportion of females visiting hosts significantly differed among experimental arenas (χ2 = 320.6, DF = 3, P = 0.0001), fly strains (χ2 = 44.9, DF = 1, P = 0.0001), and the interaction between fly strain and experimental arena was also significant (χ2 = 14.6, DF = 3, P = 0.0022). In all arenas, a higher proportion of wild females visited the hosts compared to laboratory females, with the highest values observed in area 1 of CABIM3, followed by the orchard, and lower values were recorded in areas 2 and 3. Laboratory females showed greater activity in the orchard and area 1 of CABIM3 compared to areas 2 and 3 of CABIM3 (Fig. 3A).
Ovipositions per visit
The proportion of females that oviposited relative to those that visited the hosts did not vary significantly among experimental arenas (χ2 = 1.2, DF = 3, P = 0.7435) or fly strains (χ2 = 1.1, DF = 1, P = 0.2841), and the interaction between the two factors was also not significant (χ2 = 2.8, DF = 3, P = 0.4213). The overall mean proportion (± E.E.) of females that visited and oviposited was 0.22 ± 0.03.
The number of ovipositions per female also did not vary significantly among arenas (χ2 = 2.9, DF = 3, P = 0.4121) or fly strains (χ2 = 0.8, DF = 1, P = 0.3694), and the interaction between the two factors was not significant (χ2 = 2.7, DF = 3, P = 0.4321). On average, females oviposited 1.6 ± 0.14 times per host.
Clutch size did not exhibit significant differences among experimental arenas (χ2 = 3.0, DF = 3, P = 0.3941), but differences were observed between fly strains (χ2 = 11.9, DF = 1, P = 0.0006). Laboratory females laid larger clutches (12.0 ± 1.1) compared to wild females (7.6 ± 0.5). The interaction between arena and fly strain was not significant (χ2 = 1.4, DF = 3, P = 0.7057).
Oviposition behavior of Anastrepha obliqua
Proportion of visits
There was a significant effect of experimental arena (χ2 = 193.5, DF = 3, P = 0.0001), fly strain (χ2 = 41.2, DF = 1, P = 0.0001), and the interaction between the two factors (χ2 = 34.9, DF = 3, P = 0.0001) on the proportion of females visiting hosts. The proportion of females arriving at the hosts varied depending on the arena and fly strain. Laboratory females exhibited the highest activity in the orchard, with lower activity in the CABIM3 areas. Inside CABIM3, no differences were observed between areas 1 and 3, while area 2 had the lowest proportion of visits. For wild females, the highest proportion of visits occurred in area 1, followed by the orchard, area 3, and finally, area 2, which had the lowest values (Fig. 3B).
Ovipositions per visit
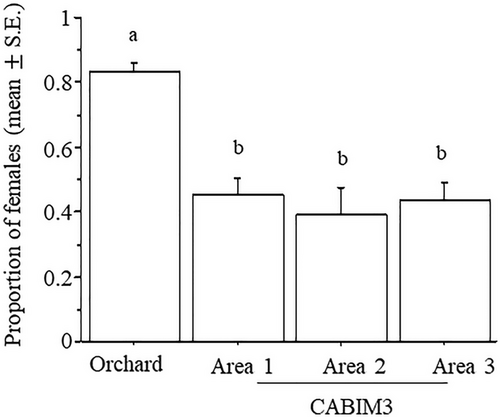
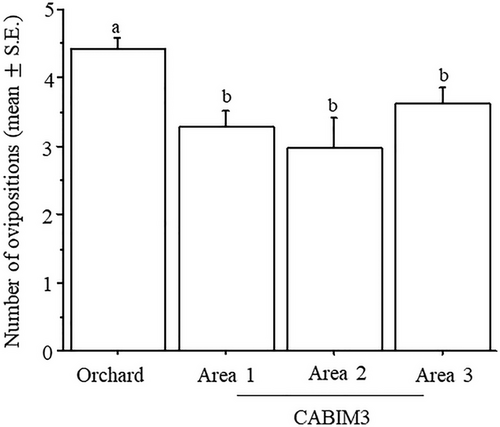
The proportion of females ovipositing relative to those arriving at the hosts varied significantly among experimental arenas (χ2 = 123.6, DF = 3, P = 0.0001). The orchard had the highest proportion of ovipositions compared to any CABIM3 area (Fig. 4). No significant differences were detected between fly strains (χ2 = 3.6, DF = 1, P = 0.0576), and the interaction between arena and fly strain was also not significant (χ2 = 5.7, DF = 3, P = 0.1281).
Statistical differences were observed in the number of ovipositions per host among arenas (χ2 = 17.1, DF = 3, P = 0.0007), with the orchard exhibiting the highest number of ovipositions compared to any CABIM3 area (Fig. 5). No significant differences were found between fly strains (χ2 = 0.07, DF = 1, P = 0.3916), and the interaction between arena and fly strain was not significant (χ2 = 0.7, DF = 3, P = 0.8846).
Environmental conditions
Environmental conditions were analyzed for two periods: evening (07:30–14:00 h) and daytime (15:00–19:00 h).
The analyses of the environmental conditions during the evening period showed very similar results, with slight variations in RH. Temperature and light intensity varied between arenas (P < 0.05) and observation hours (P < 0.05), and the interaction between the two factors was significant (P < 0.05). Relative humidity showed differences between arenas (P < 0.05) and observation hours (P < 0.05), but the interaction between the two factors was not significant (P > 0.05) (Table 2). Temperature, relative humidity, and light intensity were higher in the orchard than in CABIM3, except during a short period during the first hours, where temperature and light intensity were lower in the orchard. Temperature and light intensity increased gradually during the observation period, while relative humidity decreased in a similar way (Fig. 6).
| Environmental conditions | ||||
|---|---|---|---|---|
| Period | Factor (d.f.) | Temperature | RH | Light intensity |
| Evening |
Arena (F3, 273) |
7.2, P = 0.0016 | 11.4, P = 0.0001 | 33.1, P = 0.0001 |
|
Observation hour (F13, 273) |
35.2, P = 0.0001 | 128, P = 0.0001 | 8.2, P = 0.0001 | |
Arena × hour (F39, 273) |
7.2, P = 0.0001 | 0.7, P = 0.9062 | 4.5, P = 0.0001 | |
| Daytime |
Arena (F3, 280) |
6.7, P = 0.0011 | 13.4, P = 0.0001 | 2.3, P = 0.1083 |
|
Observation hour (F8, 280) |
6.7, P = 0.0011 | 38.1, P = 0.0001 | 8.8, P = 0.0001 | |
Arena × hour (F24, 280) |
6.7, P = 0.7156 | 1.3, P = 0.1396 | 0.4, P = 0.9942 | |
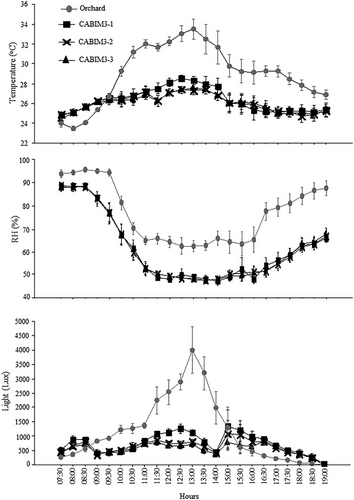
For the daytime period, the analyses of the environmental conditions also showed similar results, with the exception of light intensity. There were differences between arenas (P < 0.05) and observation hours (P < 0.05), and the interaction between the two factors was not significant (P > 0.05) (Table 2). Temperature and humidity were higher in the orchard than in CABIM3, while light intensity did not vary between arenas. Temperature and light intensity decreased gradually throughout the daytime period, while humidity increased in a similar way (Fig. 6).
Discussion
The results demonstrated significant differences in male sexual performance and female oviposition behavior between the CABIM3 areas and the orchard. Overall, mating and oviposition activities were higher under natural conditions than in CABIM3. These differences can be attributed to variations in the environmental conditions, including higher light intensity, temperature, and relative humidity in the orchard compared to CABIM3, which likely had a direct impact on fly behavior.
The primary objective of CABIM3 is to improve the behavioral repertoire of mass-reared flies by enriching their breeding environment, mitigating the negative impacts of confinement on behavioral traits. This concept is not novel; for instance, Leppla et al. (1983) found that Ceratitis capitata (Wiedemann) individuals reared in a plant-enriched environment exhibited greater competitiveness compared to those reared in an unenriched environment. Similarly, enriched environments enhanced the behavioral repertoire of Drosophila melanogaster (Meigen) (Dukas & Mooers, 2003). In this study, mating activity in CABIM3 was lower than in the orchard for both species and strains. Interestingly, RSI values did not differ for A. ludens but did for A. obliqua, with sterile males of A. obliqua performing better in the orchard.
This result aligns with previous observations in A. ludens when these parameters were evaluated in field cages under laboratory and natural conditions. Wild flies showed reduced performance in laboratory settings compared to natural conditions, whereas laboratory flies maintained a consistent mating performance across scenarios (Meza-Hernandez & Díaz-Fleischer, 2006). In contrast, A. obliqua exhibited greater variability, with sterile males performing better in the orchard than in CABIM3 areas. These interspecies differences can likely be attributed to their mating schedules: A. ludens mates exclusively at dusk, while A. obliqua primarily mates at dawn and, to a lesser extent, at dusk (Aluja et al., 2000). Differences in light conditions between CABIM3 and the orchard, particularly during the evening period, may have impacted A. obliqua males more significantly than A. ludens males. Differences in the mating performance of wild, but not laboratory, A. ludens flies were observed when field cage assays were carried out in the field or inside a mass-rearing facility (Meza-Hernandez & Díaz-Fleischer, 2006).
This study represents the first comparison of oviposition patterns in these two species of flies under greenhouse conditions with high environmental complexity and orchard conditions. Oviposition behavior differed significantly among species, strains, and scenarios. Females exhibited greater activity under orchard conditions compared to CABIM3. Both species displayed similar host-searching and oviposition activity patterns across scenarios; however, A. obliqua females made more visits and oviposited more frequently than A. ludens females. These interspecific differences can be attributed to the higher activity levels of A. obliqua females in response to changes in host density and condition (Aluja et al., 2000; Díaz-Fleischer & Aluja, 2003a, 2003b). Regarding fly strains, females of wild origin were more active than laboratory-reared females for both species. These findings suggest that microenvironments play a critical role in shaping behavioral responses. Environmental stimuli such as light, temperature, and relative humidity appear to affect laboratory and wild flies differently (Inskeep et al., 2021).
To conclude, despite the differences observed in fly behavior and environmental conditions between CABIM3 and the orchard, having a breeding facility with greater spatial complexity could be a valuable tool for enhancing the attributes of insects used in SIT programs. Liedo et al. (2007) demonstrated that even minor adjustments to adult maintenance conditions, such as adding plexiglass pieces to increase resting areas, significantly mitigated the negative effects of mass rearing on the sexual performance of sterile males in C. capitata. Additionally, environments with greater spatial complexity have been shown to benefit not only male mating performance but also female behavior.
For instance, A. ludens females housed with natural or artificial foliage exhibited reduced copulation latency and shorter mating times compared to females without such stimuli (Díaz-Fleischer et al., 2009). Similarly, in D. melanogaster, rearing in spatially complex environments enhanced female fecundity and reduced reproductive latency (Malek & Long, 2019; Talagala et al., 2024). Thus, employing CABIM3 for fly rearing could improve male behavioral quality and increase female egg production, both of which are critical for the successful application of the SIT.
Acknowledgments
We extend our gratitude to Ramón Fuentes Morales, Henry Esteban Calderón, Marco Polo Pérez, and Juan Heliodoro for their invaluable technical assistance. We also thank Maritza Juárez Durán, Directora del Programa Nacional de Moscas de la Fruta, and Francisco Ramírez y Ramírez, Director General de Sanidad Vegetal at the Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria (DGSV-SENASICA-SADER), for their support and encouragement. This research was funded through contract D42017 by the International Atomic Energy Agency (IAEA) in collaboration with SENASICA-DGSV and the Programa Nacional de Moscas de la Fruta.
Disclosure
The authors have not disclosed any competing interests.




