Role of eicosanoids in insect immunity: new insights and recent advances
Abstract
Viruses, bacteria, fungus, protozoans, and different metazoan parasites and parasitoids present a constant threat to insects. Insect immunity has two components: humoral and cell mediated. Humoral immunity can be achieved by various antimicrobial proteins, namely, cecropins, sarcotoxin, defensin, attacin, etc. The cell-mediated immunity comprises various cells having immune functions fostering nodulation, phagocytosis, microaggregation, encapsulation etc. Eicosanoids play a crucial role in insect immunity comparable to other animals. The above-mentioned are signaling molecules derived from polyunsaturated fatty acids and they exert numerous physiological effects, namely, inflammation, immune modulation, and regulation of cellular processes. The review article elucidates various roles of eicosanoids, namely, nodulation reaction, Toll signaling pathway, nitric oxide (NO) generation, Ca2+ mobilization, production of reactive oxygen species (ROS), actin polymerization and aquaporin activation. Eicosanoids can function in immune priming in insects drawing hemocytes. An agent named Duox was also identified serving as ROS generator in insect gut. Moreover, role of Repat gene in insect immunity was also studied. However, recently the role of prostacyclin (PGI2) was found to be negative as it inhibits platelet aggregation. In this brief review, we have tried to shed light on the various functions of eicosanoids in immunity of insect those have been discovered recently. This concise study will allow to decipher eicosanoids’ function in insect immunity in a nutshell, and it will pave the way for more researches to understand the key players of insect immunity which may eventually help to develop novel vector and pest control strategies in near future.
Introduction
Diversity of insects on earth is vast and for this reason, insects face exposure to a broad array of pathogens. Almost every familiar habitat and niche are occupied by uncountable insect species, while a small number of marine species are there (Stanley et al., 2009). Susceptibility of insects to various infectious agents due to their wide range of diversity and also colonization of pathogens results in deleterious effects in host. Insects lack an adaptive immune system and are therefore entirely reliant on their innate immune system to combat microbial infections (Sheehan et al., 2020). Innate immunity occurs naturally; it is nonspecific and is not influenced by any prior infection. Action of innate immunity in insects starts with the first step of interaction of Pattern Recognition Receptors (PRRs) and Pathogen Associated Molecular Pattern (PAMP), which is the specific configuration found in microbial surface (Lin et al., 2020). Strong innate immune system contributes to their resilience over time. In course of evolution before the activation of innate immunity, insects developed first line of defense that is composed of their tough exoskeleton and peritrophic matrix. Stanley (2011) reported that, integument in insect species serves as potent physical barrier against various microbial agents. GI tract or gastrointestinal tract primarily serves as the entrance to the insect body for several microbial agents, but the fact is that it is not an only path for entry (Stanley, 2011). Many studies reported that insects’ innate immunity comprises humoral immunity and cell-mediated immunity (Ferrandon et al., 2007; Lemaitre & Hoffmann, 2007). Humoral immunity includes antimicrobial peptides, those are produced by genes. Pathogens those come into contact with insect bodies through hemocoel, are generally eliminated by both host's humoral and cell-mediated immune response. Therefore, hemocytes, salivary glands, fat bodies, midgut all of these manifest a strong immune response to the insect (Hillyer, 2016).
A brief overview of eicosanoids (Figs. 1 and 2)
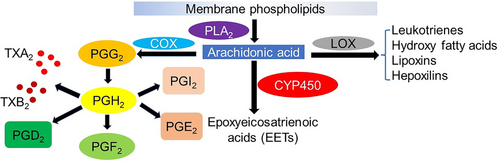
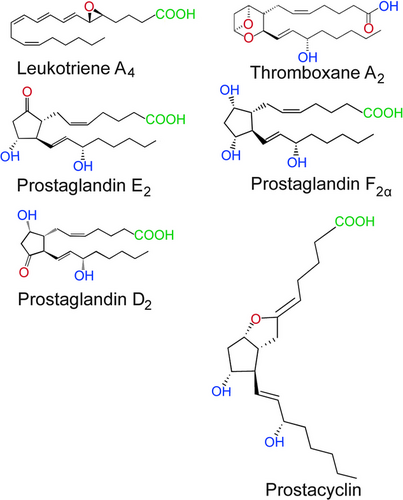
The etymology “eicosanoid” was derived from “είκοσι” (eíkosi), a Greek word, referring to the number 20 (Corey et al., 1980). Recognition of eicosanoids already was initiated in previous times when various researches intimated the fact that, one type of lipoidal acidic substance is generated by the prostate gland and the substance was recognized by von Euler (1936) as prostaglandin (Stanley, 2011). Eicosanoids are major regulators of inflammation and immunity (Tilley et al., 2001). Arachidonic acid or AA (20:4n-6) and two additional C20 polyunsaturated fatty acids (PUFAs) are oxygenated and produce prostaglandins and other eicosanoids. The epoxyeicosatrienoic acids (EET), lipoxygenase (LOX) metabolites, and PGs or cyclooxygenase (COX) are the three major groups of eicosanoids. Von Euler (1936) first discovered the prostaglandins during his research on human reproductive physiology. It is undeniable that PGs and other eicosanoids are important for lower vertebrates and invertebrates as well as humans and other mammals. The role of eicosanoids in few faunal groups varies as ion transport physiology modulators, ovulatory pheromones, and cellular immune reactions in vertebrate and invertebrate, certain fish species and insects respectively (Stanley & Miller, 2006). Prostaglandins can be biosynthesized from AA by enzymatic oxygenation process. The enzyme phospholipase acts upon phospholipid and hydrolyses AA and kick-starts the biosynthesis of PGs. Stanley & Miller (2006) stated that except few steps, PG biosynthetic pathway in mammals and insects are much similar.
Insect immunity in a nutshell
Immune response is the defensive mechanism mediated by innate and adaptive immune systems. Innate immune response functions throughout the entire body; it is nonspecific and regarded as the first defensive line against microbial pathogens (Sheehan et al., 2018). Insect immune response comprises cell-mediated and humoral responses. Where Hemocytes acting like mammalian neutrophils and performing several functions like phagocytosis, the release of certain enzymes are manifested by cell-mediated immune response. On the other hand, humoral immune response generally includes antimicrobial peptide (AMP) secretion, clotting, melanization (Browne et al., 2013), etc. Insect does not possess adaptive immune response where there is a potentiality to memorize prior pathogens and to respond more prominently against respective outbreak (Cooper & Alder, 2006). A class of transmembrane protein (type 1) known as Toll like receptors (TLRs) have role in humoral immune response in insects as well as in mammals (Sheehan et al., 2018). Since Toll and TLRs are preserved in due course of the evolutionary process, therefore, these proteins are common in animals as well as plants. The cytoplasmic TIR (Toll/IL-1R) domain in Drosophila Toll receptor can be perceived as homologous with mammalian Toll receptor and the characterization of cytoplasmic TIR domain can be made by the presence of LRRs (leucine-rich repeats) (Zhang & Ghosh, 2001). Mammals and dipteran insects both possess genomes encoding roughly ten of these receptors (Imler & Zheng, 2004). After pathogen infestation in insects, TLR gets activated by the presence of a ligand—Spätzle (Spz)—a cysteine-knot growth factor (Kojour et al., 2020). Imler & Zheng (2004) reported the fact that immune functions carried out by Toll/TLR are not the results of closely related progenitors, but might be considered as evolutionary convergence. Prematurely synthesized version of Spz gene is broken down by the serine protease, and the Toll receptor gets activated by the attachment of the cysteine-knot motif of the liberated carboxyl terminal fragments (Alpar et al., 2018). Drosophila melanogaster, a fruit fly, has provided valuable understandings into the process of innate immune activation. Apart from the Toll signaling pathway, the immunological response in Drosophila is controlled by an additional signaling cascade that has been preserved throughout evolution: the immune deficiency (Imd) pathway activating NF-κB. The Imd pathway is crucial for maintaining a healthy immune system in Drosophila, as it regulates the expression of the majority of antimicrobial peptides (AMPs) (Myllymäki et al., 2014).
Eicosanoid biosynthesis in insects
Eicosanoids are typically synthesized within cells and released into the hemolymph. They exert their effects through cell surface receptors via autocrine or paracrine mechanisms (Stanley & Kim, 2019a). In insects, the biosynthesis of prostaglandins (PGs) involves three distinct stages. Initially, phospholipase A2 (PLA2) releases polyunsaturated fatty acids (PUFAs) from membrane phospholipids (Linoleic acid, 18:2n-6) which is transformed to AA by elongases and desaturases (Kim & Stanley, 2021). Insects often maintain very low levels of AA and high levels of linoleic acid, which may lessen oxidative damage to phospholipids in cells (Stanley & Kim, 2020). According to Stanley & Kim (2019a), a protein with two catalytic sites called cyclooxygenase (COX) biosynthesizes PGG2s from AA and PGG2 is subsequently transformed into PGH2 by a hydroperoxidase. PGH2 is converted by enzymes specific to individual cells into a variety of chemicals including thromboxanes, PGI2 (also known as prostacyclin), PGA2, PGB2, PGD2, PGE2, and PGF2 (Stanley & Kim, 2019b). Insect genomes lack genes for cyclooxygenase (COX) enzymes, which produces PGs from C20 PUFAs (Stanley & Kim, 2019b). Peroxynectin (Pxt), a COX-like heme peroxidase, is in charge of PG production in insects (Tootle & Spradling, 2008; Tootle et al., 2011; Park et al., 2014). PGH2, which changes into various PGs by enzymes specific to individual cells, is produced from AA by the Pxts (Stanley & Kim, 2019b). Anopheles gambiae has been found to consist two Pxts, HPX7, and HPX8, which are probably related to PG production and function as a mediator of the gut immunity against a malarial parasite infection (Park et al., 2014). However, information about lipoxygenases and their byproducts in insects is scarce (Stanley & Kim, 2019a). Epoxyeicosatrienoic acids (EETs) function as proinflammatory signals in immune signaling and are generated from AA by cytochrome P450s (Kim & Stanley, 2021). A comprehensive study on eicosanoid signal transduction in invertebrates has been done by Stanley (2000) and Stanley & Kim (2014) also delineated the biosynthetic pathways of eicosanoids in insects and provided chemical structures in detail.
Background studies of eicosanoid on insect immunity
Stanley-Samuelson et al. (1991) demonstrated the role of eicosanoids in insect immunity. They showed eicosanoid can clear bacterial cells in hemolymph of Munduca sexta. The investigators used dexamethasone, a PLA2 inhibitor, and when the insect larvae were infected with red-pigmented strain of Serratia marcescens, the insects were unable to clear the bacterial load, which led them to claim that early immune responses are mediated by eicosanoids. After three years, Miller et al. (1994) hypothesised that reactions which lead to microaggregation and nodulation are fuelled by eicosanoids. They performed similar experiment by the eicosanoid biosynthesis inhibitor dexamethasone and applied into S. marcescens infected tobacco hornworm. Stanley & Miller (2006) tabulated 21 insects species belonging to the six insect orders which display cellular immune response upon immune challenge in both larvae and adult stages. According to Lord et al. (2002) and Dean et al. (2002) after B. bassiana and M. anisopliae infection, M. sexta manifests signal transduction followed by antimicrobial responses which led to the nodulation reaction. Franssens et al. (2005) used laminarin (a common cell wall component of fungus) to treat grey flesh fly larvae (Neobellieria bullata) and observed nodulation reaction. Dexamethasone, inhibitor of phospholipase A2; naproxen, inhibitor of cyclooxygenase and esculetin, inhibitor of lipoxygenase were used for the treatment and it was evidenced that the insect's nodule formation was significantly hampered by both dexamethasone and naproxen and addition of eicosanoid precursor AA resumes the nodule formation reaction. This study also confirms intricate relationship of insect immunity and eicosanoids. Garcia et al. (2004) discovered that immunity can be achieved by eicosanoids against Typanosoma rangeli—a protozoan parasite to blood-sucking insect Rhodnius prolixus. However, according to Figueiredo et al. (2008) platelet activating factor and eicosanoids act jointly in hemocyte phagocytosis in R. prolixus. Leptopilina boulardi, a parasitoid wasp infects larvae of Drosophila melanogaster, and the research of Carton et al. (2002) demonstrated that upon the parasitoid infection when D. melanogaster larvae were treated with dexamethasone, one of the inhibitors of eicosanoid biosynthesis resulted in significant reduction of the melanotic encapsulation. The findings of this study imply that prostaglandins and other eicosanoids have a role in D. melanogaster larvae hemocytic encapsulation reaction as cell-signaling molecules (Carton et al., 2002). Büyükgüzel et al. (2007) used Bovine herpes simplex virus-1 (BHSV-1) to infect larvae of greater wax moth (Galleria mellonella) and showed nodulation reaction to occur which was due to the action of eicosanoids. Injection of indomethacin—inhibitor of COX-1 and COX-2 prior to the viral infection resulted in significantly reduced number of nodules (approximately by 4-parts) and nodulation and phenoloxidase activity also decreased by 10-parts and 3-parts respectively by increasing indomethacin dose in diet by 100 folds (from 0.01% to 1%) (Büyükgüzel et al., 2007), confirming the eicosanoids’ function in immune response generation against viral infection.
Role of eicosanoid in insect immunity
Nodulation reaction
Tunaz et al. (2020) infected Spodoptera littoralis larvae with Beauveria bassiana 6646 and Metarhizium anisopliae 3293 fungi and studied the nodulation reaction and the role of eicosanoid in the process. The infection of the larvae resulted in the nodule formation and when the inhibitors of eicosanoid biosynthesis were injected by the researchers, the nodule formation was severely hampered (Tunaz et al., 2020). Tunaz et al. (2020) used six eicosanoid biosynthesis inhibitors, namely—dexamethasone (DEX), ibuprofen (IBU), naproxen (NAB), indomethacin (IND), esculetin (ESC), and phenidone (PHE). DEX inhibits phospholipase A2 enzyme; IBU, NAB, and IND block cyclooxygenase enzyme; ESC blocks lipoxygenase enzyme and PHE inhibits both cyclooxygenase and lipoxygenase enzymes (Stanley, 2000). After the application of the inhibitors (6 h PI) the authors dissected the larvae and investigated for nodule formation and confirmed that the cyclooxygenase and lipoxygenase pathways of eicosanoid biosynthesis have a biological effect on insect immunity. DEX application in S. exigua also resulted in inhibition of nodule formation (Shrestha & Kim, 2009). The research of Shafeeq et al. (2018) also reflected that eicosanoid biosynthesis block followed by nodule formation inhibition can be resulted by knocking down the Toll signaling molecules: MYD88 and Pelle in S. exigua. According to Park & Kim (2012), RNAi treatment against Toll in S. exigua inhibited nodulation reaction which can be rescued by adding AA. Further, Kim et al. (2020) in S. exigua larvae showed suppression of nodulation is possible by knocking down PGE2 receptor (PGE2R) resulting in reduced F-actin growth in hemocytes. Thus, it can be easily inferred that the Toll signaling pathway modulates eicosanoid production and that eicosanoid, particularly PGE2, contribute to nodule formation (Suzuki et al., 2022). Stanley & Miller (2006) also hypothesized that microaggregation and nodulation are controlled by eicosanoids. Their hypothesis is based upon the findings of Miller et al. (1994), where the authors treated tobacco hornworms (Munduca sexta) with DEX and the control insects were treated with ethanol (EtOH). The researchers demonstrated a reduced nodulation reaction (about 25% to that of control) after the infection of the treated tobacco hornworms with Serratia marcescens. The authors stated that the effect of DEX was dependent upon dose and the nodulation process can be rescued if the insects are treated with polyunsaturated fatty acids, which are eicosanoid precursors. Further, the in vitro rescue experiments done by Stanley & Miller (2006) reported that microaggregation reactions are aided by COX products, but not LOX as PGH2 can rescue the DEX effect by increasing the microaggregation reaction significantly and PGD2 and PGE2 did not increase the reaction significantly. So, Stanley & Miller (2006) hypothesized the role of PGH2 as an active eicosanoid in insects aiding in microaggregation and nodulation.
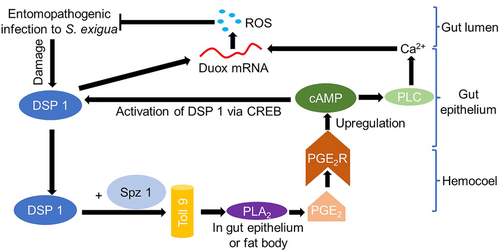
Does insect gut in combination with eicosanoid aid in immunity? (Fig. 3)
PGs are essential for protecting the insect midgut against microbial infections (Kim & Stanley, 2021). In insect gut, reactive oxygen species (ROS) can be produced and used as an agent in immunity by the NADPH family member Dual oxidase (Duox) (Lee et al., 2018). The investigators demonstrated that enteric infection results in lipid catabolism by enterocytes and homeostasis of Duox. Sajjadian & Kim (2020a) also confirmed the role of Duox in midgut cells of late larval instars of S. exigua in producing ROS after Serratia marcescens treatment. This molecule is supposed to be a transmembrane protein having high similarity with Drosophila homolog (Sajjadian & Kim, 2020a). However, after bacterial infection if PLA2 is inhibited, it hampered the Duox expression and it is rescued after adding the catalytic product (PGE2 and PGD2) and in particular, the expression of Duox was markedly inhibited by the PG synthesis inhibitor (Sajjadian & Kim, 2020a). The authors also stated that cAMP and downstream components are activated by PG signaling. Adenylate cyclase, protein kinase A inhibits Duox expression, and moreover, production of Duox was suppressed by particular RNAi that were specific to the PGE2R, AC, PKA, or cAMP-responsive element-binding protein (CREB) implying that insect midgut immunity can be stimulated through the cAMP signaling pathway by PGs promoting Duox expression and elevated ROS levels. Damage-Associated-Molecular-Pattern (DAMP) was first evidenced by Mollah et al. (2021) in Spodoptera exigua where the authors confirmed the production of eicosanoids is manifested by PLA2 activation. In nearly all eukaryotic cells, a nuclear protein, High-Mobility Group Box 1 (HMGB1), functions as an effector of DAMP (Yang et al., 2013). However, in another study on mealworm beetle (Tenebrio molitor), Dorsal switch protein 1 (Tm-DSP1) was identified as HMGB1 homolog (Mollah & Kim, 2021). The researchers confirmed the elevated expression of Tm-DSP1 in midgut cells along with hemocytes and fat body through expression studies. RNAi knockdown experiment against Tm-DSP1 resulted in inhibition of expression of AMPs and nodule formation in the larvae. Apart from this, the knockdown experiment also caused a significant reduction in PLA2 activity in the larvae, which is the key player in eicosanoid biosynthesis (Kim et al., 2018). Though it has been more than a decade, the role of gut in insect immunity through eicosanoids was also confirmed by García Gil de Muñoz et al. (2008). The investigators did a fantastic experiment to track the role of PGE2 in midgut cells and fat body of Anopheles albimanus by at first treating the insect with antibiotics, collecting the midgut, fat body and ovarioles for culture and quantifying the PGE2 by enzyme immunoassay. After that, 6000 Gram-positive Micrococcus luteus or 6000 Gram-negative Klebsiella pneumoniae (both heat-killed) were incubated with the cultured midguts during 60 min at 22 °C and again PGE2 level was determined (García Gil de Muñoz et al., 2008). After 60 min, the result showed a rise of PGE2 in normal midgut cell from 14 pg/mg protein to M. luteus infected midgut 15.62 pg/protein (García Gil de Muñoz et al., 2008). García Gil de Muñoz et al. (2008) also reported that midguts and cultured fat bodies produce mRNAs for AMPs, such as Aa-Gambicin, Aa-Cecropin, and Aa-Attacin, synthesis of which also reduced if DEX was added and addition of AA rescued the effect, confirming the role of midgut cells in immune function.
Eicosanoids modulating Toll signaling pathway
Studies have shown that in lepidopteran insect Spodoptera exigua, toll signal pathway can result in immune response upon a fungal infection by activating eicosanoid biosynthetic pathway (Roy & Kim, 2022). The immune reactions include activation of phospholipase A2 (PLA2), phenoloxidase (PO) enzyme and upregulation of genes encoding cecropin, hemolin, gallerimycin (Roy & Kim, 2022). Roy & Kim (2022) infected S. exigua with an entomopathogenic fungus Metarhizium rileyi. The researchers reported that S. exigua genome encodes ten toll receptors, among these seven are having role in immunity against M. rileyi. The Toll signaling also depends on two Spätzles, which are the ligands for Toll. Individual RNAi screening revealed that fungal infection provoking S. exigua immune responses through the Toll signal pathway depends on three pattern recognition receptors (βGRP-1, βGRP-2, and GNBP3) and five serine proteases (ModSP, HP21, HP5, HP6, and HP8), confirming that in S. exigua, eicosanoid biosynthetic pathway is crucial for firing different types of immune function against fungal infection, which is triggered by upstream components (pattern recognition receptors, serine proteases) of Toll signaling pathway (Roy & Kim, 2022). However, Toll signaling can also activate the eicosanoid biosynthetic pathways by activating PLA2s. Shrestha & Kim (2010) proved the link between eicosanoids and Toll/Imd signaling pathway in red flour beetle (Tribolium castaneum). The authors challenged the insect with Xenorhabdus nematophila K1, Bacillus subtilis, Escherichia coli, and Flavobacterium sp. and upon dsRNA treatment against Toll/Imd genes, they demonstrated the inhibition of the synthesis of AMPs (antimicrobial peptides) which are critical for the insect immunity. This experiment proves the role of toll signaling pathway in AMP production. However, dsRNA treatments against Toll/Imd genes also inhibited the PLA2 gene expression (Shrestha & Kim, 2010). The immunofluorescence assay of PLA2 in control larvae showed entry of PLA2s near to hemocyte cell membrane but in case of the dsRNAs treated larvae the translocation of PLA2s did not occur establishing order of immunogenic effect: recognition of bacteria, Toll/Imd pathways, activation of PLA2s associated to immune effect in T. castaneum (Shrestha & Kim, 2010).
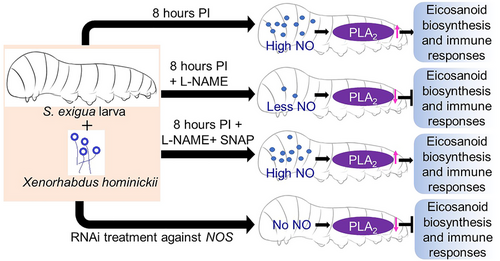
NO and eicosanoid cross-talk (Fig. 4)
In insects and vertebrates, nitric oxide (NO) functions as a mediator in the neurological and immune systems (Rivero, 2006). It is a small, membrane-permeable signal molecule. NO synthase (NOS) acts upon L-arginine and synthesizes NO. It shows multifaceted role, namely, functioning as potent vasodilator and platelet aggregation inhibitor in blood-sucking insects (Ribeiro et al., 1993), long-term memory of insect associated with visual and chemical signals and neural processing of insect (Müller, 1997), inducing cellular and humoral immune response in Drosophila (Nappi et al., 2000; Foley & O'Farrell, 2003). We already know that PLA2 is an important agent which forms eicosanoid by hydrolysing AA. The relation between PLA2 activity and NO was proved by Sadekuzzaman et al. (2018). The investigators chose S. exigua as a model organism and infected it with Xenorhabdus hominickii, an entomopathogenic bacteria isolated from the nematode Steinernema monticolum and demonstrated that infection of the bacterium significantly increased the NO concentration in the larva. According to Sadekuzzaman et al. (2018), the hemocytes and fat body all showed NO concentrations of roughly 0.1 μmol/mg protein (determined as nitrate) constitutively, rising by five to eight times in just 8 h PI and the increase of NO in plasma was less. However, when NO inhibitor Nω-nitro-L-arginine methyl ester hydrochloride (L-NAME) was applied NO production (determined as nitrate) decreased severely measuring 0.2 μmol/mg protein (Sadekuzzaman et al., 2018). The researchers also stated that the dose-dependent increase in PLA2 activity in hemocytes and fat body also went down after adding L-NAME and further addition of a NO releaser SNAP rescued the PLA2 activity and nitrate concentration. In addition to this, NOS mRNA decreases approximately to 25% 72 h PI when RNAi treatments were performed against NO synthase gene (NOS) (Sadekuzzaman et al., 2018). Therefore, it can be deduced that increased NO concentrations stimulate PLA2 and subsequently eicosanoid production leading to aiding in immune function, which in turn drives the molecular cross-talk processes amid NO and eicosanoid signaling. According to the study of Mohamed et al. (2018), hemocytes are the primary site of biosynthesis of NO and de novo production of it indicates its immune response against bacteria. Infecting a flesh fly Sarcophaga (Liopygia) argyrostoma with Micrococcus luteus boosted up the production of NO and immune-reactive lysozymes (IrLys) in the larval hemocyte and fat body cell (Mohamed et al., 2018). The authors found reduced NO and IrLys production upto 72 h PI when they applied three eicosanoid biosynthetic inhibitors: DEX, IBU and PHE and the NO production rises significantly when the treatment is coupled with the bacteria (significant differences with that of saline injected larvae, P < 0.05). Thus Mohamed et al. (2018) confirmed the role of eicosanoid as a mediator and coordinator in iNOS and lysozyme synthesis. However, the experiment also confirmed the elevated NO biosynthesis after treatment with biogenic monoamines (BMAs) such as octopamine (OA) and serotonin (5-hydroxytryptamine [5-HT]) (Mohamed et al., 2018).
Combat against fungal infection and rise of Ca2+ mobilization
Roy et al. (2021) discovered the role of eicosanoid in combating fungal infection in insects. They also chose S. exigua as the model organism. Causative agent of green muscardine of S. exigua in Korea—Metarhizium rileyi, an entomopathogenic fungus was procured as spores and cultured by the authors. The researchers showed that 40% mortality was achieved if the fungus applied topically, but if administered through injection the mortality increased to 64% and within 5 min of infection PLA2 activity rises indicating synthesis of eicosanoids. When coinjection treatment with DEX was performed, the mortality increased more than 90% and nodule formation by hemocytes and phagocytosis were also hampered. The researchers identified that TXA2 and TXB2 having the central role in immune function in S. exigua against fungal pathogen (Roy et al., 2021). In the same study, administration of Napoxen (NAP)—a COX inhibitor, severely affected the Ca2+ signaling showing hemocytes, which were Fura-positive at reduced level and combined effect of NAP+PGE2 rescued the inhibitory effect. Effect of DEX also showed reduced aggregated hemocytes and hemocytes which were Fura-positive and the effect was opposite when DEX was combined with prostanoids (Roy et al., 2021). Inhibitory effect of dazoxiben (DAZ)—thromboxane biosynthesis blocker and terutroban (TTB)—antagonist of thromboxane receptor also lowered the aggregated hemocytes by 20% and 35%, respectively and Fura-positive hemocytes by 10% and 20% respectively (Roy et al., 2021). This experiment also confirms the Ca2+ mobilizing effect of eicosanoid aiding in insect immunity. However, novel Ca2+ independent PLA2s are also discovered, which can steer insect immunity (Park et al., 2015; Sadekuzzaman et al., 2017).
Repat—a new gene family facilitating eicosanoid signaling pathway
A new role of a novel gene family named “Response to Pathogen” or Repat is revealed by Hrithik et al. (2021). Repat gene family is consisting of 46 genes in S. exigua. The investigators infected the insect with Escherichia coli, Enterococcus mundtii, and Metarrhizium rileyi, then extracted RNA and prepared cDNA library from epidermis, hemocytes, fat body, and midgut cells and confirmed its expression in last larval instar. Subsequently, Repat sequence analysis and protein domain structure prediction were also performed which revealed Repat comprises three groups (Group I–III), where Group I represents the largest and Group III corresponds to the smallest (Hrithik et al., 2021). Treatment with DEX subsequently hampered the Repat33 expression confirming the gene's expression is controlled by eicosanoids; Ibuprofen and Esculetin which are Prostaglandin and Leukotriene biosynthesis inhibitors respectively were seen to suppress the expression of Repat33 significantly which also confirmed the role of eicosanoid in Repat33 expression (Hrithik et al., 2021). Hrithik et al. (2021) also proved that after the bacterial challenge the RNAi treatment against Repat33 opposes the hemocyte spreading behavior, which was due to the fact that antimicrobial protein (AMP) gene cecropin was inhibited by RNAi and addition of PGE2 also failed to rescue the effect of Repat33 indicating its downstream role in PGE2 signaling pathway. Kwon et al. (2020) recently discovered that in Munduca sexta a specific receptor is involved in binding with PGE2 and eventually increasing the cAMP level. Sajjadian et al. (2020) showed that cAMP response element binding protein (CREB) is activated by protein kinase A in S. exigua, which was prior activated by cAMP and the CREB activates target genes after translocating to the nucleus. Based on this, Hrithik et al. (2021) also proposed the signaling pathway as PGE2–CREB–Repat33, which requires further investigation.
Systemic immune priming
In insects, immune-priming occurs following a previous exposure to a pathogen. Barletta et al. (2019) did a beautiful experiment on mosquito to see whether prostaglandin has any role in immune priming on the Plasmodium berghei infection or not. The authors let Anopheles gambiae to feed upon P. berghei-infected mice. The researchers demonstrated that female mosquitoes that fed on infected mice exhibited distinct behavior compared to those that fed on healthy mice: the PG levels increased 6 folds, 24 h post feeding (PF) and ookinete invasion induced PG release in the presence of mosquito gut microbiome. Barletta et al. (2019) demonstrated that midgut possesses 10 times higher PG and immunofluorescence study (6 h PF) also revealed strong evidence of presence of PGE2 in the female midgut upon bacterial exposure. Finally, it can be inferred that the immune priming mechanism works in the following way: bacteria that come into touch with epithelial cells when ookinetes infiltrate the midgut cause the expression of heme peroxidases (HPX7 and HPX8), which stimulate the midgut epithelial cells to synthesis PGE2, release of which eventually draws hemocytes, amplifies their activity during patrolling, and initiates immunological priming (Barletta et al., 2019). Currently Haraji et al. (2024) also proved the role of eicosanoid in immune priming in S. exigua and they showed infection by heat-killed E. coli can manifest immune response against a second and different bacterial infection. The authors demonstrated that in addition with the apolipoprotein D (Se-ApoD3) eicosanoids also aid in immune priming. In their experiment, inhibition of LOX resulted in suppression of immune priming and addition of lipoxin A4 or B4 rescued the priming activity (Haraji et al., 2024).
Eicosanoid in reactive oxygen species production
According to Hou et al. (2019) and Leonard et al. (2020), the insect midgut has a physical barrier against infections—peritrophic matrix containing microorganisms that detoxify xenochemicals and aid digestion by supplementing nutrients. However, antimicrobial activities can also be performed by producing reactive oxygen species (ROS) (Ryu et al., 2008; Buchon et al., 2013). According to Kim & Lee (2014) dual oxidase (Duox) catalyses ROS production by Drosophila midgut epithelium. Roy et al. (2022) demonstrated increased level of ROS in S. exigua gut lumen upon infection of Steinernema feltiae—an entomopathogenic nematode. The authors proposed a model showing that as soon as the infection occurs, dorsal switch protein 1 (DSP1) after releasing to the plasma from the nucleus binds to Spätzle1 and activates Toll9 receptor and it in turn activates PLA2 (Mollah et al., 2021). PLA2 increases PGE2, which binds to the PGE2R eventually leading to activation of cAMP, switching on the Se-Duox (Sajjadian & Kim, 2020a). PLC is also activated by cAMP leading to rise of IP3, which upon binding with its receptor releases the Ca2+ from endoplasmic reticulum and creates calcium burst phenomenon by ryanodine receptor, which ultimately leads to activation of Duox and production of ROS (Ahmed & Kim, 2021). The involvement of eicosanoids in reactive oxygen species (ROS) production was substantiated through experimental validation. Specifically, the addition of a PLA2 inhibitor (DEX) and PGE2 had contrasting effects: DEX blocked ROS production, while PGE2 rescued it. Notably, the eicosanoid responsible for ROS production was identified as COX, as reported by Roy et al. (2022). Additionally, in studies conducted by Sajjadian & Kim (2020a,b) on S. exigua and Plutella xylostella respectively, it was observed that eicosanoids produced by activated PLA2 upregulated the expression and activity of the Duox gene. So, the study infers that PGE2 activates Duox, leading to increased Ca2+ signaling and ROS generation. Ahmed et al. (2022) conducted a study on Aedes albopictus, commonly known as the Asian tiger mosquito, which produces a protein called dorsal switch protein 1 (Aa-DSP1), acting like a potential damage-associated molecular pattern (DAMP). Initially this protein can be found in unaffected larval midgut epithelial cells (in the nuclei). Aa-DSP1 relocates to the hemocoel and activates phospholipase A2 (PLA2) upon infection by the pathogenic bacterium Serratia marcescens. This activation leads to an increase in PGE2 levels within the gut, triggering a calcium ion (Ca2+) signal that induces the production of reactive oxygen species (ROS) via dual oxidase (Duox). Ahmed et al. (2022) attempted to inhibit Aa-DSP1 through specific inhibitors or RNA interference but failed to elevate PGE2 and Ca2+ signals during bacterial infection. Consequently, inhibitors, which hinder biosynthesis of eicosanoid, effectively inhibited the upregulation of ROS generation in the gut, resulting in increased postinfection mosquito mortality. However, adding PGE2 successfully restored those inhibitory effects, indicating that Aa-DSP1 plays a crucial role in the mosquito gut's immune response as a DAMP, initiating the DSP1/PLA2/Ca2+/Duox signaling pathway (Ahmed et al. 2022).
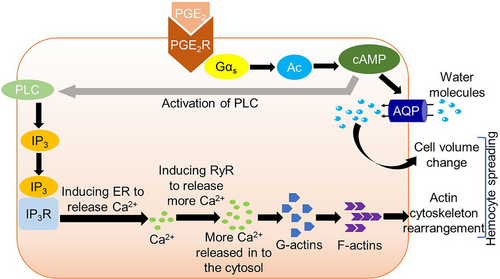
Actin polymerization and activation of aquaporin (Fig. 5)
According to Ahmed & Kim (2019a) and Fauvarque & Williams (2011), hemocyte spreading, an extensive cytoplasmic expansion, requires cytoskeletal reorganization by actin and cell volume change for creating pseudopodia empowering the hemocytes to encapsulate large sized pathogens (Lavine & Strand, 2002). Ahmed & Kim (2021) in S. exigua reported that PGE2R binds with PGE2 and increases cAMP which finally induces G proteins followed by polymerization of actin and aids in hemocyte volume change. The spreading behavior and Ca2+ signal of hemocyte were dependent on cAMP as Ca2+ mobilization hindered upon RNAi treatment against PGE2R expression or inhibition of adenylate cyclase (Ahmed & Kim, 2021). Aquaporins (AQPs) transport water and few other inert materials across the plasma membrane in nearly all organisms (Gonen & Walz, 2006). Ahmed & Kim (2019b) in their previous study identified the AQP, which functions in hemocyte volume changing. However, Ahmed & Kim (2021) confirmed that cAMP modulated the AQP function, which was increased by PGE2 through adenylate cyclase. The activated AQP permits water entry into the cytosol and manifests cell volume change. Joint action of both actin polymerization and water entry by AQP results in cell volume change and hemocyte spreading behavior.
Role of prostacyclin (PGI2)
Moncada et al. (1976) discovered prostacyclin or PGI2. The findings of Ahmed et al. (2021) and Mitchell & Kirkby (2018) confirmed that PGI2 functions as inhibitor of platelet aggregation and vasodilator defining its cardioprotective role as an eicosanoid. Fat body of S. exigua showed an elevated level of PGI2 (around 4 times) upon challenge with bacteria (Ahmed et al., 2021). The investigators confirmed the synthesis of an enzyme: S. exigua PGI2 synthase (SePGIS) in adult fat body and hemocytes and in larval gut. Ahmed et al. (2021) further corroborated their initial findings by employing dsRNA construct to silence the gene expression, resulting in the absence of detectable expression. The authors also found that hemocyte spreading can be achieved by PGs other than PGI2, as treatment with dexamethasone (DEX) can reduce the spreading but DEX+ PGI2 treatment does not show the same result. Their observations led them to deduce that PGI2 plays a suppressive role in hemocyte spreading (Ahmed et al., 2021).
Conclusion
There is much to be discovered if eicosanoid activities in insect defenses are investigated further. However, because of meager functional data on PG receptors, the mechanistic knowledge of PG in immunological activities in insects is not complete. Although a number of predicted GPCRs are considered as PG receptors in insect genome annotations, functional characterization of these in arthropods are wanting (Kwon et al., 2020). According to Stanley & Kim (2011) role of eicosanoid in insect immunity is studied among around 29 insect species belonging to 7 orders. So, more research should be conducted using more model organisms to decipher general principles involving eicosanoid activity in insect immunity. There is a link between Toll/Imd signaling pathway and eicosanoids in red flour beetle (Shrestha & Kim, 2010) but still there is no robust proof of eicosanoid mediating JNK signaling pathway in insect which is another important pathway in innate immune response in insect (Yu et al., 2022). Repat33 was first time identified by Hrithik et al. (2021) in S. exigua larvae, which aids in immunity by eicosanoid signaling pathway. However, presence of this protein is not yet discovered in other insects. Eicosanoids can also function in immune priming. Barletta et al. (2019) and Haraji et al. (2024) confirmed this in mosquito and armyworm respectively. Still, it is not clear whether this priming effect is evident in other insects or not. According to Goodman et al. (2021) immortal arthropod cell lines can be used to carry out research on immunity as these are high-throughput, consistent, and cost effective. The future investigators can adopt this technique in coming days so that least number of insects can be used as model organisms and the biodiversity can be secured. Future works should also be directed to study the cell-type specific immune reactions manifested by eicosanoids, so that detailed idea of a particular immune reaction involving eicosanoids specific to a cell can be understood.
The idea that PGs and other eicosanoids play a critical role in insect immunological responses was initially proposed approximately more than 30 years ago. Identifying precise and targeted techniques to modify eicosanoids should be a major topic of focus for the government, industry, and academic community because these substances influence multiple domains of human and veterinary pathophysiology. Among these are the traditional aspirin and other NSAIDs (nonsteroidal anti-inflammatory medications) which block the key enzyme involved in the manufacture of PG–COX. Much milder NSAIDs are being developed and are currently available across the marketplace. Eicosanoid-mediated insect immunity can also be altered. Researches showed in experimental insects that had their eicosanoid production inhibited are fatally incapable of eliminating bacterial infections from their hemolymph and it has been proved that in insects, infection stimulates the manufacture of eicosanoid compounds. Therefore, the discoveries in various aspects might pave the way to control insect pests in specific cases. It has been already proved (Stanley & Kim, 2014) that insect immunity compromised by RNA interference therapies intended to suppress PG signaling genes. PGs along with various eicosanoids operating under emergency conditions could be obvious targets for creation and application of innovative insect pest management solutions and controlling the insects, which function as vector of many diseases. As, PLA2 is the key player in production of PGs and aiding in insect immunity, this enzyme can be blocked to suppress insect immunity. Shrestha et al. (2010) confirmed this concept by silencing the genes for sPLA2s thus impairing insect immunity. Further, pharmaceutical inhibitors of PLA2s, COX, and LOX also blocked the immunity. Further, increasing the PGI2 synthesis may also be an effective way to suppress insect development as it is negative regulator of insect immunity (Ahmed et al., 2021). The creation of new pest control technology will support global food security as human population is expanding quickly. However, detailed studies in this field are still required to further shape up our understanding.
Acknowledgments
The authors are thankful to Dr. Zarqua Jamal, assistant professor, B.B College, Asansol for her spontaneous help in betterment of the manuscript. The authors would also like to sincerely thank the anonymous reviewers for their insightful feedback that helped to shape the content of the manuscript.
Disclosure
The authors state that they have no conflict of interest.




