Transcriptome analysis and functional study of phospholipase A2 in Galleria mellonella larvae lipid metabolism in response to envenomation by an ectoparasitoid, Iseropus kuwanae
Abstract
There is abundant evidence that parasitoids manipulate their hosts by envenomation to support the development and survival of their progeny before oviposition. However, the specific mechanism underlying host nutritional manipulation remains largely unclear. To gain a more comprehensive insight into the effects induced by the gregarious ectoparasitoid Iseropus kuwanae (Hymenoptera: Ichneumonidae) on the greater wax moth Galleria mellonella (Lepidoptera: Pyralidae) larvae, we sequenced the transcriptome of both non-envenomed and envenomed G. mellonella larvae, specifically targeting genes related to lipid metabolism. The present study revealed that 202 differentially expressed genes (DEGs) were identified and 9 DEGs were involved in lipid metabolism. The expression levels of these 9 DEGs relied on envenomation and the duration post-envenomation. Further, envenomation by I. kuwanae induced an increase in triglyceride (TG) level in the hemolymph of G. mellonella larvae. Furthermore, silencing GmPLA2 in G. mellonella larvae 24 h post-envenomation significantly decreased the content of 4 unsaturated fatty acids and TG levels in the hemolymph. The content of linoleic acid and α-linoleic acid were significantly decreased and the content of oleic acid was significantly increased by exogenous supplement of arachidonic acid. Meanwhile, the reduction in host lipid levels impairs the growth and development of wasp offspring. The present study provides valuable knowledge about the molecular mechanism of the nutritional interaction between parasitoids and their hosts and sheds light on the coevolution between parasitoids and host insects.
Introduction
Parasitoid wasps belonging to the order Hymenoptera used to be known as a kind of critical biocontrol agent in pest management (Wang et al., 2019). Parasitoids can be divided into 2 classes, named ectoparasitoids and endoparasitoids. Generally, the ectoparasitoids paralyze the host permanently by injecting venom prior to oviposition. They lay eggs on the surface of hosts where juvenile parasitoids hatch and acquire nutritional resources for development until they have matured. Conversely, the endoparasitoids usually inject venom into the host hemocoel first and then lay eggs inside the host bodies (Vinson, 1988; Moreau & Asgari, 2015). The developmental patterns of these 2 parasitoid cohorts highlight the crucial role of host nutrient availability and the regulation of host nutrient metabolism by parasitoids for the optimal growth and development of their offspring (Visser et al., 2010; Zhang et al., 2011).
For successful parasitization, parasitoids have developed a smart array of mechanisms to manipulate the host by injecting several virulence factors into the host's body. These so-called parasitization factors, including venom, have been reported to not only suppress the immune response of hosts, but also have effects on host metabolism and development (Beckage & Gelman, 2004; Huang et al., 2008). Available studies regarding the function of venom have primarily concentrated on the immune interaction between parasitoids and their hosts, with particular emphasis on induction of the host's innate immune response encompassing both cellular and humoral immunity (Carton et al., 2008; Yang et al., 2020; Wang et al., 2023). Although the regulation of host nutrient metabolism by parasitoids is a widely observed phenomenon, the specific mechanism involved in the manipulation caused by venom has not been extensively investigated.
Recently, the expression level of genes related to host nutrient metabolism before and after envenomation have been assessed using omics techniques. For instance, 2 differentially expressed genes (DEGs) of Sarcophaga crassipalpis which are related to lipid metabolism were found after being envenomed by Nasonia vitripennis (Danneels et al., 2013).
Phospholipase A2 (PLA2) is a large superfamily of proteins able to cleave oxidized lipids at the sn-2 position on phospholipid (PL) molecules to produce polyunsaturated fatty acids (PUFAs) (Burke & Dennis, 2009). PLA2 is classified into 3 types: Ca2+-independent PLA2s (iPLA2s), Ca2+-dependent PLA2s (cPLA2s), and secretory PLA2s (sPLA2s) (Winstead et al., 2000). sPLA2s mediate cellular immune responses, and iPLA2s have an activity site that is similar to cPLA2s. In mammalians, PLA2s mediate a variety of biological functions, such as lipid digestion, cell membrane remodeling, signal transduction, host immune defense, and the production of various lipid mediators. In vertebrates, PLA2 is associated with the biosynthesis of PUFAs and arachidonic acid (AA). PLA2s play an important role in insect homeostasis, immunity, reproduction, and body fluid secretion (Stanley & Kim, 2014). sPLA2s have traditionally been considered as a constituent of venom proteins in hymenopteran insects (Dudler et al., 1992; Xin et al., 2009; Baek & Lee, 2010). The non-venomous PLA2s were first identified from Tribolium castaneum, and were linked to insect immunity (Shrestha et al., 2010). SeiPLA2A, the first identified insect iPLA2 discovered in Spodoptera exigua, exhibits a functional correlation with the cellular immune reaction (Park et al., 2015). However, whether PLA2 acts as an essential regulator of host lipid metabolism in parasitoids and their hosts associations remains unknown.
Iseropus kuwanae (Hymenoptera: Ichneumonidae) is a preponderant gregarious ectoparasitoid of several lepidopteran pests, such as Rondotia menciana (Lepidoptera: Bombycidae), Chilo suppressalis (Lepidoptera: Pyralidae), and the greater wax moth Galleria mellonella (Lepidoptera: Pyralidae). Given that I. kuwanae female wasps lay their eggs after envenoming their hosts and fully paralyzing them, in this study, we mainly focused on the impact of envenomation on the host prior to oviposition. In the present study, we conducted Illumina sequencing to investigate the global transcriptional responses in G. mellonella larvae which were envenomed by I. kuwanae and identified the DEGs related to lipid metabolism. Meanwhile, we used RNA interference (RNAi) to further explore the function of PLA2 in the lipid metabolism of envenomed G. mellonella larvae. The present results contribute to understanding the host response mechanism for the envenomation of ectoparasitoids and provide fundamental data for further revealing the interaction between parasitic insects and their hosts.
Materials and methods
Insects rearing
The laboratory stock of I. kuwanae was established by collecting cocoons containing parasitized larvae of R. menciana in mulberry fields located at Jiangsu University of Science and Technology, Zhenjiang city, Jiangsu province, China. G. mellonella larvae purchased from Jiyuan Baiyun Biopesticide Co., Ltd (Henan, China) were used as the hosts. The insects were raised in a controlled environment under the following conditions: 25 ± 2 °C, 60%–80% relative humidity and a 14 L : 10 D photoperiod. The adult wasps were raised in glass tubes (12 cm long × 3 cm diameter) where they received daily nourishment consisting of a solution made up of honey diluted to a concentration of around 10%. To maintain the parasitoid colony, female wasps that had already mated were utilized to attack prepupae of G. mellonella (Lepidoptera: Pyralidae) that had been wrapped in cocoons (Ueno, 2016). For mating procedure, 1 female and 1 male wasp were placed together into the glass tube, The male wasp rapidly flapped its wings and chased the female, then the male climbed onto the female and started mating with its externalia inserting into the female's genital orifice. The separation of the couples means the end of mating behavior and the female wasps were removed. One mated female wasp and a single 6th–7th instar host larva were positioned in a plastic box (11.7 cm × 7.1 cm × 5.9 cm) together. After 24 h, the parasitized host larva was put in a Petri dish (8.2 cm diameter × 1.4 cm height) for the development of parasitoid offspring.
Sample collection
Female I. kuwanae wasps typically paralyze their hosts prior to oviposition by inserting ovipositors into the host body and injecting venom. Hence, the occurrence of envenomation behavior we assessed by observing the probing behavior of the parasitoid ovipositor toward the host. Subsequently, 1 female wasp and 1 host were placed in a plastic box. The parasitic wasp was removed from the box once the host had been envenomed. Six hours post-envenomation, the host samples were collected and stored at −80 °C for future RNA sequencing (RNA-seq). Healthy hosts that were not exposed to parasitoids were taken as the control group. Each sample consisted of 3 host larvae, and 3 samples were collected for each treatment.
RNA isolation and complementary DNA (cDNA) library construction
According to the manufacturer's instructions, the mirVana micro RNA (miRNA) Isolation Kit (Invitrogen, USA) was used to isolate total RNA of each group. The quantification and purity of total RNA was estimated by a Nano 2000 spectrophotometer (Thermo Scientific, USA). RNA integrity was assessed with an Agilent 2100 Bioanalyzer (Agilent Technologies, USA). A total amount of 1 µg RNA from each sample was used as an input material to make sample preparations. Subsequently, TruSeq Stranded messenger RNA (mRNA) LT Sample Prep Kit (Illumina, USA) was used to construct the libraries. OE Biotech Co., Ltd. (Shanghai, China) conducted the transcriptome sequencing and analysis.
Quality control and cDNA sequencing
An Illumina HiSeq X Ten platform was used to sequence the libraries to generate 150 bp paired-end reads. The fastq format raw data which eliminated low-quality reads used Trimmomatic (LEADING: 3 TRAILING: 3 SLIDINGWINDOW: 4 : 15 MINLEN:50) (Bolger et al., 2014) to process. After removing adaptor and low-quality sequences, the clean reads were assembled into expressed sequence tag clusters (contigs) and de novo assembled into transcripts by using Trinity (version: 2.4, –seqType fq –SS_lib_type RF) (Grabherr et al., 2011) in paired-end methods. The longest transcript was chosen as a unigene based on the similarity and length of a sequence for subsequent analysis. HISAT2 (–rna-strandness rf –fr) (Kim et al., 2019) was used to map the clean reads to the G. mellonella reference genome (GCA_026898425.1). The clean paired-end sequence dataset has been uploaded to the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) Database under accession numbers SRR27693291–SRR27693293 for non-envenomed larvae groups (biosample accession numbers SAMN39557054–SAMN39557056) and SRR27693288–SRR27693290 for envenomed larvae groups (biosample accession numbers SAMN39557057–SAMN39557059).
Differential gene expression analysis
The fragments per kilobase of transcript per million mapped reads (FPKM) value of each gene was calculated using cufflinks (–compatible-hits-norm) (Trapnell et al., 2010), and the read counts of each gene were obtained based on htseq-count (-s reverse) (Anders et al., 2015). The differential expression analyses between envenomed and non-envenomed G. mellonella larvae were identified using the DESeq (2012) R package (Anders & Huber, 2013) to function estimateSizeFactors and nbinomTest. The threshold criteria set as a P-value < 0.05 and |log2 fold change| > 1 were used to define significant differential expression. Hierarchical cluster analysis of DEGs was conducted to investigate gene expression patterns. Gene Ontology (GO) enrichment and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway (Kanehisa et al., 2008) enrichment analysis of DEGs were performed using R based on the hypergeometric distribution.
Quantitative reverse transcription – polymerase chain reaction (qRT-PCR)
To measure the expression pattern of the GmPLA2 gene after envenomation by I. kuwanae, G. mellonella larvae were collected at 1, 6, 12, 24, 36, 48, 72, and 96 h after envenomation and non-envenomed larvae were also collected as controls at the same time intervals. There were 3 biological replicates for each sampling time point and each biological replicate consisted of tissues from multiple individuals. Further, they were all the same genotype. Tissues (epidermis, hemolymph, head, silk gland, fat body, Malpighian tube, foregut, midgut, and hindgut) of G. mellonella were also collected at 24 h after envenomation. The RNA of each sample was isolated using RNAiso Plus reagent (Takara, Japan). Subsequently, the concentration and quality of RNA were assessed with a Nanodrop 2000 spectrometer (Thermo Scientific, USA) and an Agilent Bioanalyzer 2100 (Agilent Technologies, USA). Meanwhile, 1% agarose gel electrophoresis was used to check the RNA integrity. One microgram total RNA was used to reverse transcribe the cDNAs by HiScript® III 1st Strand cDNA Synthesis Kit (+gDNA wiper) (Vazyme, China). The cDNAs which were diluted to 60 ng/µL with RNase-free H2O were prepared for qRT-PCR and stored at −20 °C.
Nine DEGs (GmFAS1, GmFAS2, GmDesat, GmPLA2, GmLDAP1, GmUGT2B9, GmJHE, Gmlipase3, and Gmlipase1) involved in G. mellonella lipid metabolism were selected to ensure the effectiveness of RNA-seq and we detected the expression pattern of GmPLA2 gene in the whole body and different tissues of envenomed G. mellonella by qRT-PCR. The elongation factor 1 alpha (EF1-α) (LOC113518486) was employed as the reference gene for normalizing the relative expression of genes (Grizanova et al., 2019). The open reading frame finder (orffinder: https://www.ncbi.nlm.nih.gov/orffinder/) was used to identify the ORF sequences of these DEGs. The primers used in qRT-PCR analysis were designed with Primer-BLAST (https://www.ncbi.nlm.nih.gov/tools/primer-blast/) and are listed in Table S1. Every 20 µL reaction system consisted of 0.8 µL of forward primer, 0.8 µL of reverse primer, 10 µL of 2 × ChamQ SYBR qPCR Master Mix (Vazyme, China), 6.4 µL of ddH2O and 2 µL of template cDNA. The PCR program was as follows: 95 °C for 5 min followed by 40 cycles of 95 °C for 15 s and 60 °C for 31 s. The 2−ΔΔCT method was used to determine the relative gene expression levels.
Bioinformatics analysis of PLA2 gene in G. mellonella
The sequence of GmPLA2 was identified from the constructed G. mellonella comparative transcriptome database. We used orffinder (https://www.ncbi.nlm.nih.gov/orffinder/) to predict the ORFs of GmPLA2 gene. The NCBI Conserved Domain Search website (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) was used to predict the protein functional domains. The amino acid sequences encoded by the PLA2 gene of other species were downloaded from NCBI and multiple alignments of various protein sequences were employed by GeneDoc. Phylogenetic analysis was conducted with the neighbor-joining method by using the Molecular Evolutionary Genetic Analysis 11.0 (Tamura et al., 2021) software with 1 000 bootstrap replications. The circular phylogenetic tree was generated by the Interactive Tree Of Life (iTOL) online tool (https://itol.embl.de).
RNAi in envenomed G. mellonella larvae
The BLOCK-iTTM RNAi Designer (https://rnaidesigner.thermofisher.com/) was used to design the oligonucleotide sequences (Table S2). A transcription T7 kit (Takara, Japan) was employed to synthesize siRNA in vitro according to the guidelines provided by the manufacturer. Additionally, siRNA derived from the green fluorescent protein (GFP) gene was also synthesized as a negative control. Subsequently, the concentration and quality of RNA were assessed by Nanodrop 2000 spectrometer (Thermo Scientific, USA) and an Agilent Bioanalyzer 2100 (Agilent Technologies, USA). Meanwhile, the integrity of RNA was checked with 1% agarose gel electrophoresis.
One microliter (1 000 ng/µL) of siRNA was injected into G. mellonella larvae at 24 h after envenomation using a microsyringe (Drummond Scientific, USA). The same method was used to supply AA (80 µg/larva) to G. mellonella larvae at 24 h after siRNA injection. The samples were collected at 24 h post-siGFP and siGmPLA2 injection. Subsequently, total RNA of samples was extracted, and the cDNA was synthesized to determine the RNAi efficiency using qRT-PCR. There were 3 biological replicates for each treatment (siGFP and siGmPLA2) and each replicate consisted of samples from multiple individuals.
Measurement of triglyceride (TG) level
The TG content of G. mellonella larvae hemolymph was estimated by using a TG enzyme-linked immunosorbent assay (ELISA) kit (BYabscience, China) at 12, 24, 36, and 48 h after envenomation by I. kuwanae and 24 h after siRNA injection, according to the guidelines provided by the manufacturer. In short, G. mellonella larvae hemolymph was collected, and 0.1 g sample was used for the experiment. The samples were centrifuged for 10 min at 12 000 × g at 4 °C, and the supernatants were transferred to 1.5 mL Eppendorf (EP) tubes for detection. Subsequently, the test reagent and sample were added to the new EP tubes in accordance with the provided instructions, and then incubated under ambient conditions for 20 min in a dark environment after being mixed. Then 200 µL of the mixture was transferred into a 96-well plate, and measured with a spectrophotometer (Thermo 1500, USA) at 510 nm. There were 3 biological replicates for each treatment (siGFP and siGmPLA2) and each replicate consisted of samples from multiple individuals.
Determination of fatty acid content
The method for measuring the fatty acid content was based on Wang et al. (2022) with slight modifications. Briefly, 400 μL of hemolymph from envenomed G. mellonella larvae was collected at 24 h after siGFP and siGmPLA2 injection, then transferred to a 10 mL centrifuge tube and 1 mL n-hexane was added. Following blending, an additional 1.5 mL of n-hexane and 2 mL of 0.5 mol/L KOH-CH3OH solution were added to the mixture. Incubation at 60 °C for 1 h ensued. After cooling to room temperature, 4 mL of ultrapure water was added and the resulting mixture underwent centrifugation at 10 000 × g for 10 min. The supernatant was transferred to 2 mL EP tubes in preparation for gas chromatography (GC) analysis.
The contents of palmitic acid, oleic acid, linoleic acid, and α-linoleic acid were measured with gas chromatograph (8890 GC system, Agilent, USA). Chromatographic separation was conducted through a HP-INNOWax column (30 m × 0.32 mm × 0.25 µm) (Agilent, USA). Initially, the oven temperature was set at 200 °C for 1 min before gradually increasing to 240 °C at the rate of 1.5°C/min and held for another minute. The detector was operated at 280 °C and the injector was operated at 250 °C. Nitrogen served as the carrier gas at a flow rate of 1 mL/min. The split ratio was 50 : 1 and the injection volume was 2 μL per sample.
Nile red staining
Nile red staining was used to observe lipids in hemolymph from envenomed G. mellonella larvae 24 h post-siGFP and siGmPLA2 injection. The same volumes of hemolymph (200 µL) were dissected from siGFP and siGmPLA2-treated groups, followed by centrifugation at 12 000 × g for 5 min. The supernatant was removed and then 4% paraformaldehyde was used for fixing to a glass slide for 30 min at a temperature of 4 °C. Following fixation, the sample was washed 3 times with 1 × phosphate-buffered saline (PBS) solution each for 5 min. To stain lipids, the sample was exposed to a diluted solution of Nile red (1 mg/mL) in 1 × PBS at a ratio of 1 : 1 000 for a duration of 30 min, followed by 3 additional washes using 1 × PBS. The samples were visualized under a fluorescent microscope (IX 73, OLYMPUS, Japan). ImageJ (Schneider et al., 2012) was applied for fluorescence intensity analysis of lipid droplets in the hemolymph of G. mellonella larvae from the siGFP group and siGmPLA2 group.
Development of I. kuwanae offspring
To explore the influence of GmPLA2 on the development of I. kuwanae progeny after envenomation, the number of I. kuwanae larvae, prepupae and adults were recorded to calculate the rate of pupation, and the rate of wasp emergence for each group. In addition, the weight of I. kuwanae larvae and prepupae were measured to compare the differences between each treatment. First, to collect enough eggs for the experiment, healthy G. mellonella larvae were exposed to I. kuwanae female wasps with 7 d parasitic experience for 1 h. After siRNA injection, 10 eggs were removed and placed on the surface of envenomed G. mellonella larvae at 24 h post-injection. Each G. mellonella larva with 10 eggs of I. kuwanae was positioned in a Petri dish until the wasp emerged. The rate of pupation was calculated as the number of prepupae divided by the number of larvae. The rate of wasp emergence was calculated as the number of wasp adults divided by the number of eggs.
Statistical analysis
All statistical analyses were performed using R version 4.0.0 software. One-way analysis of variance (ANOVA) followed by Tukey's test, with a significance threshold set at P < 0.05, was used to analyze differences in the relative GmPLA2 mRNA levels, the TG content, and the weight of I. kuwanae prepupae and adults. Student's t-test was used to determine the significance of the relative GmPLA2 mRNA levels, the TG content, the content of fatty acids, and fluorescence intensity of lipid in G. mellonella larvae between the siGFP and siGmPLA2 groups. The larval hatching, pupation, and wasp emergence rates were analyzed with 2 × 2 Chi-square test, with a significance threshold of P < 0.05.
Results
Transcriptome of G. mellonella larvae after envenomation
A sum of 42.48 G clean reads was acquired. Each sample yielded an effective data volume ranging from 6.66 G to 7.48 G, with a quality Q30 value of ≥93.52% and an average GC content of 42.20% (Table S3). These reads were then mapped to G. mellonella reference genome (GCA_026898425.1), resulting in a mapping rate of 87.21%–88.53% for each sample.
Functional annotation and classification
To describe the function of protein and uncover the global effect of envenomation on host larvae, we conducted a GO classification and performed KEGG pathway analysis on the assembled genes. We annotated a total of 9 434 genes and assigned them to GO terms. In terms of the biological process categories, the majority (7 016 genes, 74.37%) were associated with “cellular process”. In the cellular component and molecular function, the most prevalent group were “cell” (7 785 genes, 82.52%) and “binding” (5 849 genes, 62.62%), respectively (Fig. S1). Sample-to-sample cluster analysis results are showed in Fig. S2 and this analysis is based on the correlation coefficient between samples. Calculation was performed using the overall gene expression levels from the samples with Pearson correlation algorithm.
Enrichment analysis of DEGs
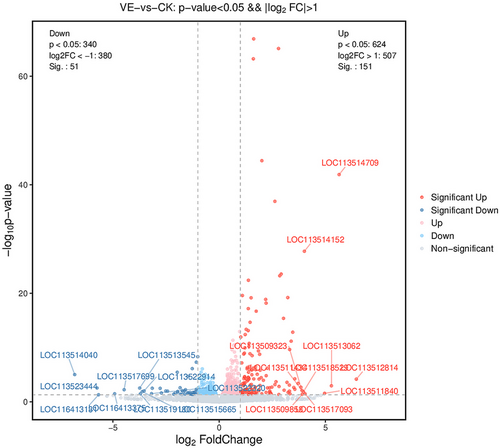
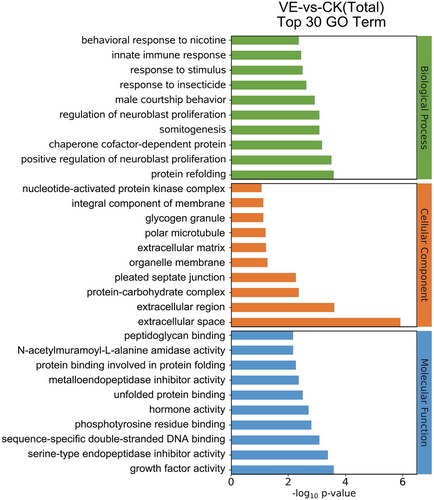
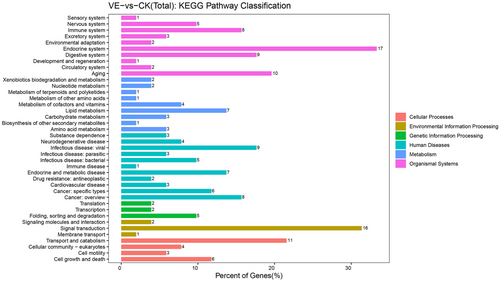
After envenomation, the RNA-seq data screened 202 genes as DEGs, 151 were upregulated, and 51 were downregulated compared to the non-envenomed group (Table S5, Fig. 1). The GO enrichment analysis enriched 120 significant DEGs (P < 0.05) in 3 main clusters and the top 30 terms are shown in Fig. 2. Among these subcategories, “integral component of membrane” includes the largest number of DEGs (29, 24.2%), followed by “extracellular region” (19, 15.8%). We noted 51 significantly enriched DEGs in 40 sub-pathways (Fig. 3). DEGs-enriched KEGG pathways are showed in Fig. 3. Interestingly, the sub-pathways “lipid metabolism”, “amino acid metabolism”, and “carbohydrate metabolism” were enriched, suggesting envenomation significantly influenced the nutrition metabolism in the host larvae.
Effects of envenomation of lipid metabolism-related genes in G. mellonella larvae
We identified 9 lipid metabolism-related DEGs with 6 DEGs upregulated and 3 DEGs downregulated (Table 1). Based on the molecular functions, these DEGs mainly encode enzymes that participate in fatty acid biosynthesis, glycerophospholipid metabolism, steroid biosynthesis, steroid hormone biosynthesis, biosynthesis of unsaturated fatty acids, and lipid transport. There were 2 upregulated fatty acid synthase genes, and we also found that envenomation upregulated 2 genes related to the biosynthesis of unsaturated fatty acids, namely acyl-CoA Delta (11) desaturase (GmDesat) and GmPLA2. UDP-glucuronosyltransferase 2B9-like (GmUGT2B9) involved in steroid hormone biosynthesis and lipase 1-like (Gmlipase1) involved in steroid biosynthesis were upregulated in G. mellonella larvae after envenomation. Moreover, we discovered that low density lipoprotein receptor adapter protein 1 gene (GmLDAP1) was downregulated in the host after envenomation. Meanwhile, the gene encoding juvenile hormone esterase (GmJHE) was downregulated.
| Acc. no. | Description | Log2 fold change | P-value | Up/Down | Pathway |
|---|---|---|---|---|---|
| LOC113509353 | Calcium-independent phospholipase A2-gamma-like | 1.299 | P < 0.05 | Up | Biosynthesis of unsaturated fatty acids |
| LOC113512308 | Acyl-CoA Delta (11) desaturase-like | 1.243 | P < 0.01 | Up | |
| LOC113515167 | Fatty acid synthase-like | 1.879 | P < 0.01 | Up | Fatty acid biosynthesis |
| LOC113515168 | Fatty acid synthase-like | 2.081 | P < 0.001 | Up | |
| LOC113522417 | Low-density lipoprotein receptor adapter protein 1-like | −1.181 | P < 0.05 | Down | Lipid transport |
| LOC113511434 | UDP-glucuronosyltransferase 2B9-like | 3.898 | P < 0.01 | Up | Steroid hormone biosynthesis |
| LOC113509809 | Juvenile hormone esterase-like | −1.076 | P < 0.01 | Down | Glycerophospholipid metabolism |
| LOC113518373 | Lipase 3-like | −1.949 | P < 0.05 | Down | Steroid biosynthesis |
| LOC116413369 | Lipase 1-like | 1.569 | P < 0.01 | Up |
qRT-PCR
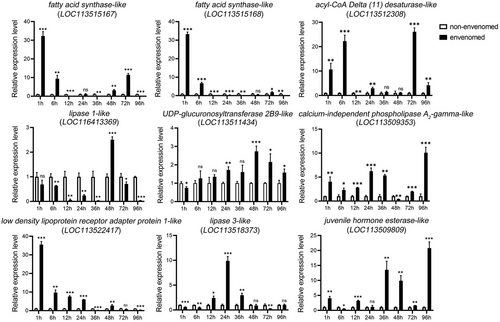
We selected 9 DEGs (GmFAS1, GmFAS2, GmDesat, GmPLA2, GmLDAP1, GmUGT2B9, GmJHE, Gmlipase3, and Gmlipase1) related to lipid metabolism for qRT-PCR. To explore the expression patterns of DEGs at various time intervals following envenomation, the expression levels at 1, 6, 12, 24, 36, 48, 72, and 96 h were also estimated (Fig. 4). First, 6 DEGs were more highly expressed in the envenomed group within a short period. Two fatty acid synthase genes (LOC113515167 and LOC113515168) and GmLDAP1 (LOC113522417) were upregulated more than 30-fold at 1 h after envenomation. GmDesat (LOC113512308) was upregulated more than 20-fold and GmFAS1, GmFAS2, and GmLDAP1 were upregulated close to 10-fold in the envenomed group at 6 h after envenomation. Because parasitoid larvae usually hatch 24–48 h after the eggs are laid, we focused on gene expression at 24 and 48 h after envenomation. Among these selected DEGs, Gmlipase3 (LOC113518373) was upregulated 10-fold at 24 h after envenomation and GmJHE (LOC113509809) was upregulated 10-fold at 48 h after envenomation. In the envenomed group, GmPLA2 expression was higher at almost each time point compared to the non-envenomed group.
Bioinformatics analysis of the GmPLA2 gene in G. mellonella
The sequence of GmPLA2 contained complete ORFs of 1 758 bp which encoded 585 amino acid residues. Protein motif analysis showed that the C-terminal region contained a patatin domain (Fig. 5A). Phylogenetic analysis of other PLA2s revealed that GmPLA2 is clustered with the iPLA2-specific group (Fig. 5B). We found that the lipase (GxSxG) and nucleotide-binding (GxGxxG) domains are conserved in both groups (Fig. 5C).
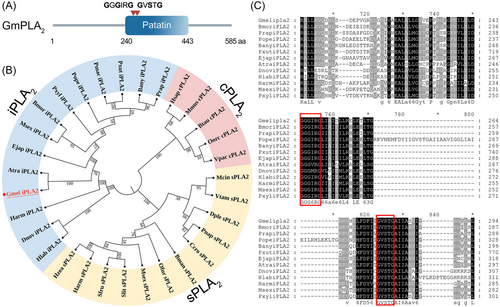
Tissue expression pattern of GmPLA2
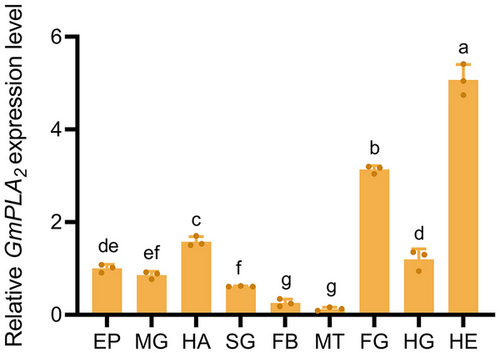
We estimated the expression level of GmPLA2 in different tissues of G. mellonella at 24 h after envenomation. GmPLA2 demonstrated the highest expression in the head, followed by the foregut and hemolymph, and the lowest expression in Malpighian tubules (Fig. 6).
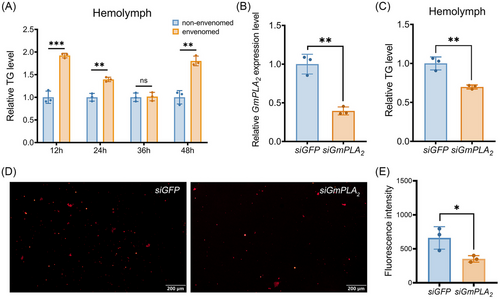
Effect of silencing GmPLA2 on lipid accumulation in G. mellonella larvae
To investigate the impact of I. kuwanae envenomation on the lipid metabolism in G. mellonella larvae, we estimated the TG content of G. mellonella larvae after being envenomed within 48 h. Compared to the non-envenomed group, the TG level was dramatically increased in the hemolymph (Fig. 7A). To investigate the influence of silencing GmPLA2 on lipid accumulation in G. mellonella larvae hemolymph, RNAi-mediated knockdown of GmPLA2 was performed. At 24 h after the injection of siGmPLA2, there was a significant downregulation of GmPLA2 mRNA levels (Fig. 7B). Furthermore, we detected a significant decrease of the TG content in the hemolymph of G. mellonella larvae after siGmPLA2 injection (Fig. 7C). Nile red staining indicated a notable decrease in lipid droplets density at 24 h after the injection of siGmPLA2 (Fig. 7D, E). GC analysis showed that the content of 4 unsaturated fatty acids (palmitic acid, oleic acid, linoleic acid, and α-linoleic acid) decreased in the hemolymph of G. mellonella larvae after silencing GmPLA2 (Fig. 8).
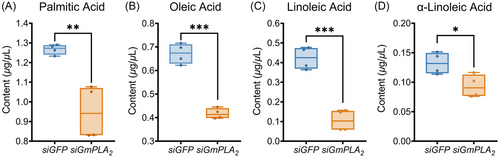
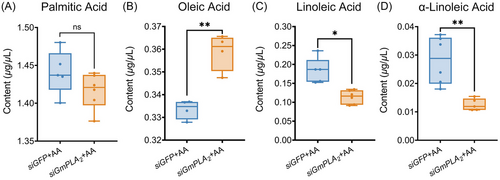
To further validate the impact of GmPLA2 silencing on the specific phospholipid hydrolysis reaction in G. mellonella larvae following envenomation, we successfully silenced GmPLA2 and administered exogenous AA through injection. GC analysis revealed a significant decrease in the content of linoleic acid and α-linolenic acid, while the content of oleic acid significantly increased, and the content of palmitic acid remained unchanged (Fig. 9).
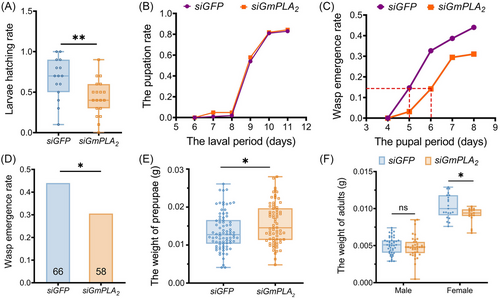
Effect of silencing GmPLA2 on the development of I. kuwanae offspring
Silencing GmPLA2 had a significant effect on the larvae hatching rate, with a decrease in the siGmPLA2 injection group (Fig. 10A), while there was no notable influence on the larval period and the pupation rate of I. kuwanae (Fig. 10B). Most importantly, I. kuwanae in the siGmPLA2 injection group exhibited a developmental delay in pupal stage compared with the siGFP injection group (Fig. 10C). We also found a significant difference in the wasp emergence rates between each treatment: the GmPLA2 injection group had its emergence rate reduced by 13.5% (Fig. 10D). In addition, we measured the body weight of prepupae and adult wasps. The weight of prepupae exhibited a significant difference between the 2 groups, with the siGmPLA2 injection group demonstrating a higher weight compared to the control group. Furthermore, the body weight of adult female wasps was significantly reduced in the siGmPLA2 injection group than in siGFP injection group (Fig. 10E, F).
Discussion
Successful biocontrol practices require a comprehensive understanding of the interaction between insect pests and their natural enemies. Parasitoid wasps rely on their hosts as their main nutritional resource to guarantee their growth and development (Rajendran et al., 2017). Envenomation by parasitoids induces complicated regulation in the host that involves a series of alterations in the immune system, nutrient metabolism, development, and reproduction (Pennacchio et al., 2014; Shi et al., 2019). Serine protease, lipase, phosphatase A1, glucosidase, enolase, and other enzymes that are important for digestion or catabolism are commonly found in the venom of parasitoids and may also have the function of regulating host nutrition metabolism (Teng et al., 2017; Xin et al., 2017; Zhao et al., 2017; Lin et al., 2019). The lipase or esterase in venom may be related to the degradation of the host fat body (Richards, 2012; Heavner et al., 2013). Research indicated that dipeptidyl peptidase IV (DPPIV) is a major venom component of an ectoparasitoid, Scleroderma guani, and has been shown to be associated with host lipid synthesis and metabolism (Wu et al., 2023).
Recent research findings have indicated that endoparasitoids have developed strategies to influence their host nutrient levels, including lipid metabolism. This evolution is crucial for fulfilling the dietary needs essential for the growth and development of their offspring (Wang et al., 2021a, 2021b; Song et al., 2022a, 2022b). Different from endoparasitoids, the ectoparasitoid I. kuwanae paralyzes the host first by inserting an ovipositor to inject the venom and then lay eggs on the surface of the host. Thus, it can be speculated that the changes in G. mellonella larvae post-venom injection should be closely monitored, as this could help to further understand the function of the venom.
Here, we employed RNA-seq to identify the DEGs of G. mellonella larvae after envenomation by I. kuwanae. In total, 202 DEGs were identified from the non-envenomed and envenomed groups. Nine DEGs were found to be related to lipid metabolism in G. mellonella larvae. Interestingly, the amount of DEGs identified in this study is relatively small compared with what has been reported in other parasitic systems (Zhu et al., 2013; Tang et al., 2014; Sheng et al., 2021). For instance, 1 861, 962 and 108 DEGs were found in Spodoptera frugiperda at 2, 24, and 48 h, respectively, after parasitization by Microplitis manilae (Gulinuer et al., 2023). However, only 1 gene was differentially expressed in S. crassipalpis at 3 h post-envenomation and 128 genes expressed differentially at 25 h post-envenomation of N. vitripennis (Danneels et al., 2013). We suspect that the number of DEGs in the I. kuwanae and G. mellonella parasitic system is related to the time from which the host was injected with the venom. In contrast to the previous studies focusing on the changes in hosts post-oviposition by parasitoids, we investigated the early stage of interaction of the I. kuwanae–G. mellonella interaction, so we collected G. mellonella samples after the injection of parasitoid venom but prior to egg laying. Therefore, our findings solely represent the initial phase of the G. mellonella response toward I. kuwanae venom.
The main reason for the changes in host nutritional substances is that parasitization upregulates or downregulates the transcriptional levels of nutritional metabolism-related genes in the parasitized host. For example, in parasitized Aphis gossypii, the mRNA level of sterol carrier protein 2, phospholipid-transporting ATPase, acetyl-CoA acyltransferase, 3-hydroxyacyl-CoA dehydrogenase, and enoyl-CoA hydratase are all upregulated (Gao et al., 2020). The upregulation of genes associated with the production of lipids could potentially elucidate the mechanism behind the significant elevation in TG levels observed in G. mellonella larvae hemolymph following envenomation by I. kuwanae (Fig. 7A). The abundance of enoyl-CoA hydratase and 3-hydroxyacyl-CoA dehydrogenase, which participated in fatty acid metabolic processes increased in Helicoverpa armigera at 6 h following parasitization by Microplitis mediator (Lin et al., 2019). Our findings are similar to previous work on different parasitic systems such as A. gossypii–Lysiphlebia japonica (Gao et al., 2020) and S. exigua–Chelonus inanitus (Kaeslin et al., 2005), for which the focus is on parasitoid manipulation of host lipid metabolism.
Among the 9 DEGs related to lipid metabolism, 6 exhibited upregulation and 3 demonstrated downregulation. GmFAS and GmDesat were found upregulated in G. mellonella larvae post-envenomation which have been reported to be associated with lipogenesis (Gondim et al., 2018). Fatty acid synthetase is a multifaceted enzyme which catalyzes fatty acid synthesis and involves the utilization of malonyl-CoA to progressively incorporate 2-carbon units onto 2-carbon units to an acetyl-CoA primer (Maier et al., 2010). According to a recent investigation, Δ11 desaturation showed a crucial role in the production of pheromone components, which are synthesized de novo from acetyl-CoA in the pheromone gland through fatty acid synthesis (Yoshiga et al., 2000). Furthermore, we found DEGs related to unsaturated fatty acid biosynthesis. PLA2s in insects are closely related to the biosynthesis of PUFAs and eicosanoic acids. Category II decanoic acid mainly includes prostaglandins, lipoxygenase, and cyclooxygenase (Valentin & Lambeau, 2000). In short, these findings imply that venom injection may have induced alterations in the production of unsaturated fatty acids in the host. In the present study, 3 DEGs, GmLDAP1, GmJHE, and Gmlipase3, were downregulated. Due to the lipid synthesis-elated genes and their regulation models being abundant, it is possible that the expression levels of DEGs may either increase or decrease. Further studies are required to validate the detail regulation mechanism of these downregulated DEGs.
Another interesting finding was the significant upregulation of GmPLA2 after envenomation. Given that most research related to insect PLA2s has focused mainly on their function in immunity, we aimed to evaluate the effect of PLA2 on host lipid metabolism after envenomation. GmPLA2 was notably elevated in G. mellonella larvae 24 h post-envenomation. We previously found that the egg stage of I. kuwanae is 1.51 ± 0.5 d (unpublished data), and the results showed that expression level of GmPLA2 was significantly upregulated at 24 h post-envenomation. These changes can perhaps be explained by an increase in the abundance of nutrients to help parasitoid juveniles develop. In the envenomed G. mellonella larvae, GmPLA2 expressed highest in the head, which is similar with BmiPLA2B (Hu et al., 2022). The highest expression level of GmPLA2 in the head may be explained in that GmPLA2 plays vital roles in the physiological processes in the head. Because the host is paralyzed and unable to feed, we hypothesized that the envenomation of I. kuwanae regulates lipid accumulation of the host, inducing the mobilization of nutrients in the head of hosts to maintain its own lipid metabolism and synthesis. Meanwhile, the impact of envenomation on the nervous system of G. mellonella larvae may be responsible for this outcome as well.
Studies have shown that PLA2 affects lipid levels in insects. For example, silencing PLA2 influenced the development of lipid droplets in fat body cells which indicated that PLA2 affects the function of fat body tissue in Bactrocera dorsalis (Li et al., 2017). Culturing S. exigua midgut in vitro and silencing Se-sPLA2 significantly inhibited the activity of sPLA2 in the intestine of S. exigua larvae and reduced the lipid digestibility of S. exigua larvae (Sajjadian et al., 2019). The PLA2 enzymes have been identified as key regulators of lipid droplet homeostasis (Guijas et al., 2014). Similarly, we successfully knocked down GmPLA2 in G. mellonella at 24 h after envenomation and a significant reduction in TG level in the hemolymph was observed. Additionally, PLA2s catalyzed phospholipid decomposition, resulting in the production of unsaturated fatty acids (Guijas et al., 2014). The quantification of 4 unsaturated fatty acids in G. mellonella larvae hemolymph also revealed a decrease following the injection of siRNA. Similarly, Song et al. (2022a, 2020b) reported that silencing SlFAS1 induced a decrease in α-linoleate content in Spodoptera litura larvae. Knocking down BgACC in Blattella germanica also significantly downregulated the total free fatty acid level (Pei et al., 2024).
Meanwhile, the content of linoleic acid and α-linoleic acid in the hemolymph of G. mellonella larvae were decreased significantly and the oleic acid content was significantly increased by exogenous supplement of AA. The obtained results indicate that exogenous supplementation of AA can facilitate the reversible reaction of GmPLA2 specific hydrolysis of PLs, which necessitates further experimental validation in the future. Taken together, the above results indicate that GmPLA2 plays a crucial role in lipid accumulation in G. mellonella.
Our study also reveals that the development of I. kuwanae was impaired, when emerging from the siGmPLA2-injected G. mellonella larvae in which lipid content was lower than that of the control (Fig. 10). Parasitoids emerged from the siGmPLA2 injection group, and exhibited a significantly reduction in the larvae hatching rate and emergence rate compared to the control group. Additionally, a prolonged pupal stage development period and lower weight in female offspring of I. kuwanae were also observed in the siGmPLA2 RNAi-treated group. However, we found that the GmPLA2 silencing resulted in an increase in the weight of wasp prepupae which deviated from our hypothesis. One possible explanation is the inability to distinguish male and female parasitoids during the pre-pupal stage. Moreover, it is commonly observed that female parasitoids exhibit a larger body size throughout their growth and development, which could be attributed to variations in the male-to-female ratio between the treatment group and control group. Our findings align with previous studies demonstrating that alterations in the nutritional status of the host exert substantial impacts on the fitness of parasitoid offspring. The development and parasitism rate of Cotesia vestalis were significantly affected, which emerged from dsPxTK- and dsPxTKR RNAi-treated Plutella xylostella larvae with higher TG levels than normal (Wang et al., 2021a). Suppressing the ecdysis of Pseudaletia separata results in a reduction in the amount in food resource (e.g., fat body) for the larvae of Cotesia kariyai. The findings indicated that the growth of the host was impeded, leading to a decrease in host quality and exerting negative impacts on the fitness of female parasitoids (Nakamatsu et al., 2006). Knocking down SiTret1 or SiTret2 induced a significant decrease in trehalose activity and the glucose content in the host larvae, with the parasitoid offspring fitness also being negatively affected, including the reduction in emergence rate, increased proportion of abnormal adults, and smaller body size (Song et al., 2022b). Further, with the aid of gut microbiota, female L. boulardi activate the insulin signaling pathway of its host Drosophila melanogaster, inhibiting lipid degradation and promoting increased lipid accumulation of the host to support the growth and development of parasitoid offspring (Zhou et al., 2022).
In summary, we constructed a transcriptome database of DEGs in G. mellonella larvae envenomed by the ectoparasitoid I. kuwanae. We identified 202 DEGs, of which 9 were related to lipid metabolism of G. mellonella larvae. We discovered that I. kuwanae significantly enhanced the lipid level in the host hemolymph by upregulating the expression of GmPLA2 and demonstrated that GmPLA2 plays a crucial role in the developmental of wasp offspring. This study offers a novel perspective on the mechanism of regulating host lipid metabolism by ectoparasitoids and contributing understanding for further research on the interaction between ectoparasitoids and their hosts.
Acknowledgments
This work was supported by the Key Research and Development Program (Modern Agriculture) of Zhenjiang City (NY2022005), the Qing Lan Project of Jiangsu Province (year of 2021), the Earmarked Fund for CARS-18, and the Postgraduate Research & Scientific Research Innovation Program of Jiangsu Province (SJCX23_2232).
Disclosure
All the authors have reviewed and agree with the contents of the manuscript. We confirm there are no conflicts of interest, including specific financial interest, relationships and affiliations relevant to the subject of the manuscript.
Open Research
Data availability statement
The data that support the findings of this study are openly available in Zenodo repository at http://doi.org/10.5281/zenodo.12633552.




