Transgenic silkworm expressing bioactive human ciliary neurotrophic factor for biomedical application
Abstract
Ciliary neurotrophic factor (CNTF) acts as a potent neuroprotective agent in neuronal survival and regeneration, and can also induce the differentiation of several stem cells into neurons, which highlights the broad application of CNTF in biomedicine. However, large-scale production of bioactive recombinant human CNTF protein remains to be explored. Herein, this study aims to express a bioactive human CNTF protein on a large scale by genetically engineering a silk gland bioreactor of silkworm. Our results showed that CNTF protein was successfully expressed in the middle silk gland (MSG) of silkworm, which can be secreted into the silks with the amount of 3.2 mg/g cocoons. The fabrication of human CNTF-functionalized silk material was able to promote proliferation and migration of neural cells when compared to the natural silk protein. Importantly, this functional silk material could also facilitate neurite outgrowth of mouse retinal ganglion cell (RGC-5) cells. All these data demonstrated a high bioactivity of the recombinant human CNTF protein expressed in the MSG of silkworm. The further fabrication of different silk materials with CNTF bioactivity will give biomedical applications in tissue engineering and neuroregeneration.
Introduction
The silkworm Bombyx mori, as a completely domesticated insect with highly efficient production of cocoon silk, has been considered an excellent lepidopteran model for biological research (Xia et al., 2004; Xia et al., 2014). The cocoon silk spun by silkworm is mainly composed of a core of silk protein, silk fibroin, and an outer layer of silk protein, silk sericin, which has been used as a textile material for thousands of years (Xia et al., 2004). In addition to the use of silk materials in the traditional textile field, due to its mechanical performance, biocompatibility, biodegradability, and water permeability characteristics (Vepari & Kaplan, 2007; Talukdar et al., 2011; Chen et al., 2019; Chen et al., 2023), recent research has expanded the application of cocoon silk into new fields, such as biopolymers, biomaterials, cosmetics, and biomedicine (Kundu et al., 2013; Zhang et al., 2015; Ma et al., 2023).
Moreover, owing to the huge ability of silkworm synthesizing abundant silk proteins in silk glands, the silkworm together with available transgenic technology has been regarded as an ideal bioreactor to express various recombinant foreign proteins accompanying the synthesis of silk proteins (Tamura et al., 2000; Xia et al., 2004; Tomita, 2011; Xia et al., 2014), which further endows the silkworm with large-scale and cost-effective production of foreign proteins when compared to other expression systems, such as transgenic animals and plants. For instance, human type III procollagen, human serum albumin, human acidic fibroblast growth factor, human lactoferrin, human platelet derived growth factor and human neurotrophin-4 (Tomita et al., 2003; Ogawa et al., 2007; Wang et al., 2015; Chen et al., 2018; Xu et al., 2021; Zhang et al., 2022), have been successfully expressed in silk glands, which shows excellent biological activities of expressed proteins and exhibits potential applications in novel fields (Wang et al., 2024). In turn, the expression and addition of active foreign proteins into the cocoon silk, not only helps to produce recombinant foreign proteins on a large scale, but also further promotes the functional application of silk materials.
Given the important neuroprotective role of ciliary neurotrophic factor (CNTF) in the central and peripheral nervous system in glial cells and neurons (Stockli et al., 1991; Winter et al., 1995), the administration of human recombinant CNTF protein to patients with neurodegenerative diseases, such as amyotrophic lateral sclerosis, retinal degenerative disease and Huntington's disease, has accelerated the great demand for CNTF in biomedical applications (Miller et al., 1996; ALS CNTF Treatment Study Group, 1996; Wen et al., 2012). Therefore, to produce the human CNTF protein on a large scale, we here sought to express the recombinant human CNTF protein in the silkworm bioreactor by using the silk sericin expression system and to investigate its potent application in biomedicine (Wang et al., 2013).
For the first time, we established a transgenic silkworm strain that could specifically express foreign human CNTF in the middle silk gland (MSG) and efficiently secret it into the cocoon silk. The present work showed that recombinant human CNTF protein was highly expressed in the MSG and the recovered CNTF from the cocoons was up to 3.2 mg/g of the cocoon shell weight. Furthermore, the application of silkworm cocoon extract containing the CNTF solution to culture neural cells, including mouse retinal ganglion cell (RGC-5) and mouse neural stem cell (NE-4C), the data showed that it could promote cell proliferation and migration. Moreover, we found that the administration of CNTF protein was able to facilitate the neurite outgrowth of RGC-5 via the induction of growth-associated protein-43 (GAP43) signaling. Taken together, these data confirmed the utility of the silkworm bioreactor for efficient expression of human CNTF protein with natural bioactivity, which could produce protein on a large scale and generate various biomaterials containing CNTF for biomedical application.
Materials and methods
Silkworm strain
The silkworm D9L strain was used to generate transgenic expression of recombinant human CNTF protein (Accession No. NP_000605). The transgenic silkworms were reared on fresh mulberry leaves or artificial diet under appropriate conditions.
Cell lines
The mouse NE-4C (Procell, Wuhan, China) and mouse RGC-5 cells (Cellverse, Shanghai, China) were cultured in Dulbecco's modified Eagle's medium (DMEM, Gibco, MA, USA), supplemented with 10% (v/v) fetal bovine serum (Gibco, MA, USA) at 37 °C in a 5% CO2 atmosphere.
Plasmid construction
The optimized human CNTF gene according to the silkworm codon usage bias (Table S1) was synthesized by BGI company (Shenzhen, China). The synthesized CNTF gene with His-tag (8×His) in the C-terminus was amplified by primers listed in Table S2 and inserted into the sericin-1 expression vector pSL1180[Hr3Ser1PSer1PA] digested by BamHI/NotI restriction endonucleases (Wang et al., 2013). The plasmid containing CNTF gene was further digested by AscI and inserted into the transgenic vector piggyBac[3×P3DsRed] to obtain the transgenic expression vector pBac[DsRed, CNTF]. All plasmids were verified by sequencing.
Generation of transgenic silkworm
Generation of transgenic silkworm was performed according to a previously reported method (Tamura et al., 2000). Briefly, pBac[DsRed, CNTF] plasmid and pHA3PIG helper plasmid were mixed at a mole ratio 1: 1 with a final concentration of 500 ng/μL, and the mixture of plasmids were microinjected (Eppendorf, Hamburg, Germany) into the non-diapause D9L silkworm embryos within 2 h after oviposition. The hatched G0 larvae were reared to oviposit the G1 eggs by intervarietal-cross. After development for 6 d, the positive G1 eggs were fluorescently screened through eyes where DsRed was specifically expressed on a fluorescence stereomicroscope (Olympus, Tokyo, Japan). DsRed fluorescence was specifically stimulated by the transgenic line expressing CNTF protein in the MSG of silkworm. The G1 positive individuals were reared to the moth stage, and crossed to generate stable transgenic silkworms. The cocoons from transgenic silkworms were collected for CNTF protein expression analysis.
Crude extraction of CNTF from the cocoon silk
The crude extraction of CNTF from the cocoon silk was performed according to our previously reported method (Wang et al., 2015; Zhang et al., 2022). Briefly, the cocoons from the CNTF transgenic silkworms were ground and extracted with a concentration of 30 mg/mL using lysis buffer (50 mmol/L Tris-HCl, 8 mol/L urea, pH 7.0) at 4 °C for 2 d. The crude extract was then centrifuged at 20 000 × g for 20 min, and the supernatant was filtrated with 0.45 μm Durapore® PVDF Membrane (Millex®-HV) and dialyzed (cellulose dialysis membranes, molecular weight cut-off 14 000 Da, Sangon, China) against the dialysis solution (50 mmol/L Tris-HCl, pH 7.0, 250 mmol/L NaCl) for 3 d with the replacement of dialysis solution every 12 h to remove the urea. The dialysis-completed extract containing the recombinant CNTF protein was dispensed into a small volume and stored at −80 °C.
Quantitative real-time polymerase chain reaction (qRT-PCR) analysis
To detect the messenger RNA (mRNA) expression of the exogenous CNTF, the total RNAs from the MSG of wild-type (WT) and transgenic silkworms were extracted using a total RNA kit (Omega, Biel/Bienne, Switzerland). Two micrograms of each sample were used for reverse transcription to obtain the corresponding complementary DNA (cDNA) by a GoScript Reverse Transcription System (Promega, Madison, WI, USA). Subsequently, an equal amount of the cDNA was subjected to qRT-PCR assay using the SYBR Premix Ex TaqTM II kit (TaKaRa, Dalian, China) on the Applied Biosystems 7500 Fast Real-Time PCR System (Thermo, CA, USA). The expression of eukaryotic translation initiation factor 4A (eIF-4a) was used as an internal control. All experiments were independently performed with 3 biological replicates, and the relative expression levels were calculated using the 2−ΔΔCt method. All primers used for qRT-PCR are listed in Table S2.
Western blot analysis
To detect the protein expression of the exogenous CNTF, cocoons from WT and CNTF silkworms were frozen in liquid nitrogen, powdered, and then the powder with a concentration of 30 mg/mL was suspended by lysis buffer containing 50 mmol/L Tris-HCl and 8 mol/L urea at pH 7.0 under 80 °C for 30 min. The extracts were centrifuged at 20 000 × g for 5 min to collect the supernatant. After quantification of proteins in cocoon extraction by the Bradford method, the expression levels of CNTF protein were detected by western blot analysis. Thirty microliters of protein samples were separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electronically transferred to the polyvinylidene fluoride (PVDF) membrane, followed by the immunoreaction with anti-His antibody (Beyotime, Shanghai, China) at a dilution of 1 : 5000 and secondary horse radish peroxidase (HRP)-labeled goat anti-rabbit immunoglobulin G (IgG) (Beyotime, Shanghai, China) at a dilution of 1 : 10 000. The immunoreacted PVDF membrane was finally visualized using chemiluminescence ECL western blotting kit (Amersham Biosciences, Little Chalfont, UK), and the images were recorded using a Chemiscope Series (Clinx Science Instruments, Shanghai, China). For the detection of GAP43 expression, RGC-5 cells treated with different cocoon extracts were extracted by radioimmunoprecipitation assay (RIPA) cell lysate (Beyotime, Shanghai, China). The proteins were immunoreacted with anti-GAP43 antibody (Beyotime, Shanghai, China) and anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody was used as an internal control. The images were acquired using a Chemiscope Series.
Quantification analysis of CNTF
To quantify the expression of CNTF protein, the Coomassie brilliant blue (CBB) analysis and 30 μL of the protein samples, together with 100, 200, 400, and 800 ng of bovine serum albumin (BSA) standard (Beyotime, Shanghai, China) were used. After electrophoresis, the gels were stained with CBB. The content of CNTF in the cocoon shell weight was quantified by analyzing the intensity of the extracted CNTF with BSA standard on the CBB by using the ImageJ software (https://imagej.nih.gov/ij/).
Scanning electron microscopy
The morphology of WT and CNTF cocoons was observed using a scanning electron microscope (SEM). The samples were dried in a constant temperature dryer. The dried samples were gold coated with Au (MCI000, Tokyo, Japan) and observed under an SEM (Hitachi SU3500, Tokyo, Japan).
Phenotypic observation
The cocoons and pupae from WT and CNTF silkworms were observed under a camera (Nikon, Tokyo, Japan). The economic characteristics of WT and CNTF silkworms including total cocoon weight, cocoon shell weight, and pupal weight were measured.
Immunofluorescence staining
To detect the distribution of CNTF in the cocoon silk, the silk fibers were fixed overnight with 4% (v/v) formalin and frozen by Tissue-Tek® O.C.T.™ compound (Sakura Finetechnical Co., Ltd., Chuo-ku, Japan). After crosscutting into 7 μm thick sections by a freezing microtome, the silk slices were incubated with the anti-His antibody and followed by the Cy3-labeled goat anti-rabbit IgG (Beyotime, Shanghai, China). Images were acquired using a fluorescence microscope (Olympus, Tokyo, Japan).
Cell proliferation assay
For the Cell Counting Kit-8 (CCK-8) assay, RGC-5 and NE-4C cells were seeded in a 96-well plate and incubated with the extract solution from CNTF cocoon at a final concentration of 100 ng CNTF protein in 1 mL medium for 24 h. As a comparison, the same volume of extract solution from WT cocoon or phosphate-buffered saline solution as CNTF cocoon extraction was added to the culture medium. Meanwhile, the final concentration of 100 ng/mL commercial recombinant human CNTF standard protein (SinoBiologica, Beijing, China) was used as a positive control. After the addition of CCK-8 (Beyotime, Shanghai, China) solution at 37 °C for 1 h, the absorbance was measured at 450 nm in a Glomax Multi Detection System (Promega, Madison, WI, USA).
For the EdU (5-Ethynyl-2-deoxyuridine) staining, RGC-5 and NE-4C cells were seeded in a 24-well plate. After different treatments, cells were labeled by BeyoClick™ EdU-488 (Beyotime, Shanghai, China) at a concentration of 2 μmol/L at 37 °C for 2 h and 4ʹ,6-diamidino-2-phenylindole (DAPI; Beyotime, Shanghai, China). The proliferated cells were photographed under a fluorescence microscope (Olympus, Tokyo, Japan).
Wound healing assay
RGC-5 and NE-4C cells were grown to confluence in 6-well culture plates. The cell layer was scraped with a sterile pipet tip. Cells were then treated with the extract of CNTF silkworm cocoon or WT silkworm cocoon according to the procedure of the cell proliferation assay. Initial wounding and the migration of the cells in the scratched area after 24 h were captured using a microscope (Olympus, Tokyo, Japan). The relative migration rate of RGC-5 and NE-4C cells with different treatments was determined using the ImageJ software.
Neurite outgrowth assay
RGC-5 cells treated with different cocoon extracts were fixed by 4% paraformaldehyde and permeabilized with 0.5% Triton® X-100 at room temperature for 20 min. The cells were then stained with Actin-Tracker Red-555 (Beyotime, Shanghai, China) and DAPI. Images were acquired using a fluorescence microscope (Olympus, Tokyo, Japan). The length of the neurites extending directly from the cell body was calculated using the ImageJ software.
Statistical analysis
Data are presented as the mean ± standard deviation (SD) of 3 independent biological replicates by using GraphPad software. Statistical significance (P-value) was analyzed by Student's t-test. Statistical significance is denoted as follows: *P < 0.05, **P < 0.01, and ***P < 0.001.
Results
Transgenic expression of human CNTF protein in the MSG of silkworm
To produce the human CNTF on a large scale, we here used the silk gland bioreactor of silkworm to express the foreign protein. A piggyBac transgenic expression vector of human CNTF protein was constructed by using the MSG expression system (Wang et al., 2013). The CNTF expression plasmid pBac[DsRed, CNTF] (Fig. 1A) together with the transposase expression plasmid pHA3PIG were microinjected into 800 non-diapause silkworm embryos. From these, 52 G0 injected embryos were hatched and fed to lay 33 broods of the G1 progenies (Fig. 1B). The obtained G1 progenies were developed to the 7th d at 25 °C. Eight broods had positive individuals with specific excitation of red fluorescence in the ocelli of the body pigmentation stage of the embryos and the compound eyes of the moths were screened by a fluorescence microscope (Fig. 1B, C), indicating the successful transformation of the CNTF expression cassette into the silkworm genome.
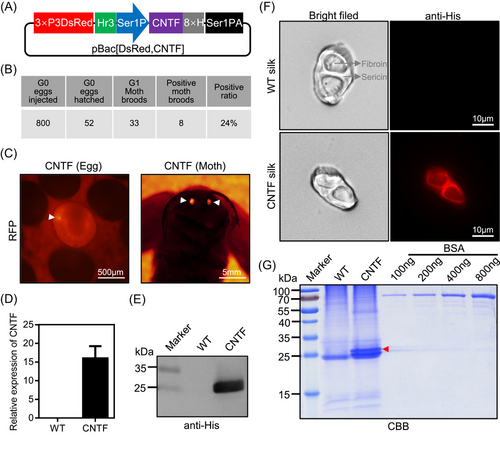
To confirm the expression of CNTF in the MSG of silkworm, total RNA and protein from the MSG were extracted. The CNTF transcript was detected by qRT-PCR and the protein was analyzed by western blot. As shown in Figs. 1D, E and S1, CNTF was successfully expressed in transgenic silkworms. Moreover, we performed immunofluorescence of CNTF by His-tag antibody in the cocoon silk. It was clearly shown that the signal of CNTF was detected mainly in the sericin layers of the silk (Fig. 1F). All these results thus demonstrated that the CNTF can be expressed in the MSG and secreted into the sericin layers of the cocoons.
To quantify the content of CNTF protein in transgenic silkworm cocoons, we extracted the cocoon proteins from the expression system of the MSG and analyzed this by SDS-PAGE. Gel staining by CBB showed that there was a significantly visible protein band of CNTF near 25 kDa when compared to WT extraction (Fig. 1G), which was consistent with its calculated molecular weight of 24.5 kDa and western blot result in Fig. 1E. Meanwhile, it was found that there was 1 obvious band near 55 kDa, which suggested that CNTF protein might form the dimer in the silk gland expression system. In addition, the different concentrations of BSA protein were also analyzed. After drawing the standard curve by the gray values of 100, 200, 400, and 800 ng of BSA protein bands, the content of CNTF protein was evaluated. We found that the content of CNTF protein in the cocoons from the transgenic silkworm strain could reach 3.2 mg/g of the cocoon shell weight. Taken together, these data showed that we have established the transgenic silkworm strain for expressing human CNTF protein.
Effects of CNTF expression on morphological properties of silkworm cocoons
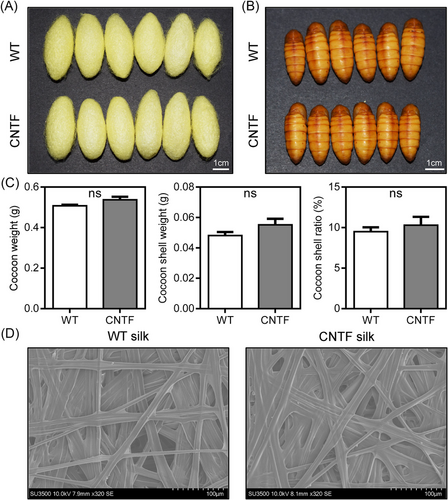
To characterize whether the expression of human CNTF had effects on the development of silkworms and economic characteristics of cocoons, we surveyed the cocoons and pupae obtained from the WT and CNTF silkworms. As shown in Fig. 2A, B, there was no difference on the morphological properties of cocoons and pupae between the WT and CNTF silkworms. We also measured the economic characteristics of cocoons, including cocoon weight, cocoon shell weight, and cocoon shell ratio. Compared with the WT silkworms, all these economic characteristics showed slight increases but no significant difference (Fig. 2C). Moreover, SEM observation of the CNTF cocoon shell showed similar substructure morphology to the WT cocoon shell (Fig. 2D). In addition, the transgenic CNTF expressing silkworm strain could normally lay eggs across generations as well (Fig. S2). All these results indicated that the expression of human CNTF in the MSG did not influence silkworm development and cocoon characteristics, supporting our proposal that we can use the silk gland bioreactor of silkworm to produce human CNTF on a large scale.
Cell proliferation activity of recombinant CNTF protein in RGC-5 and NE-4C
After obtaining the silkworms that can efficiently produce the recombinant CNTF protein, we next identified whether the expressed CNTF protein possessed the bioactivity. We first extracted the silk material containing CNTF protein from transgenic cocoons and analyzed its effects on cell proliferation in RGC-5 and NE-4C. The starved RGC-5 and NE-4C cells were treated with silkworm CNTF cocoon extract and WT cocoon extract for 24 h. The CCK-8 proliferation assay of RGC-5 and NE-4C cells showed an increased absorbance at 450 nm for both the CNTF cocoon extract and the commercially purchased CNTF standard protein when compared to the WT cocoon extract and control groups (Fig. 3A, C). Moreover, the bioactivity of CNTF cocoon extract was very similar to the CNTF standard, indicating that the silkworm expressing CNTF protein had the bioactivity of promoting cell proliferation.
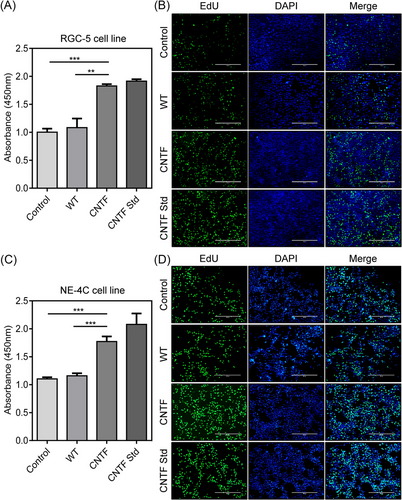
Meanwhile, we also monitored the newly synthesized DNA status by EdU incorporation assay in RGC-5 and NE-4C cells. Within the same treatments, it was shown that only a small number of RGC-5 and NE-4C cells from the control group and the WT cocoon extract exhibited green fluorescence signals, by contrast, more cells treated by the CNTF cocoon extract and the CNTF standard group emitted intensive signals (Fig. 3B, D), which was in accordance with that of CCK-8 results. These results strongly suggested that the CNTF protein expressed in the MSG of silkworm possessed the mitogenic activity to promote cell proliferation and DNA synthesis of neuron cells.
Cell migration ability of recombinant CNTF protein in RGC-5 and NE-4C
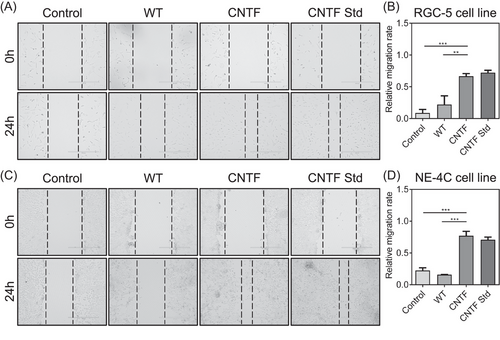
To further examine the cell migration behavior of neuron cells by recombinant CNTF protein, RGC-5 and NE-4C cells were cultured with silkworm cocoon extract containing CNTF protein and WT cocoon extract for 24 h. The wound healing assay was carried out to investigate the effect of CNTF on neural cell migration. As shown in Fig. 4A, C, the wounds were significantly wider in the control and WT groups than in the CNTF and CNTF Std groups both in RGC-5 and NE-4C cells, revealing that the neuron cells treated by CNTF migrated much more quickly into the scratch regions than WT treatment. Also, the migrating rate from the CNTF and CNTF Std groups were calculated to be higher than that of the control and WT groups (Fig. 4B, D). These results demonstrated that the recombinant CNTF protein could increase the migration ability of neuron cells, which further revealed the CNTF activity in promoting neural cell migration.
Neurite outgrowth activity of recombinant CNTF protein in RGC-5
In addition to the promotion of neuron cell migration by CNTF, we also observed an extension of neurite outgrowth in RGC-5 cells. To identify whether CNTF indeed plays a stimulative role on neurite outgrowth, we performed an immunofluorescence assay of RGC-5 cells by Actin-Tracker Red staining. Under the treatments with different silkworm cocoon extracts, the results showed that a large number of RGC-5 cells in the CNTF and CNTF Std groups exhibited more neurite extension in comparison to the control and WT groups (Fig. 5A). Moreover, quantitative data of the neurite length in different groups further confirmed the above observation (Fig. 5B). These results indicated that CNTF could lead to a notable promotion of neurite outgrowth.
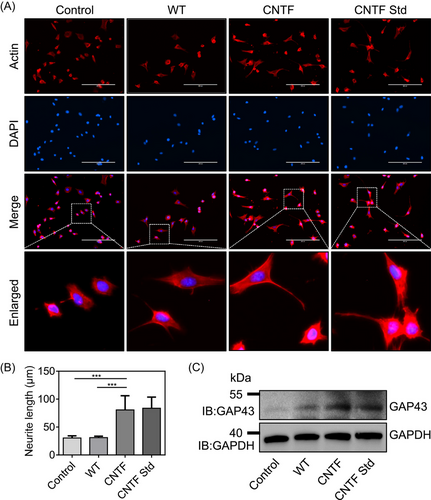
As CNTF had such a strong effect on neuronal morphology and neurite outgrowth, we asked whether the treatment of CNTF affected the expression of GAP43, a neuronal marker gene that plays an important role in the activation of transduction signals related to the actin cytoskeletal remodeling of neurites or axon neurons, thus resulting in neurite outgrowth (Benowitz & Routtenberg, 1997; Korshunova & Mosevitsky, 2010; Chato-Astrain et al., 2023). We collected the RGC-5 cells under different treatments and carried out western blot analysis by using GAP43 antibody. It was shown that GAP43 expression profile exhibited remarkable differences among distinct treatments, and recombinant CNTF and CNTF Std groups clearly induced the expression of GAP43 (Fig. 5C). Taken together, all these data revealed that the recombinant CNTF protein expressed in silk glands was able to promote neurite outgrowth via the induction of GAP43 signaling in RGC-5 cells.
Discussion
It is well-known that CNTF plays key roles in neuronal survival and regeneration, which has been recognized as a potent neuroprotective agent (LaVail et al., 1992; Sango et al., 2007; Zeng et al., 2020; Do Rhee et al., 2022). CNTF can promote the survival of different neurons and induce the differentiation of several stem cells into neurons (Lee et al., 2013; Zeng et al., 2020). There is increasing application of CNTF in clinical trials to treat hereditary and age-related retinal degenerative diseases (Zhang et al., 2011; Do Rhee et al., 2022). However, great demand for CNTF in the biomedical field and low yield of active CNTF sources in vitro limit its applications. Large-scale and cost-effective production of recombinant CNTF protein remains to be explored.
The silkworm, as a promising bioreactor by the great synthesis ability of silk glands, has been used to express various foreign proteins (Xia et al., 2014; Wang et al., 2015). Importantly, foreign proteins can be easily secreted into the cocoon silk which possesses good mechanical properties and great biocompatibility, which in turn gives foreign proteins novel function in biomedicine application (Kundu et al., 2013; Chen et al., 2023; Ma et al., 2023). Based on this silkworm system, we here sought to express the recombinant human CNTF protein in silk glands and explore its biological activity.
We therefore established a silkworm strain that can stably express human CNTF in the MSG of the silkworm. It was shown that CNTF protein could be successfully secreted into the cocoons and the crude extract of CNTF protein from silkworm cocoons could reach 3.2 mg/g, which is comparable with the Escherichia coli expression system which can produce 9–10 mg recombinant CNTF protein in 1 mL culture medium (Xu et al., 2017). Moreover, the high expression of CNTF in silkworm did not affect the quality and structure of silk cocoons. These evaluations, including the highly efficient expression in silkworm and low effect of foreign CNTF protein on silkworm development, demonstrated that we could obtain recombinant human CNTF protein on a large scale in silkworms.
To analyze whether the recombinant CNTF protein expressed in silkworm was active or not, we first examined CNTF activities on cell proliferation and DNA synthesis in neuron cells. Our experimental results showed that the recombinant CNTF protein was the most beneficial for promoting cell proliferation and DNA synthesis in both RGC-5 and NE-4C cell lines, which was similar to the activity of commercial CNTF standard protein. Moreover, the recombinant CNTF protein could also promote the migration of neuron cells. All these data revealed that our silkworm expression system was able to produce active CNTF protein. The activities of the expressed human CNTF protein on proliferation and migration in this work further supported the roles of CNTF in previous studies (Zeng et al., 2020).
It has been reported that CNTF can be released after nerve injury to promote neuroregeneration (Sango et al., 2007; Zeng et al., 2020). Neurite outgrowth is a crucial process in neuroregeneration and injury remodeling (Lindsley, 2010; Zheng et al., 2011). We wondered if the addition of foreign CNTF protein could promote neurite outgrowth. The RGC-5 cell line is a transformed retinal ganglion cell line which has been used to detect neurite outgrowth, which is considered as a potential model system to observe the neuroregenerative effect of nerves (Lindsley, 2010; Zheng et al., 2011). After the RGC-5 cells under CNTF treatment, it clearly showed that CNTF was able to stimulate more neurite extension compared to the control and WT groups in RGC-5 cells. Moreover, we found that GAP43, a protein related to remodeling of neurites (Benowitz & Routtenberg, 1997; Korshunova & Mosevitsky, 2010; Chato-Astrain et al., 2023), was significantly induced in CNTF treatment. These results suggest that neurite outgrowth promoted by CNTF might depend on the activation of GAP43 signaling (Benowitz & Routtenberg, 1997). However, the detailed mechanism for CNTF promotion in this process needs to be investigated in the future.
Taken together, the present study confirms an efficient strategy for expressing recombinant human CNTF protein by using the silk gland of transgenic silkworm, which can easily obtain active CNTF on a large scale. What is more, the natural silk can be fabricated to produce various biomaterials, such as silk hydrogel, silk film, silk sponge, 3D silk scaffold, and others (Wang et al., 2021; Zhang et al., 2022). As CNTF has been reported to exert a neuroprotective action on photoreceptors, the cells responsible for sensing light in the retina, the regenerative potential of CNTF also suggest clinical trials for its use in retinal degenerative diseases (Mittoux et al., 2000; Do Rhee et al., 2022). However, effective delivery of CNTF to target sites in the eye seems to be a challenging work due to the barrier properties of the eye. In combination with silk biomaterials such as degradable hydrogel and CNTF, the delivery and release of the active CNTF to trigger regeneration of the damaged retina should be an encouraging therapy. Therefore, our next work would like to fabricate these functional silk biomaterials with CNTF bioactivity for generating different silk materials such as hydrogels to deliver CNTF into the retina for treating retinal degenerative diseases.
Acknowledgments
This work was supported by National Key Research and Development Program of China (No. 2022YFD1201600), National Natural Science Foundation of China (Nos. 32030103 and 32172798), Natural Science Foundation of Chongqing (Nos. cstc2020jcyj-cxttX0001 and CSTB2023NSCQ-MSX0814), and Innovation and Entrepreneurship Training Program of Southwest University (No. S202210635112). We acknowledge the support of the Academy for Advanced Interdisciplinary Studies Device Sharing Service Platform (Equipment No. 18A13430), Southwest University.
Disclosure
The authors declare they have no competing interests.




