METTL3/METTL14-mediated RNA m6A modification is involved in male reproductive development in Bactrocera dorsalis
Abstract
RNA N6-methyladenine (m6A) modification represents a pivotal epigenetic modification that facilitates the remodeling of gene expression and regulates a variety of biological processes via certain post-transcriptional mechanisms. However, the specific function of RNA m6A modification in insect male reproduction remains unclear. In this study, we explored the molecular mechanism by which METTL3/METTL14-mediated RNA m6A modification regulates male reproduction in the invasive pest Bactrocera dorsalis. The results showed that BdMettl3 and BdMettl14 were highly expressed in fat body (FB) and male accessory glands (MAGs). Knockout of BdMettl3 or BdMettl14 decreased the expression level of m6A in B. dorsalis, resulting in testicular deformities and a significant reduction of viable sperm number. Specifically, BdMettl3 or BdMettl14 knockout reduced the titer of 20-hydroxyecdysone (20E, the active form of ecdysone) in males. The messenger RNA (mRNA) of Disembodied, one of the 20E synthesis genes, was modified by m6A, and its expression increased the titer of 20E. The mRNA m6A level of Disembodied obviously decreased after the knockout of BdMettl3 or BdMettl14, suggesting that RNA m6A modification regulates testis development and fecundity by modulating 20E synthesis. Taken together, this study indicates that METTL3/METTL14-mediated RNA m6A modification presents a new regulatory mechanism for male reproduction in B. dorsalis, serving as a potential target for the control of B. dorsalis.
Introduction
In insects, males play a crucial role in determining the behavior of mating and female reproductive physiology, including egg production and hatching. It has been well established that insect endocrine hormones, including juvenile hormone (JH) (Shah et al., 2022) and ecdysone (Riddiford et al., 2017; Li et al., 2024), are critical to male reproductive development. The 20-hydroxyecdysone (20E) signaling pathway plays crucial roles in a multitude of important male reproductive processes (Ables & Drummond-Barbosa, 2010). Epigenetic modifications such as DNA methylation (Jullien et al., 2012), RNA methylation (Zheng et al., 2013), and various histone modifications (Zhang et al., 2018) are crucial for animal reproduction and development by directly regulating transcription and translation. Therefore, studying male reproductive development at the epigenetic level can help further understand the underlying mechanism for genetic sterility in insects and develop eco-friendly strategies such as sterility technique to control pests.
RNA modification is a critical step in the epitranscriptomic regulation of gene expression (Gilbert & Nachtergaele, 2023), including N6-methyladenosine (m6A), 5-methylcytosine (m5C), and pseudouracil, as well as N1-methyladenosine (m1A) (Zhao et al., 2017b). Among them, RNA m6A modification is the most abundant and common RNA modification in eukaryotic RNA, where the 6th nitrogen atom (N6) on RNA adenosine is substituted by a methyl group (Dominissini et al., 2012), contributing to the regulation of messenger RNA (mRNA) levels by RNA splicing, nuclear export, mRNA stability, translation efficiency, and RNA structure (Wang et al., 2014a; Zhao et al., 2016; Peer et al., 2017). RNA m6A modification is a reversible modification that encompasses m6A methyltransferase (referred to as “writers”), demethylase (referred to as “erasers”), and m6A binding protein (referred to as “readers”) (Meyer & Jaffrey, 2017). This special modification is initially established by a multi-protein m6A methyltransferase, which is composed of METTL3 (Bokar et al., 1994), METTL14 (Liu et al., 2014; Wang et al., 2014b), along with several other adaptor proteins (Ping et al., 2014; Schwartz et al., 2014; Yang et al., 2018). Among them, METTL3 plays a major catalytic role in RNA m6A modification; it is responsible for adding methyl groups to specific adenylate residues of mRNA, thereby enabling RNA m6A modification (Li et al., 2022). METTL14 interacts with METTL3 and forms a stable heterodimer, playing an essential role during m6A deposition on nuclear RNAs with increased catalytic efficacy (Liu et al., 2014). Previous studies have shown that METTL3/METTL14-mediated RNA m6A modification plays crucial roles in mammals (Mu et al., 2021; Ren et al., 2022), plants (Yu et al., 2021; Ren et al., 2022), zebrafish (Zhao et al., 2017a), and insects (Haussmann et al., 2016; Guo et al., 2023). The m6A also has an important function in the maternal-to-zygotic transition of zebrafish (Zhao et al., 2017a; Ren et al., 2021), and regulates embryonic stem cells (ESCs) and early embryos in mice (Chen et al., 2021). In insects, RNA m6A modification is also involved in various biological processes, such as sex determination (Haussmann et al., 2016), caste differentiation (Wang et al., 2021), labor division (Chen et al., 2024), insecticide resistance (Yang et al., 2021a), and neuronal functions (Lence et al., 2016). However, the mechanistic link between insect male reproduction and RNA m6A modification remains poorly understood.
The oriental fruit fly, Bactrocera dorsalis (Hendel), is a seriously devastating invasive pest for many fruits and vegetables. It is widespread in most Asian countries, causing huge economic losses to agriculture (Clarke et al., 2005). There is an urgent need for new approaches to control B. dorsalis given the rapid development of pesticide resistance and a variety of relevant environmental problems. In this study, we found that RNA m6A modification controls 20E synthesis by regulating the expression of Disembodied, thereby regulating spermatogenesis, testis (TE) development, and male fertility of B. dorsalis. Two methyltransferases, METTL3 and METTL14, were identified in B. dorsalis. Knockout of METTL3 or METTL14 by clustered regularly interspaced short palindromic repeats (CRISPR) / CRISPR-associated nuclease 9 (Cas9) significantly reduced sperm viability and inhibited TE development. Furthermore, we found that METTL3/METTL14-mediated RNA m6A modification regulates the expression of a 20E synthesis gene (Disembodied), thereby regulating 20E synthesis. In summary, we reveal the molecular mechanism for METTL3 and METTL14 to regulate B. dorsalis male fertility at the epigenetic level, as well as provide novel insights into the molecular mechanisms underlying epigenetic regulation of male reproductive development in B. dorsalis and new genetic control targets for B. dorsalis.
Materials and methods
Rearing of B. dorsalis
B. dorsalis was reared at the Institute of Horticultural and Urban Entomology, Huazhong Agricultural University (Wuhan, China) under relative humidity of 65%–75%, a light to dark (L : D) ratio of 12 h : 12 h, and a temperature of 27 ± 1 °C. The adults were fed with an artificial diet consisting of sugar and yeast at a ratio of 3 : 1.
RNA extraction and quantitative real-time – polymerase chain reaction (qRT-PCR)
Total RNA from fat body (FB), TE, male accessory gland (MAG), and thorax (TH) of mature adults was extracted for spatial expression profiling according to the instructions of the RNAiso Plus kit (TaKaRa, Otsu, Shiga, Japan). Furthermore, total RNA was extracted from males at 1, 3, 5, 7, 9, 11, 13, and 15 d post-eclosion (DPE) for temporal expression analysis. Subsequently, 1 µg of total RNA was reverse-transcribed into complementary DNA (cDNA) using the PrimeScriptTM RT Reagent Kit (TaKaRa, Kyoto, Japan). The cDNA samples were stored at −20 °C for subsequent experiments. Then, 384-well plates (ABI, Applied Biosystems, CA, USA) were used for qRT-PCR. The reaction volume system for qRT-PCR consisted of 0.4 µL 10 mmol/L of each primer, 2 µL cDNA (diluted 1 : 10), 2.2 µL RNase-free water, and 5 µL SYBR Green Master Mix (Low ROX) (Yeasen, ShangHai, China). The qRT-PCR program was carried out as follows: a first denaturation at 95 °C for 5 min, followed by 40 cycles of 95 °C for 10 s and 60 °C for 30 s on the ABI 7500 Real-Time PCR System. The α-tubulin gene was used as an internal reference for qRT-PCR (Livak & Schmittgen, 2001; Shen et al., 2010), and the expression of related genes was analyzed by 2−ΔΔCt method. Table S1 shows the primers used for qRT-PCR. PCR efficiency and determination coefficient (R2) characterizing each standard curve is given in Table S2.
CRISPR/Cas9 knockout of BdMettl3 or BdMettl14
The single guide RNA (sgRNA) used for CRISPR/Cas9 technology was designed using the online tool CHOPCHOP (ib.no). Then, the sgRNA was synthesized according to the instructions of the MEGAscript® T7 Transcription Kit (Promega, Madison, WI, USA). To enhance the efficacy of CRISPR/Cas9-mediated gene knockout, 1 U/µL of Cas9 nuclease and 400 ng/µL target of sgRNA were coincubated at 37 °C for 15–20 min to form ribonucleoprotein (RNP) complexes, which were injected into the posterior pole end of fresh embryos within 30 min.
The PCR amplification products at the knockout site were examined using Sanger sequencing to determine mutation efficiency. Sanger sequencing results with double peaks indicate successful knockout. The detection primers are shown in Table S1.
Morphological observation of reproductive system
Phenotypes and characteristics of the reproductive system of 10-d-old B. dorsalis adult males were observed. The images of testes were captured using a Nikon D5100 digital camera (Nikon, Tokyo, Japan) with a stereo microscope.
Reproductive capacity assay of male B. dorsalis
To determine the fertility of the mutant male, 1 mutant male was mated with 3 wild-type females. The embryos were collected every 2 d for a total of 5 times and a total of 10 d. The total number of eggs laid was calculated, and that of hatched eggs was counted 2 d after laying to calculate the hatching rate.
Sperm viability assay and viable sperm measurement
As previously reported (Liu et al., 2024), the testes of 10-d-old adult males were dissected in a solution of HEPES (10 mmol/L), NaCl (150 mmol/L), and bovine serum albumin (10%) at a pH of 7.4. The spermatozoa were released by puncturing with dissection forceps. A 5 µL out-flowing sperm sample was collected and mixed with 5 µL SYBR-14 working solution (2 µL of SYBR-14 storage solution was added to 98 µL HEPES buffer). The mixture was incubated for 10 min at room temperature in the dark. Then, 2 µL propidium iodide was added into the mixture and incubated at room temperature in the dark for 7 min. To quantify the sperm viability, a fluorescence microscope (DM6B, Leica, Japan) was employed to enumerate the number of live and dead spermatozoa. Dead sperms were red and living sperms were green. Each group contained 3 biological replicates and 5 technical replicates.
Dot blots
Total RNA was isolated from 20 adult males with a total of 3 replicates (TaKaRa, Otsu, Shiga, Japan) using the method described above. Denaturation was performed at 95 °C for 3 min followed by cooling on ice for 3 min. Then, 2 µL RNA sample was loaded onto a HybondTM-N+ membrane (Biosharp, China), and UV cross-linked at room temperature for 30 min. Then, HybondTM-N+ membrane was blocked in Tris-buffered saline with Tween 20 (TBST) containing 5% milk for 2 h at room temperature, washed with TBST 3 times for 10 min each time, and then incubated with m6A antibody (ABclone, Wuhan, China) in the blocking buffer at 4 °C overnight. The membrane was further washed in TBST 3 times with 15 min for each time and incubated with a secondary antibody in the blocking buffer at room temperature for 1 h. The ECL Highsensitive Substrate (Monad, Wuhan, China) was used to visualize the dot blots by Multi Chemiluminescent Substrate Imaging System. Finally, total RNA samples were detected by 0.02% methylene blue (Solarbio).
Western blot
The B. dorsalis males that had been injected with double-stranded RNA (dsRNA) were homogenized in RIPA buffer (GBCBIO Technologies, Guangzhou, China) containing a protease inhibitor (MedChemExpress, NJ, USA) with 5 males constituting 1 sample. Then, the supernatant was obtained by centrifugation at 12 000 r/min for Western blot experiment. The protein was mixed with loading buffer (Beyotime, Shanghai, China) at a ratio of 5 : 1 and separated on 10% sodium dodecyl sulfate – polyacrylamide gel electrophoresis gel and transferred on polyvinylidene fluoride (PVDF). The membrane was blocked in TBST with 5% milk for 2 h at room temperature, incubated with antibodies of METTL3 and METTL14 at 4 °C overnight, and then incubated with rabbit antibody sealing solution for 1 h at room temperature. The protein bands were detected using Multi Chemiluminescent Substrate Imaging System. The open reading frames (ORFs) of METTL3 and METTL14 were cloned, generating rabbit polyclonal antibodies. As a loading control for the Western blot, mouse anti-β-tubulin (Frdbio, Wuhan, China) was used. The ECL Highsensitive Substrate (Monad, Wuhan, China) was used for band visualization, and the Multi Chemiluminescent Substrate Imaging System was used for image acquisition. The density was measured by ImageJ software.
N6-methyladenosine-immunoprecipitation-qPCR (m6A-IP-qPCR)
The m6A immunoprecipitation (m6A-IP) procedure was conducted as previously recorded (Ren et al., 2022). Briefly, total RNA of 20 mature adults was extracted according to the instructions of the RNAiso Plus kit (TaKaRa, Otsu, Shiga, Japan). One-tenth of RNA sample of wild type, METTL3+/− and METTL14+/− was retained as input control (input). Then, 10 µg of normal rabbit immunoglobulin G (IgG) protein or m6A polyclonal rabbit antibody (ABclone, Wuhan, China) was coincubated with 100 µL of protein A/G Beads at 4 °C for 4 h. Subsequently, the remaining nine-tenths of RNA samples was mixed with this bead-antibody mixture and incubated overnight at 4 °C. Then, the mixture was eluted by elution buffer as m6A-containing RNA (IP). The input and IP RNA were reverse-transcribed into cDNA according to the above method. Subsequently, the mRNA expression of 20E synthetic gene was detected by RT-qPCR. The primers used in m6A-IP-qPCR are shown in Table S1.
dsRNA synthesis and RNA interference (RNAi)
Specific dsRNA was synthesized in accordance with the instructions provided in the MEGAscript® T7 Transcription Kit (Promega, Madison, WI, USA). The sequences of the primers are listed Table S1. Then, 1 µg dsRNA of corresponding gene was injected into the abdomen of frozen anesthetized 3-d-old male B. dorsalis using the PV820 micro-injector (WPI, USA). An equal volume of dsEGFP was used as the negative control. At 48 h after injection, 5 males were randomly selected and the RNAi efficiency was evaluated by RT-qPCR.
Detection of 20E titer
First, 150 µL of hemolymph was collected from the hemolymph and then added to a new tube containing 150 µL of 80% methanol. The mixture was then centrifuged for 10 min at 4 °C with 12 000 r/min, and the supernatant was collected into a new tube. Then, the supernatant was completely dried with nitrogen. Finally, it was redissolved in an enzyme-linked immunosorbent assay buffer, and enzyme-linked immunosorbent assay was performed using the EIA kit to detect the 20E titer (Cayman Chemical Co, MI, USA).
Statistical analysis
SPSS 20.0 software was used for multiple comparison Tukey post hoc test and Student's t-test (P < 0.05). Data were expressed as mean ± standard error of the mean (SEM). Graphs were drawn using GraphPad Prism 7.04.
Results
Phylogenetic analysis of BdMETTL3 and BdMETTL14
In order to investigate the role of RNA m6A modification in B. dorsalis, we examined the characteristics of methyltransferase METTL3 and METTL14 involved in RNA m6A modification (Liu et al., 2014). The full-length ORF sequences of BdMETTL3 and BdMETTL14 were cloned. The sequencing results showed that the cDNA of BdMettl3 contains a 1 980-bp ORF encoding a 660-amino acid protein (Fig. 1A), and that of BdMettl14 contains a 1 221-bp ORF encoding a 407-amino acid protein (Fig. 1B).
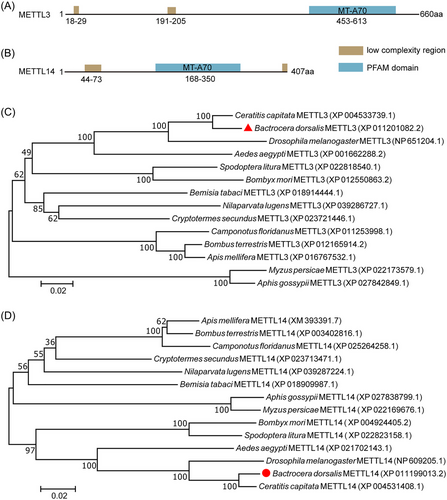
METTL3 and METTL14 had a low-complexity region and an important MT-70 region (Fig. 1A,B). Phylogenetic analysis with the neighbor-joining tree of METTL3 and METTL14 sequences from 14 species showed that BdMETTL3 and BdMETTL14 proteins were highly similar to their homologs in 3 other typical Diptera species selected in this study, including Ceratitis capitata, Drosophila melanogaster, and Aedes aegypti. Additionally, the BdMETTL3 protein had 88.08% identity with CcMETTL3 and 69.80% identity with DmMETTL3. The BdMETTL14 protein shared 96.56% amino acid sequence similarity with CcMETTL14 and 86.99% with DmMETTL14. These results indicated that BdMETTL3 and BdMETTL14 are evolutionarily conserved in Diptera insects.
Expression patterns of BdMettl3 and BdMettl14 in B. dorsalis
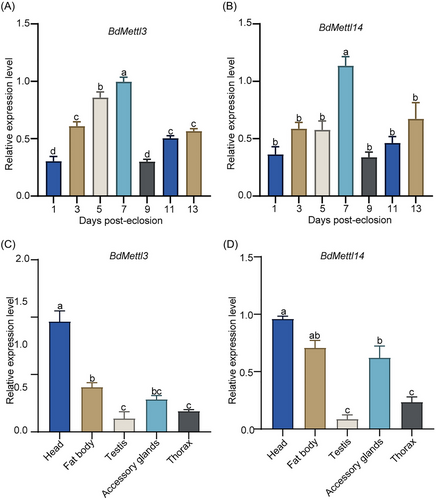
To better understand the gene function of BdMettl3 and BdMettl14 in males, the expression patterns BdMettl3 and BdMettl14 in whole males from 0 to 13 DPE (Fig. 2A,B) and in different tissues (Fig. 2C,D) of B. dorsalis were determined by qRT-PCR. The results showed that the expression level of BdMettl3 in male adults increased from 1 to 7 DPE, and reached the highest at 7 DPE (Fig. 2A). The expression level of BdMettl14 in male adults remained steady at the basic level at 1, 3, and 5 DPE, but significantly increased at 7 DPE (Fig. 2B). In addition, the expression patterns of BdMettl3 and BdMettl14 in various tissues, including the head, FB, TE, MAGs, and TH of males, were analyzed. The results showed that BdMettl3 and BdMettl14 were expressed in all tissues. The expressions of BdMettl3 and BdMettl14 were highly expressed in head and MAGs (Fig. 2C,D). Among them, accessory glands play an important role in male reproduction, so we focused on the function of BdMettl3 and BdMettl14 in male reproduction.
METTL3/METTL14-mediated RNA m6A modification is essential for male reproduction in B. dorsalis
To investigate the relationship between METTL3/METTL14-mediated RNA m6A modification and male reproduction in B. dorsalis, we generated BdMettl3 or BdMettl14 knockout mutants using the CRISPR/Cas9 system. Different types of deletion mutations or replacements are shown in Figure S1. The results showed 2 types of large fragment (−155 bp and −166 bp) deletion mutations in BdMettl3 (Fig. S1A). Four meaningful fragment deletions, including −1, −4, −8, and −13 bp deletions, and a replacement of 1 single base, were found for METTL14 (Fig. S1B). qRT-PCR results indicated that the mRNA expression levels of BdMettl3 and BdMettl14 in the heterozygous mutants (hereafter referred to as METTL3+/− and METTL14+/−) were 0.44-fold (Fig. 3A) and 0.60-fold (Fig. 3B) of those in the wild type, respectively. Western blot results showed that the protein expression levels of METTL3 and METTL14 in METTL3+/− and METTL14+/− were 0.44-fold (Fig. 3C) and 0.18-fold (Fig. 3D) of those in the wild type, respectively. The mutation rates of METTL3 and METTL14 were 33% and 22%, respectively (Table S3). Since homozygote mutation was lethal, the heterozygote mutant was used in the following experiments.
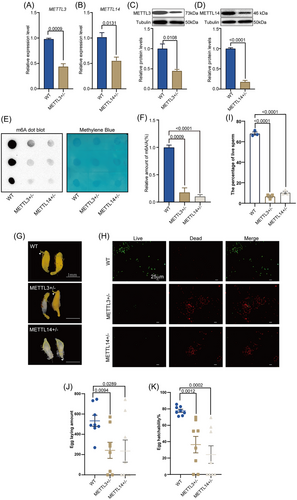
We further detected the RNA m6A modification level by m6A-specific antibodies using dot blots. The results confirmed that the level of RNA m6A modification was significantly reduced in METTL3+/− and METTL14+/− compared with that in the wild type (Fig. 3E,F). Therefore, it could be speculated that METTL3 and METTL14 are involved in RNA m6A modification. Additionally, morphological analysis revealed that 7 pairs of testes in METTL3+/− (n = 10) were deformed on 10 DPE, including 1 pair of albino testes, 4 pairs of 1 large and 1 small TE, and 2 pairs of very small TE. Similarly, 7 pairs of TE in METTL14+/− (n = 10) were deformed, including 2 pairs of albino testes, 3 pairs of 1 large and 1 small TE, and 2 pairs of very small TE (Fig. 3G and Table S4). The wild-type group exhibited normal development of TE (Fig. 3G). Since METTL3 or METTL14 knockout resulted in the deformity of TE, we further determined the sperm viability. The sperm viability in METTL3+/− and METTL14+/− was about 10% and 15% of that in the wild type, respectively, and METTL3 or METTL14 knockout led to an increase in sperm mortality (Fig. 3H,I). Furthermore, we evaluated the effects of METTL3 or METTL14 knockout on male fecundity; the fecundity of individuals in the METTL3+/−, METTL14+/−, and wild-type groups was examined by bioassays. The results indicated that the amount of eggs laid (Fig. 3J) and the rate of hatching (Fig. 3K) in the mutant groups was significantly reduced. Taken together, these findings suggest that METTL3/METTL14-mediated RNA m6A modification is essential for male reproductive development of adult B. dorsalis.
METTL3 or METTL14 knockout suppresses ecdysone biosynthesis
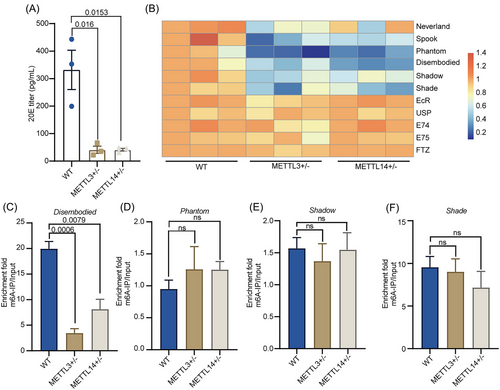
Our results showed that BdMettl3 and BdMettl14 were highly expressed in MAGs, and it has been reported that the source of 20E in insects is the MAG rather than the TE (Pondeville Emilie et al., 2008). Therefore, it can be speculated that hormones may be involved in the regulation of METTL3 and METTL14 on male reproductive development in B. dorsalis. As expected, the titer of 20E significantly decreased in METTL3+/− and METTL14+/− compared with that in the wild type (Fig. 4A). Further, we examined the expression profiles of genes involved in 20E biosynthesis and response. As a result, the mutant groups showed significantly downregulated expression of genes in the 20E biosynthesis pathway (Spook, Neverland, Phantom, Disembodied, Shade, and Shadow) except for Neverland, but no significant difference in 20E response genes (E74, E75, FTZ, EcR, and USP) compared with the wild type (Fig. 4B). To further confirm that BdMettl3 and BdMettl14 regulate the expression of genes in the 20E biosynthesis pathway by mediating RNA m6A modification, we determined whether METTL3+/− and METTL14+/− had significant differences in the mRNA m6A level of 20E biosynthesis genes (except for Neverland) from the wild type by m6A-IP-qPCR. The results showed that METTL3+/− and METTL14+/− had lower mRNA m6A levels of Disembodied compared with the wild type (Fig. 4C); no significant difference was observed in mRNA m6A level of Phantom (Fig. 4D), Shadow (Fig. 4E), and Shade (Fig. 4F); and no mRNA m6A modification was detected for Spook. Furthermore, we used SRAMP software to predict the RNA m6A modification site on Disembodied. The results showed there were 5 possible RNA m6A modification sites in the Disembodied coding sequence (CDS) region, all of which had the m6A motif sequence of RRACH. Among them, 2 sites have a high confidence. (Fig. S2, Table S5). In general, the above results indicated that METTL3/METTL14-mediated RNA m6A modification activates the mRNA expression of Disembodied, thereby increasing the titer of 20E in male B. dorsalis.
Disembodied controls male reproductive development by reducing 20E titer
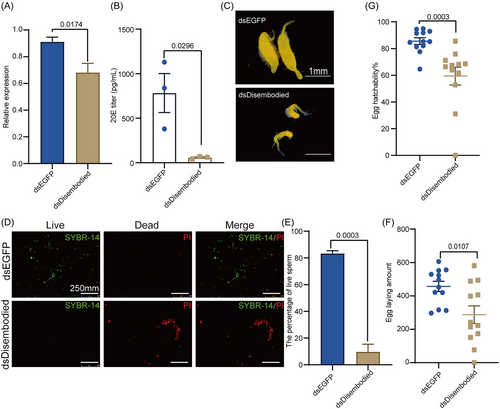
To determine whether the expression of Disembodied controls male reproductive development via regulating 20E titer in B. dorsalis, we silenced the expression of Disembodied in B. dorsalis by RNAi (Fig. 5A). Notably, dsDisembodied-injected males showed a significantly lower titer of 20E than dsEGFP-injected males (Fig. 5B). In addition, morphological analysis revealed that dsDisembodied-injected males tended to show an abnormal TE morphology, with 4 pairs of TE in the dsDisembodied group (n = 10) being deformed on 10 DPE, including 1 pair of albino TE and 3 pairs of 1 large and 1 small TE; however, the dsEGFP-injected males showed normal TE development (Fig. 5C). We further detected the sperm viability after knockdown of the expression of Disembodied. The results showed that dsDisembodied-injected males had a higher percentage of dead sperm (Fig. 5D,E). Additionally, females mated with dsDisembodied-injected males also showed a significantly reduced amount of eggs laid (Fig. 5F) and rate of hatching (Fig. 5G) compared with those mated with the wild type. Taken together, these results indicated that decreasing the expression of Disembodied affects male reproductive development by reducing the 20E titer in B. dorsalis.
Discussion
Despite a lot of studies reporting the role of RNA m6A modification in eukaryotes, a comprehensive understanding of the key enzymes involved and their biological functions remains elusive. In this study, we found that the METTL3/METTL14-mediated RNA m6A modification is important for male reproduction by controlling 20E biosynthesis in B. dorsalis. Knockout of BdMettl3 or BdMettl14 inhibited TE development and significantly decreased the fertility by reducing the synthesis of 20E. At the molecular level, qRT-PCR and m6A-IP-qPCR results demonstrated that the knockout of BdMettl3 or BdMettl14 significantly suppressed mRNA expression and the m6A modification level of 20E biosynthesis gene Disembodied. Collectively, our results demonstrated the role of RNA m6A modification in the male reproductive development of B. dorsalis.
We found that the knockout of BdMettl3 or BdMettl14, 2 genes with relatively high expression among transmethylase genes, reduced the RNA m6A modification in male B. dorsalis. It can lead to abnormal TE and reduce sperm viability, thereby decreasing progeny viability. Previous studies have indicated that RNA m6A modification is essential for insect growth and development. For example, RNA m6A modification modulates sex determination in Drosophila by affecting the pattern of Sxl (Haussmann et al., 2016; Lence et al., 2016). In addition, it plays important roles in caste differentiation and larval development in Apis mellifera (Wang et al., 2021), as well as insecticide resistance in Bemisia tabaci (Yang et al., 2021a). METTL3/METTL14-mediated m6A modification reduces the fitness cost in response to pathogenic attacks by regulating the JH titer in Plutella xylostella (Guo et al., 2023). Furthermore, it has been demonstrated that m6A is involved in the regulation of sperm tail formation in Anopheles sinensis at the omics level (Liu et al., 2022). Importantly, the ubiquitous knockdown of Mettl3 leads to the mislocalization of spermatids during the process of Drosophila spermatogenesis. Additionally, morphologically abnormal swollen tips and the misdistribution of sperm bundles within the testes have been noted (Rowe & Rockwell, 2022). These studies corroborate our conclusions. Our results suggest that METTL3/METTL14-mediated RNA m6A modification participates in male reproduction in B. dorsalis, thereby furnishing more candidate target genes for further optimizing and developing RNAi-based sterile insect techniques for large-scale field applications. Similarly, in mice, METTLl3-mediated m6A modification was found to regulate meiosis initiation and spermatogonial differentiation (Xu et al., 2017), and ALKBH5-dependent m6A demethylation is essential for spermatocyte nuclei and round spermatids by controlling the splicing and stability of long 3′-untranslated region mRNAs in male germ cells (Tang et al., 2018). In summary, RNA m6A methylation performs a variety of functions in insects, and is particularly important for male reproduction in animals.
In this study, we found that the expression of BdMettl3 and BdMettl14 was enriched in MAG in B. dorsalis. RNA m6A modification was found on the Disembodied gene, and the knockout of BdMettl3 or BdMettl14 reduced the RNA m6A modification of this gene, thereby reducing its expression level. Furthermore, METTL3/METTL14-mediated RNA m6A modification regulated the 20E titer by modulating the expression of the Disembodied gene. 20E plays a critical role in promoting animal reproduction (Roy et al., 2018; Li et al., 2024), and is essential for male reproduction. A previous study has demonstrated that Anopheles gambiae and Drosophila males produce the steroid hormone 20E from the MAG (Pondeville et al., 2008; Sharma et al., 2017). In male B. dorsalis, the 20E signaling pathway is widely involved in some important reproductive processes such as germ stem cell maintenance (Li et al., 2014), accessory gland proteins (ACPs) production (Sharma et al., 2017), and sperm morphogenesis (Ables & Drummond-Barbosa, 2010). Specific suppression of 20E receptor expression in Inka cells reduces the fecundity of Drosophila (Meiselman et al., 2017). Furthermore, knockdown of E75, a 20E response gene, inhibited TE development and sperm viability in B. dorsalis (Li et al., 2024). In this study, we only found the expression of Disembodied was regulated by RNA m6A modification. The possible m6A sites of Disembodied mRNA were predicted only by SRAMP software. Due to technical limitations, we were unable to carry out single base mutation in B. dorsalis, so we did not perform validation of potential m6A sites. In future research, we will conduct in-depth verification on the m6A site on the Disembodied gene.
Recent studies have suggested that epigenetic modifications, such as histone modification (Yang et al., 2021b), microRNA modification (Kumar & Xi, 2011), and DNA methylation (Zhao et al., 2023), play vital roles in regulating the synthesis of 20E. It has been found that histone H3K27 methylation regulates insect developmental transition by modulating 20E biosynthesis (Yang et al., 2021b). The DNA methylation gene Dnmt1 can also affect the 20E titer to regulate female reproduction in Blattella germanica (Zhao et al., 2023). The microRNA Bdo-Let-7 regulates the 20E signaling pathway through an exact dose of BdE75 gene, which is essential for normal larval/pupal development in B. dorsalis (Peng et al., 2019). To date, there has been no report about the regulation of RNA m6A modification on male insect development by mediating the titer of 20E. Thus, our results, together with previous reports, reveal an epigenetic regulatory network by which hormones control insect development.
In summary, we propose a specific mechanism by which METTL3/METTL14-mediated RNA m6A modification regulates male reproduction in B. dorsalis partly by affecting the expression of Disembodied. The regulation of RNA m6A modification on the reproductive and developmental processes can be critical for TE function. This work demonstrates that RNA m6A modification plays an important role in male reproduction of B. dorsalis by regulating the hormone level, and provides candidate target genes for the development of effective ecological pest control strategies.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (no. 32220103009), Earmarked Fund for China Agriculture Research System (CARS-26) and Hubei Hongshan Laboratory. Zhang, H.Y. and Zhang, Q.Y. conceived and designed the project. Qiao, J. performed experiments and analyzed data. Zheng, C.J., and Qiao, J. did the gut bacterial identification experiments. Li, Z.N. made the graphs. Zhang, Q.Y., Zheng, W.W., and Zhang, Q.Y. wrote the manuscript draft. All authors discussed and analyzed the data, and modified the manuscript.
Disclosure
The authors declare no competing interests.




