Entomopathogenic Bacillus cereus impairs the fitness of the spotted-wing drosophila, Drosophila suzukii
Abstract
Drosophila suzukii is a notorious pest which causes devastating damage to thin-skinned fruits, and the larvae feed on the fruit, causing extensive agricultural economic loss. The current application of insecticides to manage this pest results in serious resistance and environmental hazards, so an alternative strategy for D. suzukii biocontrol is urgently needed. Here, we reported that entomopathogenic Bacillus cereus has the potential to biocontrol D. suzukii. We isolated and identified the bacterial strain, B. cereus H1, that was detrimental to the fitness of both D. suzukii progenies and parents. D. suzukii was robustly repelled to depositing eggs on the halves with metabolites of B. cereus H1. Both males and females of D. suzukii were susceptible to B. cereus H1. B. cereus H1 significantly arrested larval development with at least 40% lethal larvae. The median lethal time (LT50) of males and females of D. suzukii challenged with B. cereus H1 was 3 and 2 d, respectively. Moreover, B. cereus H1 disrupted the intestinal integrity and pH value of D. suzukii and resulted in an increase in bacterial load of guts and hemolymph. Mechanistically, infection of B. cereus H1 led to the activation of the dual oxidase (DUOX)-ROS-Jun N-terminal kinase (JNK) pathway. The findings showed that the entomopathogen B. cereus H1 could potentially act as a biological control agent against D. suzukii, advancing fundamental concepts of integrated pest management programs against D. suzukii.
Introduction
The spotted-wing drosophila, Drosophila suzukii (Matsumura), is one of the most destructive polyphagous pests in soft-skinned fruits, including blueberries, strawberries, cherries, and raspberries (McIntosh et al., 2022; Vijayan et al., 2022; Lisi et al., 2023). It is endemic to East Asia, and currently widespread throughout the Americas, Europe, Oceania, and Asia as an invasive species (Garcia et al., 2022). Female flies are characterized by a serrated ovipositor that pierces the skin of intact and ripening fruits (Bošković et al., 2023; Puppato et al., 2023). They lay eggs inside fruit flesh, which enables their progenies to attack ripening fruit (Karageorgi et al., 2017). With a fast lifecycle of 8–9 d and a high reproductive rate of ∼450 eggs per female at optimal conditions, D. suzukii is causing an extensive agricultural economic loss in a short time (Wang et al., 2021; Hanson et al., 2023; Tungadi et al., 2023). The common strategy for management of this pest still heavily relies on environmentally hazardous chemical pesticides applied at weekly or shorter intervals to ripening fruits (Sharma et al., 2020; Disi & Sial, 2021; Ganjisaffar et al., 2022). However, the hatching larvae inside the fruit are protected from direct exposure to insecticides. Moreover, frequent insecticide applications have disturbed integrated pest management (IPM) programs and enhanced the risk of insecticide resistance (Biondi et al., 2021; Gress & Zalom, 2022).
Due to host specificity (Brodeur, 2012), insect pathogens provide one promising alternative strategy to control this pest in agriculture. Entomopathogens, such as Bacillus thuringiensis and Beauveria bassiana, have been used to control pests for decades (Babin et al., 2023; Hanson et al., 2023; Wang et al., 2023). Recently, more entomopathogens, Serratia marcescens PS-1 or Leuconostoc pseudomesenteroides, have become important biopesticides and are widely applied for the biocontrol of D. suzukii (Zeng et al., 2020; Eski et al., 2024). Despite great progress in pest biocontrol, other unidentified entomopathogens suitable for D. suzukii biocontrol urgently need to be exploited. B. thuringiensis is extensively applied to the biological control of many insects, but the Bacillus cereus group is traditionally composed of 6 species, with the presumption that specific strains of this group have potential in pest biocontrol (Morandini et al., 2024).
The intestine, representing one of the largest interplays between the host's internal and external environments, is a major entry for many pathogens, including bacteria, viruses, fungi, and parasites (Barbara et al., 2021). The intestinal epithelia generate a gut barrier that physically separates the gut from many insults by entomopathogens (Wang et al., 2024). However, pathogens have evolved a defense strategy to evade colonization resistance, making the gut susceptible to various injuries. Many entomopathogenic bacteria induce intestinal hyperpermeability and barrier dysfunction, resulting in bacterial translocation and subsequent systemic infection (Xu & Foley, 2024). Therefore, pathogens can be postulated to compromise the lifespan by accelerating the onset of intestinal barrier defects. On the other hand, the epithelia quickly respond to these aggressions and undergo turnover to maintain intestine integrity. Mounting studies found that dual oxidase (Duox)-mediated generation of reactive oxygen species (ROS) is a main immune mechanism that conservatively regulates microbe-intestine homeostasis of insects (Liu et al., 2022; Prakash et al., 2023). Duox gene expression was specifically associated with either Jun N-terminal kinase (JNK) or extracellular signal-regulated kinase, but not any of the other immune signaling modulators, in the mosquito and Drosophila midgut (JNK-dependent intestinal barrier failure disrupts host-microbe homeostasis during tumorigenesis). Moreover, JNK-dependent intestinal barrier failure disrupts host-microbe homeostasis in tumorigenesis (Zhou & Boutros, 2020; Liu et al., 2022). However, the mechanism through which immune activation leads to pathogen-specific pathology and mortality remains elusive.
To address these questions, we attempted to identify entomopathogenic bacteria that caused morbidity and mortality of D. suzukii. We tested the impact of entomopathogenic B. cereus on the survival of D. suzukii and elucidated the underlying mechanism of the insecticidal activity. Our findings highlighted a valuable alternative D. suzukii biocontrol source in agricultural production.
Materials and methods
Fly culture and stocks
Flies were reared and maintained in the condition of 25 °C, 60% humidity with a 12 h : 12 h light-dark cycle unless otherwise noted. Flies are maintained on standard cornmeal agar medium, and the standard food recipe was as follows: 105 g dextrose, 7.5 g agar, 26 g yeast, 50 g cornmeal, and 1 L purified H2O were mixed and boiled 5 times with constant agitation, and 0.25 g sodium benzoate (Sigma Aldrich, St. Louis, MO, USA) dissolved in 8.5 mL 95% ethanol and 1.9 mL propionic acid (99%, Mallinckrodt Baker) was added to the cornmeal agar medium.
D. suzukii was generously gifted by Prof. Haoyuan Hu at Anhui Normal University, and D. melanogaster Canton S was used as described (Jia et al., 2021). D. suzukii was maintained for ∼80 generations in laboratory conditions. The used stocks esg-Gal4/+; tubulin-Gal80TS, UAS-Bsk, and UAS-JunbZIP were previously described (Liu et al., 2022). The line of esg-Gal4 was combined with a ubiquitously expressed temperature-sensitive Gal80 inhibitor (esg-Gal4; tub-Gal80TS) as described (Zhai et al., 2018). Crosses and flies were kept at 20 °C and later transferred to 29 °C for 5 d to allow expression of transgenes.
Bacteria strains
The strain of Lactobacillus plantarum (Lactobacillaceae) with the GenBank accession number KY038178 was used (Jia et al., 2021) and B. cereus H1 (GenBank: OR206465) was isolated and identified from D. suzukii pupae.
Bacterial isolation and identification
The morbid pupae of D. suzukii were washed 3 times with 75% ethanol in an Eppendorf tube, fully ground in sterile water, and centrifuged at 76 g. The supernatant was plated to Luria-Bertani (LB) agar medium, and incubated at 37 °C for 36 h. Single colonies were selected and purified via streaking. Colonies were preserved at −80 °C in 20% glycerol for identification. Bacterial genomic DNA was extracted using a FastPure Bacteria DNA Isolation Mini Kit (DC103-01) according to the manufacturer's instructions. The isolated 16S rRNA fragments were amplified via polymerase chain reaction (PCR) using universal primers 27F and 1492R at 95 °C for 3 min; 95 °C, 15 s; 45.5 °C, 15 s; 72 °C, 35 s; 35 cycles; 72 °C for 5 min. The PCR products were sequenced by GENERAL BIOL, and the 16S rRNA sequences were analyzed using BLAST (http://www.ncbi.nlm.nih.gob/blast). The related bacteria are downloaded from the GenBank database. Based on the homology of 16S rRNA genes, the maximum likelihood method in MEGA7.0 was used to generate the phylogenetic tree (Vacchini et al., 2017; Luo et al., 2018; Hiebert et al., 2020; Eski et al., 2024).
Generation of germ-free and gnotobiotic flies
Germ-free flies were generated as described (Jia et al., 2021). In brief, embryos were collected on a grape agar plate within 6 h, rinsed with sterile water, and transferred into 1.5 mL Eppendorf tubes. Diluted sanitizer Walch (1: 30, Procter & Gamble Co., Cincinnati, OH, USA), 2.5% sodium hypochlorite (Sigma Aldrich), 75% ethanol, and sterile phosphate-buffered saline (PBS) containing 0.01% Triton X-100 were used to sterilize embryos successively. These eggs were transferred into LB plates. After 24 h, 1st instar larvae were selected from the LB plate to axenic fly food with 2.5% yeast. B. cereus H1 or L. plantarum with 0.1 optical density (OD) (∼107 colony-forming units [CFU]) was added to establish a gnotobiotic fly model (Schretter et al., 2018; Consuegra et al., 2020; Jia et al., 2021).
CFU counting and pH value detection of B. cereus H1 on the fly medium
To quantify the number of associated bacteria, 0.1 OD L. plantarum and B. cereus H1 were inoculated to the fly sterile artificial food, respectively. The CFU and pH values were detected at 6, 12, 24, 36, 48, and 72 h, respectively. Bacterial load was calculated by plating corresponding fold serial dilutions of the homogenates on LB agar plates and incubating the plates at 35°C for 12 h, and the bacterial count of the fly was determined as follows: CFU/mL = (1 000 μL/volume plated) × average n of colonies. CFU/mL at the origin = CFU/mL × dilution factor. CFU/g fly food = (CFU/mL at the origin × volume in which fly food is homogenized)/n of food homogenized. The pH value of L. plantarum or B. cereus H1 was detected by a pH meter (Xima, PH818M). All the material to manipulate bacteria was sterilized before use as described (Clark et al., 2015; Jia et al., 2021).
Drosophila developmental timing assays
To test whether B. cereus H1 was the pathogen of D. suzukii and D. melanogaster, 20 germ-free larvae 2 d after hatching were transferred to fly food with 0.1 OD B. cereus H1. The numbers of pupae and adults were recorded daily, and the formula used to calculate the pupation and emergence rates: R = (N1 + N2 +…+ Nm)/5n, where R represents the pupation rate or emergence rate, Nm is the number of pupae or adults every day. n is the total number of treated larvae. m = 5, n = 20 (Li et al., 2020).
Survival assays
To measure the lifespan of the adults, 20 healthy males and females each were collected in vials containing 2 filter papers supplemented with 200 μL L. plantarum or B. cereus H1 suspension (100 OD, in 5% sucrose water) as described (Li et al., 2020; Bing et al., 2023). The filter paper was replaced every 2 d, and the survival rates were monitored over time. For each group, at least 5 independent biological replicates were made. The lifespan of esgTS>Syn flies treated with B. cereus H1 was conducted in the same way.
Climbing assay
This assay was conducted similarly to the survival assay, with the following modifications. The filter papers at each vial were refreshed with 200 μL B. cereus H1 with 100 OD or PBS every 24 h. The flies were exposed to bacteria suspension for 2 d (lethality rate 50%) and then 10 flies were transferred to a 20 mL graduated glass cylinder. The speed of their climbing was calculated by recording the fly's position every 1 min. Each treatment was performed in 5 biological replicates, and the whole experiment was repeated 3 times (Madabattula et al., 2015; Li et al., 2020; Schretter, 2020).
Oviposition preference assay
Two-choice egg-laying chambers were constructed in a manner as described (Liu et al., 2017; Su et al., 2019). To generate the fermented substrates, food agar was autoclaved at 121 °C and plated with either 0.1 OD B. cereus H1 or ddH2O for the controls. Flies were fed with yeast paste and kept at 25 °C for 36 h. The agar plate was evenly cut into 2 halves with a razor blade, and 2 oviposition substrates were put into a Petri dish for the oviposition chamber assembly. To assess the effect of metabolites on oviposition preference, B. cereus H1 was inoculated in liquid fly food and incubated at 25 °C for 36 h. Fermented fly food was then centrifuged at 13523 g for 10 min, and the supernatant was distributed to the fly food surface in 1 of the 2-choice halves. A total of 30 female flies with 20 males were transferred to the device. At last, the number of eggs on each half of the 2-choice site was recorded after the flies were removed, and the oviposition index (OI) was calculated with the following equation: OI = (number of eggs laid on experimental food – number of eggs laid on control food)/total number of eggs laid.
Smurf and intestinal pH assay
The intestinal integrity was determined by Smurf assay as previously depicted (Rera et al., 2012; Li et al., 2016; Li et al., 2020). More than 100 flies were first exposed to B. cereus H1 for 2 d and 20 flies were transferred to test vials with 2.5% Erioglaucine (FD&C Blue #1) and kept at 25 °C for 12 h. A fly was counted as a Smurf when dye coloration occurred outside the digestive tract. Smurf% = number of Smurf flies/(number of non-Smurf flies + number of Smurf flies) × 100%. The intestinal pH values were determined by the pH indicator as described (Li et al., 2016). In brief, 50 flies were exposed to B. cereus H1 for 2 d. Ten flies were transferred to test vials with 2% bromophenol blue sodium (Sigma Aldrich) and kept at 25 °C for 12 h. The intestinal pH was dissected and measured by pH indicator strips.
CFU counting in hemolymph and guts
Hemolymph was collected in a procedure described (Stoepler et al., 2012) with modest modifications. In brief, the thorax of anesthetized female adults was pierced with a sterile microinjector. Twenty infected or control flies were transferred to a pre-chilled 0.5 mL Eppendorf tube with 3 pores punctured with an 18-gage needle at the bottom. The 0.5 mL tubes were inserted into a 1.5 mL Eppendorf tube with 100 mL PBS. Hemolymph was collected at the bottom of the 1.5 mL Eppendorf tube after centrifugation at 2348 g for 5 min. For gut bacterial load, guts of 20 females were dissected in cold PBS, and then ground in 1.5 mL Eppendorf tubes. Bacterial load was calculated by plating the homogenates with proper dilutions on LB agar plates and incubated at 28 °C for 24 h. CFUs of each sample were recorded.
In vivo detection of ROS
ROS production in the gut was examined with the intracellular ROS-sensitive fluorescent dye dihydroethidium. After B. cereus H1 infection for 24 h, the midguts of flies were dissected in PBS, and incubated in 5 dihydroethidium (Invitrogen) for 30 min at room temperature in the dark. Next, the midguts were washed 3 times with PBS, and the guts were immobilized with 4% paraformaldehyde for 10 min. Subsequently, the tissues were washed 3 times with PBS. Finally, the gut samples were transferred to a glass slide in a drop of PBS and observed under a fluorescence microscope (Nikon, TI-DH) (Wei et al., 2017; Li et al., 2020).
H2O2 assay
Fly guts after infection were dissected in cold PBS, and 20 guts were homogenized in 200 μL lysis buffer. The samples were centrifuged at 12 000 × g at 4 °C for 5 min, and the supernatant of each sample was collected for H2O2 assay with the Peroxide Assay Kit (Sigma Aldrich, MAK311-1KT). According to the manufacturer's instructions, the concentration of H2O2 was examined by a standard curve of hydrogen peroxide as described (Liu et al., 2022).
Real-time – quantitative PCR analysis
The intestines of female D. melanogaster were dissected in cold PBS after infection with B. cereus H1 for 12 h. Total RNA was extracted with an RNA Extraction Kit (Vazyme), and the concentrations of RNA were detected by a NanoDrop spectrophotometer (Thermo Scientific). HiScript III All-in-one RTSuper Mix Kit (Vazyme) was used to reverse transcribe RNA into complementary DNA (cDNA). Up to 2 μL cDNA diluted 20-fold was mixed with 0.2 μmol of forward and reverse primers and the mixture was subjected to real-time – quantitative PCR (RT-qPCR) analysis using the ChamQ Universal SYBR qPCR master mix kit in a total reaction volume of 20 μL in a CFX96™ Real-Time System (BioRad, Hercules, CA, USA). No reverse transcription controls were carried out for each experiment to ensure that there was no chromosomal DNA contamination in each RNA sample. RNA from 3 biological replicates was analyzed and 3 technical replicates were performed. The relative expression values were calculated using the following formula: ΔCt = Ct (target gene) − Ct (reference gene), ΔΔCt = ΔCt (target gene) − ΔCt (average control target gene), and the relative expression is equal to 2−ΔΔCt (Su et al., 2019). The Actin ribosomal RNA (rRNA) protein-encoding gene was used as an internal control. The primers used for RT-qPCR analysis are listed in Table 1.
| PCR primer | Primer sequence (5'–3') |
|---|---|
| 27F* | AGAGTTTGATCCTGGCTCAG |
| 1492R | TACGACTTAACCCCAATCGC |
| Dpt F† | ACCGCAGTACCCACTCAATC |
| Dpt R | CCCAAGTGCTGTCCATATCC |
| Drs F | GTACTTGTTCGCCCTCTTCG |
| Drs R | CTTGCACACACGACGACAG |
| Duox F | CATTCCCCTGGACTCGCAC |
| Duox R | TCGTGCGATTGGGTGGAC |
| Actin F | TTGTCTGGGCAAGAGGATCAG |
| Actin R | ACCACTCGCACTTGCACTTTC |
- * 27F,1492R were the primers for 16S rDNA sequences.
- † Drs, Dpt, Duox, and Actin were primers for quantitative PCR analysis.
Immunofluorescence staining
Flies were fed with 5% sucrose solution with or without B. cereus H1 for 12 h. Intestines were dissected in PBS and fixed with 4% paraformaldehyde for 25 min at room temperature. The samples were blocked with a blocking solution (PBS with 0.3% Triton X-100, 0.2% goat serum, and 0.1% fetal calf serum) for 25 min, incubated in primary antibodies overnight at 4 °C, and washed in PBS supplemented with 0.3% Triton X-100 (PBST). Then the samples were incubated with the secondary antibodies conjugated with Alexa 488 or 568 (Thermo Fisher, 1: 1 000, Waltham, MA, USA) for 2 h at room temperature. Primary antibodies were α-syn (Novus Biologicals, 1: 1 000, Centennial, CO, USA), phospho-histone 3 (Millipore, 1: 1 000, Burlington, MA, USA), DIg (DHSB, 1: 50, Iowa City, IA, USA) and phospho-JNK (Millipore, 1: 200). Images were observed by confocal microscopy (Olympus Fluoview FV3000) and processed using Adobe Photoshop 2020.
Statistical analysis
Results are reported as means ± standard error of the mean (SEM), and the difference at P < 0.05 was regarded as a statistical significance. At least 3 independent experiments were performed in this study. GraphPad Prism 9.0 was applied to conduct statistical analysis. ImageJ was used to analyze the fluorescence intensity. Differences between treatments were determined by one-way analysis of variance followed by the t-test unless otherwise mentioned. All significant pairwise comparisons are labeled: *P < 0 .05; **P < 0.01; ***P < 0.001; ****P < 0.0001; “ns” represents not significant.
Results
B. cereus H1 was a potential entomopathogen of D. suzukii
We collected the dead pupae of D. suzukii in a grape (Vitis Vinifera “Summer Black”) orchard (lat 37.265454, long 111.787759) in Fenyang, Shanxi Province and isolated 16 strains of Drosophila-associated bacteria. To assess the pathogenicity toward hosts, we orally infected larvae by re-inoculating the isolated strains to the fly medium. We found that strain H1 merely imposed morbidity and mortality on D. suzukii at the stage of pupae. Compared to control ones (Fig. 1A, B), bodies of infected pupae became blackish and distorted (Fig. 1C–E), and ∼60% of them died before the pre-pupation stage, indicating that strain H1 was a potential entomopathogen of D. suzukii. To identify it, molecular phylogeny of the strain H1 was carried out based on the nucleotide sequences of their 16S rRNA genes. A sequence variation of <1% was found between strain H1 and B. cereus JXJGS201608-27, indicating that strain H1 shares a close phylogenic relationship with B. cereus. To distinguish this strain from others, it was henceforth termed B. cereus H1 (Fig. 1F).
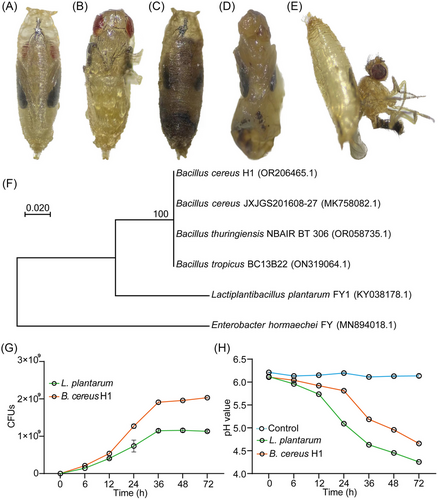
B. cereus H1 rapidly colonizes the fly medium
We next examined the growth traits of B. cereus H1 on the fly standard food. The result showed that B. cereus H1 entered the logarithmic growth phase at 12 h post-inoculation and reached the plateau value with 1.96 × 109 CFUs at 36 h post-inoculation (Fig. 1G). Moreover, the load of B. cereus H1 was 1.7-fold higher than that of L. plantarum after the plateau of the growth (P < 0.0001), suggesting that B. cereus H1 was a fast-growing pathogen. However, B. cereus H1 caused a modest decrease in the pH of the medium from 6.11 to 4.66 (Fig. 1H). Taken together, these results suggest that B. cereus H1 rapidly propagated in the medium of Drosophila and modestly generated organic acids.
Metabolites of B. cereus H1 elicit an oviposition avoidance of D. suzukii
Drosophila females choose favorable egg-laying sites to increase the survival and fitness of their progenies (Liu et al., 2017). Studies have shown that L. plantarum attracted Drosophila to lay eggs on fermented food with the 2-choice assay, eliciting an oviposition preference (Liu et al., 2017). Given that B. cereus H1 imposed morbidity on larvae, it prompted us to hypothesize that Drosophila could sense B. cereus H1 in potential egg-laying sites. However, the results show that female D. suzukii deposited about 48.5% of their eggs on halves in the presence of B. cereus H1, with an OI of −0.03. B. cereus H1 was unable to elicit an oviposition avoidance of D. suzukii (P = 0.8459). Considering that bacteria can generate an extraordinary variety of secondary metabolites to repel insects, we assessed the effect of metabolites on oviposition preference. Indeed, D. suzukii and D. melanogaster were robustly repelled to depositing the eggs on the surface of the halves with metabolites of B. cereus H1, with the OI of −0.41 and −0.435 (Fig. 2A), suggesting that secondary metabolites of B. cereus H1 more alerted the flies to the presence of toxins compared to bacteria cells (P = 0.0027).
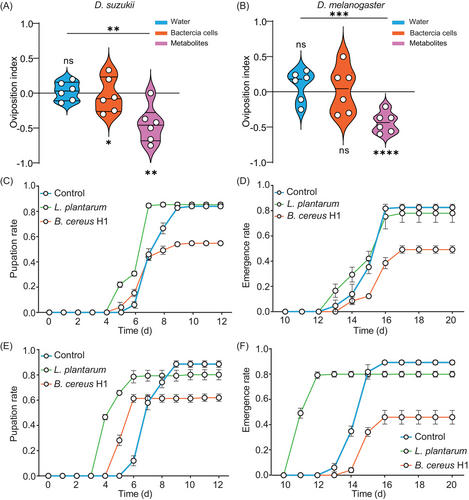
B. cereus H1 compromises the fitness of D. suzukii
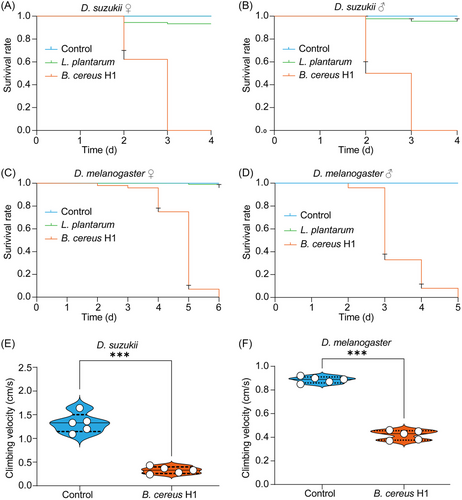
We generated germ-free and B. cereus H1 mono-associated flies to study whether B. cereus H1 could specifically affect the development of D. suzukii. We found that the rates of both pupation and eclosion were significantly reduced in the case of B. cereus H1 compared to the control group (P < 0.0001) (Fig. 2C–F). Next, we conducted oral infection to assess the pathogenicity of B. cereus H1 toward conventionally reared Drosophila. The median lethal time (LT50) of males and females of D. suzukii challenged with B. cereus H1 was, respectively, 3 and 2 d (Fig. 3A, B), while the control flies remained intact. Similarly, the median lethal time (LT50) of males and females of D. melanogaster treated with B. cereus H1 was, respectively, 5 and 3 d (Fig. 3C, D). Notably, L. plantarum-treated flies grew normally. Coordinated locomotion, including climbing, is essential for the fundamental activities of any insects (Schretter et al., 2018; Hiramoto et al., 2021), so negative geotaxis was applied to assess the climbing impairment of adult flies subjected to B. cereus H1 as described (Willenbrink et al., 2016). Indeed, the B. cereus H1-treated D. suzukii flies climbed more slowly at a velocity of 0.35 cm/s than control ones at a velocity of 0.96 cm/s (Fig. 3E). Coincidently, D. melanogaster flies also exhibited climbing impairment after being challenged with B. cereus H1 (Fig. 3F). Collectively, these results suggest that B. cereus H1 compromised the fitness of both D. suzukii and D. melanogaster.
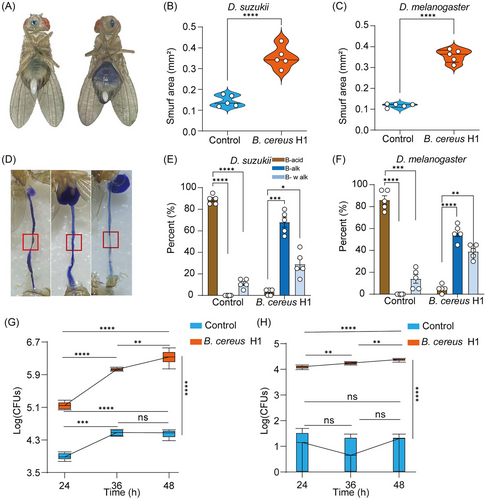
B. cereus H1 disrupts intestinal integrity and pH homeostasis in Drosophila
Wild flies routinely ingest rotten fruits containing a variety of pathogens, which can disrupt their digestive tracts by inducing lesions on the epithelium (Wu et al., 2017). To test it, we carried out histological analysis for loss of intestinal integrity utilizing an unabsorbable blue dye as described (Li et al., 2016; Zhang et al., 2023). In comparison with the control groups, B. cereus H1 resulted in a higher percentage of disrupted intestinal integrity of both D. suzukii and D. melanogaster (P < 0.0001) (Fig. 4A–C), respectively. Next, we studied B. cereus H1-caused intestinal dysfunction with the pH indicator as depicted (Li et al., 2016). We found that the normal gut had a noticeable yellowish area with acidity, but more than 95% of flies infected with B. cereus H1 exhibited pH interruption in both D. suzukii and D. melanogaster (P < 0.0001). Notably, the phenotypes were heterogeneous. For example, 56% of D. melanogaster had a highly basic intestine without an acidic region, and 39% manifested weak staining throughout the intestine (weakening blue) (Fig. 4D–F). Intestinal dysplasia was related to the increase in bacterial load in guts and hemocoel of insects (Wei et al., 2017), prompting us to examine the cultivable bacterial loads in the midgut and hemocoel of the fly with B. cereus H1 infection. Indeed, bacterial load was significantly increased in infected guts at 24, 36, and 48 h timepoints compared with controls treated with PBS (Fig. 4G). Consistently, bacterial load in hemocoel was increased in infected flies with B. cereus H1 (Fig. 4H), indicative of systemic infection. Taken together, these results demonstrate that B. cereus H1 disturbed intestinal integrity and pH homeostasis in drosophilid flies.
B. cereus H1 triggers oxidative innate immunity of Drosophila
Given that Duox is responsible for the dysbiosis-induced generation of ROS, it was conceivable that high pathogen burdens could aggravate cell death through the DUOX-ROS pathway. To issue it, we assessed the levels of ROS in the midgut of D. melanogaster and D. suzukii by dihydroethidium staining. As expected, ROS activity was significantly higher in B. cereus H1-treated flies than that of corresponding flies (Fig. 5A, B). In agreement with this finding, the level of H2O2 in B. cereus H1-treated flies was relatively higher than that of untreated flies (Fig. 5C). In addition, ROS scavenger, N-acetylcysteine (NAC), partially ameliorated the mortality of flies infected with B. cereus H1 (Fig. 5D). ROS generation was triggered by Duox, a member of the nicotinamide adenine dinucleotide phosphate oxidase family. To validate it, we tested the expression of the Duox gene through RT-qPCR. Our result showed that B. cereus H1 increased levels of Duox in the midgut of flies compared to control flies (Fig. 5E). Furthermore, the expression of the antimicrobial peptides Diptericin (Dpt) and Drosomycin (Drs) was increased in the intestine of flies in the case of B. cereus H1 treatment. Altogether, these results suggest that B. cereus H1 induced marked inflammation and oxidative innate immunity of the midgut in drosophilid species.
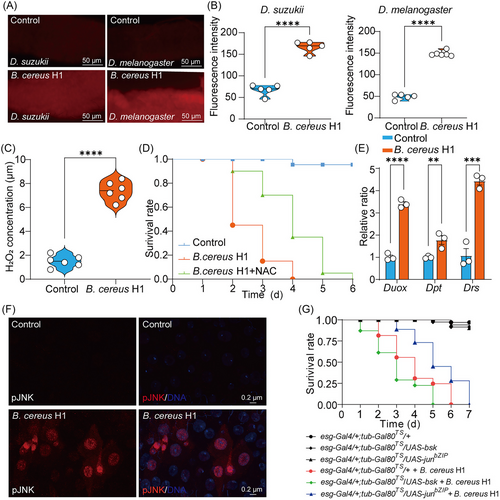
In intestinal cells, activation of the JNK pathway contributes to compensatory cell proliferation and apoptosis induced by ROS upstream in D. melanogaster. To ask whether JNK signaling in the gut was activated by B. cereus H1, we checked the level of phospho-JNK as described (Liu et al., 2022). As expected, we found elevated phospho-JNK levels in B. cereus H1-treated intestines compared with control ones (Fig. 5F). Furthermore, inhibition of the JNK signaling significantly enhanced survival of flies challenged with B. cereus H1 compared to control flies (Fig. 5G), while activation of JNK signaling reduced the survival of B. cereus H1-treated flies. Collectively, our results suggest that B. cereus H1 infection compromised the survival of Drosophila species through the DUOX-ROS-JNK pathway.
Discussion
In this study, we tested B. cereus H1 against target insects of D. suzukii through oral infection. We found that this strain was detrimental to the fitness of both D. suzukii progenies and parents (Figs. 1 and 2). Extensive studies found that the control of D. suzukii relies heavily on chemical insecticides (Lisi et al., 2023). However, the wide application of insecticides has led to pest resistance and caused environmental hazards (Deans & Hutchison, 2022). Therefore, an alternative strategy for the biocontrol of D. suzukii is urgently needed. A study found that D. suzukii larvae were susceptible to B. thuringiensis (Mastore et al., 2021). More interestingly, only few serotypes of B. thuringiensis are effective against the target D. suzukii (Cossentine et al., 2019). Fortunately, B. cereus H1 was a strain that exhibited high insecticidal activity to D. suzukii (Fig. 3), suggesting that it was a rare strain that could be used as a biological control agent. However, we could not compare the insecticidal activity of B. cereus H1 with other strains, because of differences among laboratory conditions. Our results show that B. cereus H1 results in an increase in bacterial load in the guts and translocation of this pathogen from the gut to the hemocoel (Fig. 4G, H), which facilitates killing of flies.
Entomopathogens play a critical role in regulating insect populations in the wild and have been widely used as promising agents in the biocontrol of pests for many years (Mastore et al., 2021). However, the relatively slow action of pathogens, compared with chemical insecticides, has hampered their widespread application (Wei et al., 2017). To better understand bacteria-pest interactions is critical for accelerating the speed at which a bacteria pathogen kills its host. These entomopathogens have evolved diversified lifestyles and possess dozens of secondary metabolic gene clusters to encode many secondary metabolites, which seriously threaten the survival of pests (Cao et al., 2023). According to the Natural Products Atlas 2.0, Bacillus species can generate at least 455 secondary metabolites with species specificity, mainly including lipopeptides, siderophore, polyketides, lantibiotic, bacteriocin, and macrolactone (Yin et al., 2023). Many of these compounds have insecticidal activity against pests, including D. suzukii (Liu et al., 2020; Akpor et al., 2021). For example, cereulide is a small, heat-resistant, and acid-stable peptide, and acts as a potassium ionophore that interferes with cellular and mitochondrial membranes of cells with high cytotoxic potential (Babin et al., 2023; Oliveira et al., 2023). It is proposed that specific metabolites of B. cereus H1 could underlie the morbidity and mortality of infected D. suzukii. However, specific bacterial metabolites that disrupted intestinal homeostasis of D. suzukii need to be identified in future study. Taken together, B. cereus H1 has the potential to be used for pest biocontrol against D. suzukii.
The Drosophila model system provides us with a reductionist approach to disentangle the complexity of host-microbe interactions. Selecting an appropriate place to lay eggs is an integral reproductive requirement of Drosophila females because larvae have restricted motility. Based on the hypothesis of “mother-knows-best”, female oviposition decisions have evolved to deposit eggs in sites favorable for survival of offspring (Liu et al., 2017; Babin et al., 2023). Indeed, we found that D. suzukii were strongly repelled to deposit their eggs in the place with the metabolism of B. cereus H1 (Fig. 2A, B). Moreover, pathogens generate a broad range of highly toxic metabolites that are sensed by D. suzukii. Oviposition decisions require integrated sensory modalities, including visual, gustatory, olfactory, and proprioception. It would be attractive to investigate which sensory modality contributes to the egg-laying avoidance of sites with B. cereus H1.
The intestine, a hub of communication with other organs, is the most important site where pathogens and hosts arms race with each other (Wu et al., 2017; Kamareddine et al., 2018). The innate immune response is the first line of defense to protect the organisms against the infection of oral pathogens. However, pathogens have evolved a wide array of mechanisms to cope with this defense, resulting in severe damage to the intestines (He et al., 2017; Pandey et al., 2023). Our research has shown that the dysfunction of the intestinal barrier caused by B. cereus H1 increases intestinal barrier permeability (Fig. 4). It is proposed that the massive pathogenic bacteria could penetrate the hemolymph through the impaired intestinal tract and outgrow the hemolymph, which consequently causes the death of D. suzukii (Fig. 4H). At the same time, the acid-producing region of the fore-midgut showed a pH imbalance, which might aggravate intestinal inflammation (Fig. 4D–F). ROS was an inevitable pro-inflammatory toxin that accelerated tissue injury (Fig. 5) when the amounts exceeded certain thresholds (Aviello & Knaus, 2018; Tafesh-Edwards & Eleftherianos, 2023). Moreover, B. cereus H1 in the diet of D. melanogaster could cause an increase in IMD expression in Duox and antimicrobial peptides in comparison with common feeding flies (Fig. 5). IMD/TOLL pathway activation could be explained by B. cereus H1 promoting oxidative stress in the gut and disrupting intestinal homeostasis. The results facilitate understanding the underlying mechanism underpinning the pathogenicity of B. cereus H1.
Overall, these findings provide new insights into the mechanisms of bacterial pathogenesis in insects. Understanding the interactions between B. cereus H1 and Drosophila may lead to new strategies for the biological control of D. suzukii. Although B. cereus H1 could be potentially used as a biological control agent, it could have broader host range and cast a risk to non-target insects and local communities through complex direct and indirect effects. Future studies need to evaluate the insecticidal activity of B. cereus H1 to fulfill commercial production of B. cereus H1 and requirements in terms of environmental risk.
Conclusion
In summary, we isolated and identified the bacterial strain, B. cereus H1, that was detrimental to the fitness of both D. suzukii progenies and parents. Moreover, this strain disrupted the intestinal integrity and pH value of D. suzukii. Infection of B. cereus H1 activated the DUOX-ROS-JNK pathway. The findings showed that an entomopathogen B. cereus H1 could potentially act as a biological control agent against D. suzukii, advancing fundamental progress of IPM programs.
Acknowledgments
We would like to thank all members of Liu Wei's lab for helpful discussions. This work was supported by the National Natural Science Foundation of China (31501175), Grants of Anhui Natural Science Foundation (20230302123239), and Talent Grants of Anhui Agricultural University (RC342201).
Disclosure
The authors declare no conflicts of interest.




