Male-derived phospholipase A2 enhances WD46 expression and increases fertility in Ophraella communa
Abstract
Successful bisexual reproduction requires interactions between males and females. Male-derived seminal fluid proteins (SFPs) transferred to females during mating profoundly affect females from pre- to post-mating, and the subsequent shift in female physiology enhances their fertility. SFPs have important evolutionary implications for the fitness of many insects. However, little is known about how females respond to male SFPs. In this study, we identified a male-derived SFP-phospholipase A2 (PLA2) in Ophraella communa. PLA2 is a vital enzyme in eicosanoid biosynthesis; however, it has not been identified as an insect SFP. We found that OcPLA2 is specifically expressed in males, especially in the male accessory glands (MAGs); it is transferred to the female during mating and functions as an SFP to enhance fertility. The expression of a female-derived gene encoding the WD repeat-containing protein 46 (WD46) was upregulated when OcPLA2 entered the female reproductive tract, and this contributed to female egg production by increasing triacylglycerol lipase (TGL) gene expression and the triglyceride (TG) content. This is the first study to identify PLA2 as an SFP in insects. Our findings also shed light on the regulatory role of OcPLA2 in beetle reproduction; the expression of OcPLA2 is initially correlated with female WD46 expression and later with the decline in TGL gene expression and the TG content. This represents a unique mechanism of reproductive regulation by an SFP.
Introduction
Interactions between males and females from premating through post-mating induce shifts in female physiology that increase fertility (Singh et al., 2018). In addition to direct gamete interactions, numerous other functions are indispensable for successful reproduction. During mating in insects (as well as in many other internally fertilized animals), seminal fluid from the male reproductive system (MRT) travels through the ejaculatory duct into the female reproductive tract, which mediates physiological changes (Poiani, 2006; Kerwin et al., 2020). Seminal fluid is a complex mixture of spermatozoa and male-derived proteins, such as seminal fluid proteins (SFPs). SFPs are mainly produced by male accessory glands (MAGs), including many important protein classes such as proteases and protease inhibitors, which play key roles in regulating immunity, lipid metabolism, carbohydrate interactions, and post-mating behavior (Findlay et al., 2009; Avila et al., 2011).
SFPs influence female reproductive physiology, including oogenesis, egg laying, female receptivity, and sperm storage (Avila et al., 2011). These physiological functions imply that the transfer of SFP molecules from males to females initiates important reproductive responses in females (Mack et al., 2006; Markow, 2015), and the female reproductive tract must decipher the repertoire of molecular cues received from the male during copulation to ensure high levels of egg production (Sanchez-Lopez et al., 2022). In Drosophila, the sex peptide (SP) interacts with the sex peptide receptor (SPR), which mediates the transition of the female from a premated state to an active post-mating state (Yapici et al., 2008). The long-term persistence of post-mating changes requires SP and multiple other SFPs.
Identifying the functions of individual SFPs and their interactions with female molecules has been a major challenge because of the complexity of seminal fluid. The ragweed leaf beetle, Ophraella communa (Coleoptera: Chrysomelidae), is a biological control agent of the invasive common ragweed, Ambrosia artemisiifolia (Kim & Lee, 2019; Zhang et al., 2021). O. communa can completely defoliate before pollen production, which relieves damage from Ambrosia allergies worldwide (Guo et al., 2011; Schaffner et al., 2020). We previously showed that larger males of O. communa facilitate population expansion and that SFPs potentially play important roles during population expansion in O. communa. Recent studies have analyzed the proteome of the MRT (accessory glands and testes) of O. communa. We identified a potential SFP, phospholipase A2 (PLA2), which is widespread in nature (Dennis et al., 2011). The first step in arachidonic acid (AA) synthesis is mediated by PLA2s, which can be classified into 3 types: secretory PLA2s (sPLA2s), Ca2+-dependent cellular PLA2s (cPLA2s), and Ca2+-independent cellular PLA2s (iPLA2s) (Burke & Dennis, 2009; Dennis et al., 2011). Toxic and nontoxic sPLA2s have been detected in different insects (Kim et al., 2018; Perez-Riverol et al., 2019; Vatanparast et al., 2019). PLA2s have various biological functions (Schaloske & Dennis, 2006). Recent studies have indicated that sPLA2s play a crucial role in insect immunity and development (Vatanparast et al., 2019; Ji et al., 2022). Given that sPLA2s catalyze the hydrolysis of glycerophospholipids to release fatty acids and lysophospholipids and are important for the biosynthesis of biologically active lipid mediators, they may be involved in various biological processes (Valentin & Lambeau, 2000).
However, whether PLA2 functions as an SFP and is transferred from males to females during mating in insects, as well as how PLA2 alters the gene regulatory networks of female molecules to mediate female fertility, remains unclear. Here, we analyzed the tissue expression patterns of OcPLA2 and its transfer from males to females during copulation. We sequenced the transcriptome of females that were mated with OcPLA2 knockdown males and used RNA sequencing (RNA-seq) to identify female genes activated by OcPLA2. We identified the WD repeat-containing protein 46 (WD46) gene involved in the reproduction of O. communa and found that WD46 was related to the expression of triacylglycerol lipase (TGL) genes and the triglyceride (TG) content. We found that the transfer of OcPLA2 from males to females enhances the expression of WD46, which enhances the fertility of O. communa. Our study provides new insights into the functions of SFP-PLA2 and the role of WD46–TGL in female reproduction.
Materials and methods
Insect rearing
The O. communa populations used in this study were collected from Langfang City, China, in 2020. They were reared on A. artemisiifolia at 27 ± 1 °C and a 12 h : 12 h light/dark cycle (Zhang et al., 2021). Newly emerged adults were identified under a microscope. Females were mated individually with males or kept virgins. At the end of copulation, females were separated from males, and mated bursa copulatrix (M-BC) tissues were dissected at the appropriate post-mating time.
Sample collection
Sexually mature adults were dissected to collect different tissues, including the head, thorax, mag, testis, female reproductive system (FRT), fat body (FB), and bursa copulatrix (BC). In the mating experiments, we placed single virgin females with a virgin male. The matings were observed, and the time at which mating began was recorded. Matings that occurred unusually rapidly (<15 min) were considered invalid according to the previous study (Zheng et al., 2018), given that sperm transfer takes approximately 30 min on average and an average normal mating takes 38.12 min. The M-BC tissue was collected from 30 females immediately after the end of mating (AEM); virgin BC (V-BC) tissue was collected from 30 virgin females of the same age. The samples were stored at −80 °C for subsequent RNA and protein extraction (Zhang et al., 2022). At least 3 independent biological samples were prepared for each treatment and time point for both the real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis and western blot assays.
qRT-PCR analysis
Total RNA was first isolated from the whole body or tissues of O. communa using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Ten individuals were sampled for each tissue type, and 3 biological replicates were performed for each tissue. RNA samples (1 μg of total RNA) were reverse-transcribed using the TransScript One-Step RT-PCR SuperMix kit (TransGen Biotech Co., Ltd, Beijing, China) for complementary DNA (cDNA) synthesis. Full-length cDNA sequences of Ocpla2 were amplified using specific primers (Table S1). The internal controls for qRT-PCR were the RPL4 and RPL19 genes of O. communa (Zhang et al., 2020), and the SYBR Green Master Mix (Yeasen Biotechnology Co., Ltd, Shanghai, China) was used for qRT-PCR. The relative quantitative method followed that used in our previous study (Zhang et al., 2022). The primers used for qPCR are listed in Table S1.
Protein extraction and western blot assays
We dissected the BC of unmated and mated females at different times post-mating and stored the tissue at −80 °C. There were 30 individuals for each tissue type and 3 biological replicates for each tissue. Different tissues for western blot assays were homogenized in RIPA lysis buffer and centrifuged at 12 000 × g and 4 °C for 10 min. After centrifugation, the supernatants were collected to obtain total protein. Protein concentrations were measured using a BCA Protein Assay Reagent Kit (Solarbio). The samples (8 μg of total protein) were loaded onto sodium dodecyl sulfate – polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to a polyvinyl difluoride membrane (Invitrogen) for immunoblotting. The membrane was blocked with 5% nonfat milk and incubated for 1 h, followed by overnight incubation at 4 °C with the indicated primary antibodies at 1 : 5 000. After washing the membrane with Tris-buffered saline with Tween 20 (TBS-T), it was incubated with an antirabbit immunoglobulin G (IgG) horseradish peroxidase-linked secondary antibody (1 : 5 000) (Genescript) following a previously described method (Wang et al., 2021). Western blot signals were visualized using an ECL Chemiluminescence Detection Kit (Thermo Scientific) and photographed using an ImageQuant LAS 4000. An actin polyclonal rabbit primary antibody was used as the loading control.
Immunofluorescence microscopy
Immunofluorescence microscopy was performed according to the immunofluorescence application solution kit protocol (Cell Signaling Technology) with some minor modifications. Briefly, TE, M-BC, and V-BC samples were fixed with 4% formaldehyde in phosphate-buffered saline (PBS) for 60 min, permeabilized with blocking buffer for 60 min, and incubated overnight at 4 °C with rabbit PlA2 antibody. After 5 washes in PBS-T, Alexa Fluor 594 goat antirabbit IgG secondary antibody (Cell Signaling Technology, 1 : 500) was added for 1 h at room temperature. Samples were extensively washed again with PBS-T and stained with 4ʹ,6-diamidino-2-phenylindole (DAPI: 1 μg/mL, 10 min). Images were captured and processed using a confocal laser microscope. The confocal microscopy data were processed using the ZEN 3.2 Image Manipulation Program.
RNA interference
The PLA2-specific region of O. communa was used as the DNA template for double-stranded RNA (dsRNA) synthesis. A MEGAscript T7 High-Yield Transcription Kit (catalog no. AM1334; Ambion) was used to synthesize the dsRNAs of PLA2 according to the manufacturer's instructions. The product concentration was quantified using a P330 Nanophotometer (Thermo Fisher Scientific). The method for synthesizing the dsRNA of other candidate genes in this study was the same as that for dsPLA2 synthesis. The primers used for dsRNA synthesis are listed in Table S1. The injection method was based on that described in a previous study (Zhang et al., 2022). Approximately 500 ng of dsRNA in 100 nL was microinjected into the abdomen of newly emerged adults (males and females <12 h after eclosing) using the Nanoject III Programmable Nanoliter Injector.
RNA-seq and functional study
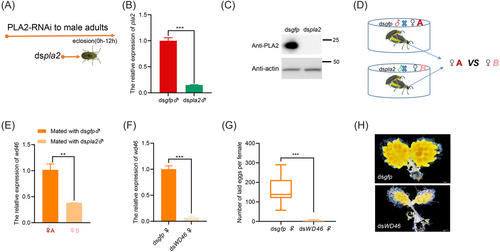
Extraction of total RNA from the mates of PLA2 knockdown males and their corresponding controls was performed using TRIzol reagent (Invitrogen, MA, USA). Library construction and RNA-seq were performed by Novogene (Beijing, China), and this was followed by computational analysis. HISAT2 software was used to quickly and accurately compare clean reads with the reference genome to obtain the location information of reads in the reference genome. Differential expression analysis was performed using the DESeq2 R package (1.20.0). We used log2|(FoldChange)| > 1 and Padj ≤ 0.05 as the criterion for differential gene screening. Ten individuals were sampled in each treatment, and there were 3 biological replicates per treatment. Transcriptome data were verified using qPCR. Next, the dsRNA of candidate genes was synthesized for functional studies, and a bioassay for O. communa fecundity was performed following a previously described method (Zhang et al., 2022). Briefly, the newly emerged O. communa adults were anesthetized on ice for 15–30 s, and dsRNA was injected into the insect abdomen using a Nanoject III system (Drummond Scientific, Broomall, PA, USA). Virgin males collected 12 h after emergence were injected with dsgfp and dspla2, reared for 2 d, and then mated with wild-type females. Virgin females collected 12 h after emergence were injected with dsgfp, dsWD46, and other candidate gene dsRNAs, reared for 2 d, and then mated with wild-type males. Each pair was reared separately and maintained on common ragweed for further bioassays. The number of eggs laid by females was counted daily. Ovarian development after injection was observed using an OLYMPUS SZX16 stereomicroscope.
TG content measurement
Female adults, female FB, and the FRT were collected after dsWD46 injection; dsgfp was used as a control, and the samples were used for TG content determination using the Triglyceride Assay Kit (Applygen Technologies, Beijing, China) following the manufacturer's instructions. Protein concentrations were analyzed using a BCA Protein Assay Kit (Solarbio) according to the manufacturer's instructions. The methods followed those described by Wang et al. (2021).
Data analysis
Statistical analysis was performed using SPSS 22.0 and GraphPad Prism 8.0. Given that the data met the assumptions of analysis of variance (ANOVA), the statistical significance of the experimental data was analyzed using one-way ANOVA with Tukey's least significant difference (LSD) test (α = 0.05). Differences were considered significant at P < 0.05 (*P < 0.05, **P < 0.01, and ***P < 0.001). Values are shown as mean ± standard error.
Results
Gene identification and sequence analysis of the pla2 gene in O. communa
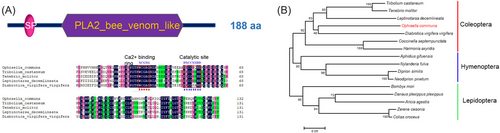
O. communa pla2 cDNA was cloned according to previously sequenced genomic data (unpublished data). The sequences of pla2 contained complete open reading frames of 567 bp, which encoded 188 amino acid residues. PLA2 belongs to the PLA2 family and has a molecular weight of 18.59 kDa. The protein sequence of PLA2 has a PLA2_bee_venom_like functional domain. PLA2 contains a 19-amino acid signaling peptide, a highly conserved Ca2+-binding site (XCGXGG), and a catalytic site (DXCCXXHD) (Fig. 1A); therefore, it is a sPLA2. To clarify evolutionary relationships among PLA2s, a phylogenetic tree was constructed using the PLA2_bee_venom_like domain proteins from insects according to their annotations. Phylogenetic analysis revealed that O. communa PLA2 was closely related to Diabrotica virgifera virgifera PLA2, which is consistent with their close phylogenetic relationship (Fig. 1B).
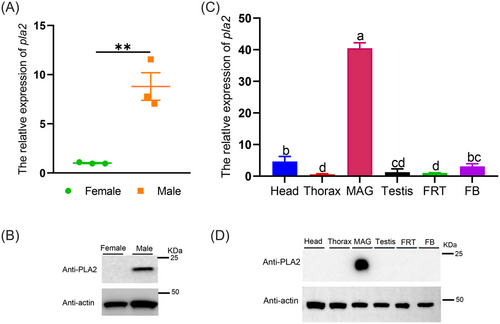
PLA2 is especially expressed in male adults and MAGs in O. communa
The distributions of genes and proteins are closely related to their functions. We investigated the expression of the pla2 gene in both sexes and different tissues by qRT-PCR analysis using a relative quantitative method and analyzed the expression of PLA2 protein using western blot. The pla2 gene and its encoded protein were expressed at higher levels in males than in females (Fig. 2A, B); specifically, the expression of pla2 was 8.77-fold higher in males than in females (Fig. 2A). Furthermore, the expression levels of PLA2 varied among tissues; pla2 expression was highest in the MAG, and it was 40.35-fold higher in the MAG than in the FRT (Fig. 2C). There were no significant differences in the expression of pla2 between the thorax, testis, and FB. The protein levels and messenger RNA (mRNA) levels in different tissues were similar (Fig. 2D).
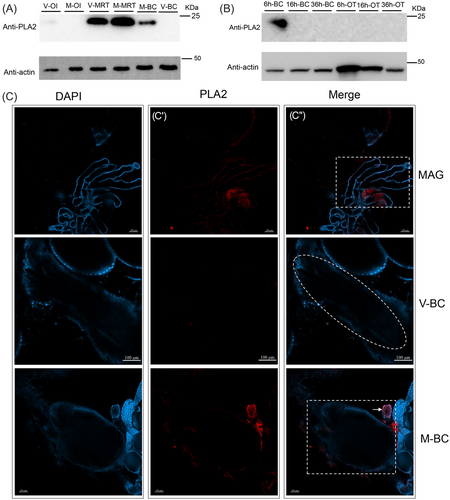
PLA2 was transferred from males to females during copulation
To determine whether PLA2 is an SFP, we measured the expression of PLA2 in different tissues before and after male and female mating using western blotting and immunofluorescence assays. High expression of PLA2 was observed in MRT (both mated- and virgin-MRTs) (Fig. 3A). As expected, PLA2 protein levels were detected in M-BCs after mating but not in the V-BCs and ovarioles (OI) of mated or virgin females (Fig. 3A). We next examined the expression of PLA2 in the BC and OI at different time points (AEM-6 h, AEM-16 h, and AEM-36 h) to determine how long it is expressed in the lower reproductive tract of mated females and found that PLA2 expression was only detectable in AEM 6 h-BC and not at 16 and 36 h (Fig. 3B). After establishing that PLA2 is transferred from males to females, we performed immunofluorescence staining to localize PLA2. The results showed that PLA2 was produced in the short accessory glands of males (Fig. 3C–C″) and was not detected in the lower reproductive tract of virgin females (Fig. 3C–C″); however, it was present in large amounts in the inner BC and the spermatheca of females after mating (Fig. 3C–C″). In addition, the size of the PLA2 protein was not altered in MAGs and M-BC, which means that PLA2 was not cleaved during its transfer or in females (Fig. 3).
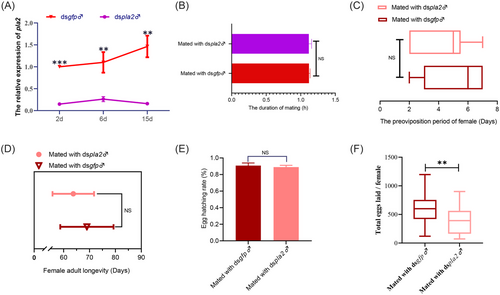
PLA2 was responsible for the reproduction of O. communa
To evaluate the significance of PLA2 as an SFP in females, we used microinjections to knock down PLA2 expression in the MRT of O. communa. The results showed that injection of dspla2 induced a 96%, 79%, and 99% reduction in mRNA levels in the MRT compared with the dsgfp control 2, 6, and 15 d after injection, respectively (Fig. 4A), and we observed no difference in mating duration between PLA2-RNAi males and control males (Fig. 4B), or in the preoviposition, longevity, and hatching rate of the offspring of females mated with PLA2-RNAi males compared with control individuals (Fig. 4C–E). However, total oviposition by mates of PLA2-RNAi males was 34% lower on average compared with that of dsgfp control individuals (Fig. 4F), implying that PLA2 is involved in the reproductive process of O. communa.
PLA2 activates the expression of WD46 in females to initiate the reproduction process
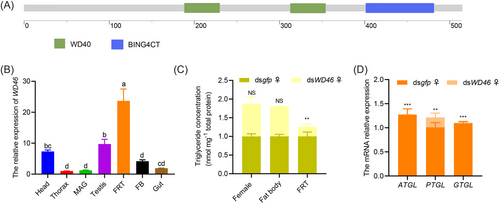
Interactions between male and female molecules are necessary for the production of offspring. To identify candidate genes activated by PLA2 in females, we constructed a differential transcriptome between females mated with dspla2-injected males and females mated with dsgfp-injected males (♀ mated with dspla2-injected ♂ vs. ♀ mated with dsgfp-injected ♂). First, dspla2 was injected into the abdomen of newly emerged males (0–12 h) (Fig. 5A), and dsgfp was injected into another group of males as a control. The expression of PLA2 was significantly silenced at both the transcriptional and protein levels by RNAi (Fig. 5B, C), and pla2 expression in males injected with dspla2 was 85% lower than that of control males. Males (the same males used for determining gene expression levels) in the treatment (injected with dspla2) and control groups (injected with dsgfp) were mated with virgin females of the same age and reared in single pairs to observe their mating (Fig. 5D). Transcriptome samples were collected from females at AEM-16 h and AEM-36 h. Summary statistics of the RNA-seq results for PLA2-RNAi-treated individuals are listed in Table S3. Transcriptome data were validated using qPCR (Fig. S1, Fig. 5E), and functional studies were conducted on 10 genes with downregulated mRNA expression in females mated with dspla2-injected males (Table S2). The expression of each of these 10 genes was silenced in mated females (Fig. S2, Fig. 5F), and only knockdown of the WD46 gene caused a reduction in female fecundity (Fig. S3, Fig. 5G); yolk synthesis in the ovaries was reduced in females with WD46 knocked down compared with control females (Fig. 5H), indicating that the activation of the WD46 gene by PLA2 is required for the reproductive process in females. WD46 in O. communa contained 2 WD40 domains (Fig. 6A) and was highly expressed in FRT tissues (Fig. 6B). Moreover, the TG content was 74% lower in FRT tissues in WD46 knockdown females than in control females, and no difference in the TG content was observed in the female FB and female adults (Fig. 6C). The decrease in the TG content in the FRT of WD46 knockdown females was accompanied by a 99% reduction in ATGL expression (adipose TGL), 80% reduction in PTGL expression (pancreatic TGL), and 98% reduction in GTGL (gastric TGL) expression (Fig. 6D).
Discussion
The initiation of the reproductive process following male–female copulation depends on the release of SFPs (Bromfield, 2014). SFPs interact with female molecules, neurons, and pathways to transduce the female from a “poised” premated state to an active post-mating state, which leads to high levels of egg production (Avila et al., 2016). PLA2 is involved in the first rate-limiting step in eicosanoid biosynthesis, and products such as AA and lysophospholipids are precursors for signaling molecules (Schaloske & Dennis, 2006). However, few studies have investigated the role of PLA2 in insect reproduction. We present evidence suggesting that PLA2 is a vital SFP for male reproductive success in O. communa.
The PLA2 of O. communa was discovered in the proteome of the MRT and was highly abundant (unpublished data). Our results showed that it was expressed specifically in male adults of O. communa and accumulated only in the MAG according to analysis of transcript and protein levels (Fig. 2). However, the expression patterns of PLA2 in other insects are vastly different from those observed in O. communa. For example, PLA2 is highly expressed in the hemolymph and gonads (testis and ovary) of Bombyx mori (Li et al., 2023), the FB and gut of Ostrinia furnacalis (Ji et al., 2022), and the testis tissue of Bactrocera dorsalis (Li et al., 2017). This study provides the first evidence that PLA2 is a male accessory gland-specific transcription factor (Fig. 2). The accessory glands of male insects are secretory tissues of the genital tract and are composed of several distinct cell types. These glands secrete components of ejaculatory fluid in Drosophila (Avila et al., 2016). Additionally, these glands are also the site of prothoracicotropic hormone-controlled ecdysone synthesis (Hentze et al., 2013). Proteins secreted by MAG are usually considered candidate SFPs. Western blotting results indicated that PLA2 of O. communa is present in the MRT (M-MRT or V-MRT) and M-BC of females but absent in the V-BC and OI (M-OI or V-OI) of females (Fig. 3); therefore, PLA2 was confirmed to be a novel SFP which is transferred from males to females during mating. The fate of PLA2 in mated females was different from that of other SFPs. In Drosophila melanogaster, 2 SFPs (msP 355a and msP 355b) are transferred to the female genital tract between 5 and 10 min after the beginning of copulation, and msP 355a is subject to rapid and specific cleavage within the female genital tract (Monsma et al., 1990). During normal mating, SFPs were only transiently present in mated females. However, in contrast to msP 355a and msP 355b, the PLA2 of O. communa could still be clearly detected in the 6 h-BC samples of mated females but was not detected in the 16 h-BC samples after being transferred (Fig. 3), which is consistent with the state of the BC (M-BC was full in females within approximately 6 h of mating but reverted to a virgin-like state after approximately 6 h). Similarly, during mating, a coagulated mating plug that packaged the SFPs is digested for 1–2 d in Anopheles gambiae (Gabrieli et al., 2014). The immunofluorescence results from our study indicated that SFP-PLA2 was located in the lumen of O. communa MAG and was transferred to the inner BC and the spermatheca of mated females (Figs. 2 and 3). Therefore, the retention time of SFPs within the female genital tract varies with protein type and among species.
Following their transfer during mating, SFP molecules exert wide-ranging effects on female reproductive activity and increase the male's chances of siring a significant proportion of female offspring (Avila et al., 2011). Two of these changes include increased egg production and reduced mate longevity. Using RNAi to knock down the expression of SFP-PLA2, we showed that the mating duration of O. communa females mated to pla2-RNAi males and control males (females that mated with dsgfp-injected males) did not differ; similar results were observed for the female preoviposition period, female adult longevity, and egg hatchability of offspring. However, oviposition was lower in females that were mated to males with interfered pla2 expression compared with control females (Fig. 4); these findings are consistent with the results of a previous study of Rhinella arenarum showing that PLA2 activation induces oocyte maturation (Ortiz et al., 2014). In contrast to our results, silencing of the pla2 of B. dorsalis, which is preferentially expressed in the testis, resulted in a reduction in the egg hatchability of the mate but did not affect the number of eggs (Li, 2018). This finding indicates that the survival rate of sperm was reduced. Most previous studies on PLA2 have reported that it is involved in the immune response of some insects (Li et al., 2017; Ji et al., 2022), which is consistent with its biased expression in the hemolymph. Tissue-specific transcription of accessory gland genes may modulate female behavior (Dottorini et al., 2007). This is consistent with our results; the first reported SFP-PLA2 is vital for O. communa reproduction, which might stem from its specific expression in the MAG (Figs. 2 and 3).
The mechanism by which males and females alter their physiological state has become clarified as our understanding of the roles of specific SFPs has improved (Sirot, 2019). Some important SFPs, such as msP 355a (Monsma et al., 1990), the glycoprotein Acp36DE, and the prohormone ovulin (Avila et al., 2011; Gabrieli et al., 2014), are proteolytically cleaved either during or after mating to perform their functions (Ravi Ram et al., 2006; LaFlamme et al., 2012). However, in our study, we found that PLA2 of O. communa was not cleaved in the female reproductive tract. Thus, its effects on females must be mediated by another pathway and female molecules.
In model insects, the effects of SFPs on transcriptional changes in mated females have been examined by microarray analyses, and the expression of over 1 700 transcripts is altered 1–3 h post-mating in females (Mack et al., 2006). Virtually nothing is known about the contribution of these genes to the post-mating behavior of females. We silenced male PLA2 expression, constructed a female differential transcriptome (♀ mated with dspla2-injected ♂ vs. ♀ mated with dsgfp-injected ♂), and identified 10 downregulated genes that might be correlated with PLA2 to verify their function (Table S2, these genes are involved in AA metabolism, lipid transport, insulin metabolism, and protein interactions). We found that the silencing of WD46 greatly reduced female fertility (Fig. 5), and few eggs and only immature eggs were observed in female ovaries (Fig. 5). The long-term phenotypes of females mated with PLA2 knockdown males were similar to those of females that failed to express WD46, an important WD repeat family protein involved in signal transduction, RNA processing, and gene regulation during development (Hollmann et al., 2002). In general, these proteins have regulatory functions, and no member of this class of proteins has been shown to have a major effect on female fecundity. In our study, the reduced fecundity of WD46 knockdown females suggests that it plays a role in reproductive regulation (Fig. 5). Lipids are essential for egg synthesis and development, and the inhibition of WD46 expression might hinder the accumulation and invasion of lipids, thereby hindering egg formation and ovary development. Furthermore, in light of the absence of yolk in the ovaries of WD46-silenced females, we found that the lipid content of the ovaries was significantly reduced, and the expression of the TGL genes (ATGL, PTGL, and GTGL) decreased, suggesting that WD46 might mediate the synthesis and invasion of lipids to regulate female reproduction.
According to these results, we propose that PLA2 is a novel SFP for regulating the reproduction of O. communa. Once PLA2 is transferred to the female during mating, the expression of a female-specific gene, WD46, is activated, and lipids for egg production and development are synthesized. A similar model has been demonstrated for Drosophila SP. Specifically, the SP is released into the female; it interacts with the SPR, which induces a shift in females from a premated state to an active post-mating state; and females continue to lay eggs at a high rate (Hopkins & Perry, 2022). The interactions between male- and female-derived molecules are complex and require long-term studies. Our study reveals a new pathway (SFP-PLA2-WD46-lipid) that regulates insect SFPs and suggests that SFP cascades of female molecules and lipid signaling may be a mechanism for the reproduction of post-mating responses in females; these findings provide new insights that could aid future research on SFP evolution theory. Study of the novelly identified SFP-PLA2s could potentially provide insights that could improve the fecundity of O. communa, thereby enhancing their potential for the control of ragweed.
Acknowledgments
We thank Liwen Bianji (Edanz) (www.liwenbianji.cn) for editing the English text of a draft of this manuscript. This study was supported by the China-EU joint action for promoting innovation and development of integrated pest management in agriculture (2023YFE0104800) and China Postdoctoral Science Foundation (Nos. 2023M743842).
Disclosure
All the authors declare no conflicts of interest associated with this work.




