Current paradigms and future challenges in harnessing gut bacterial symbionts of insects for biodegradation of plastic wastes
Abstract
The ubiquitous incorporation of plastics into daily life, coupled with inefficient recycling practices, has resulted in the accumulation of millions of metric tons of plastic waste, that poses a serious threat to the Earth's sustainability. Plastic pollution, a global problem, disrupts the ecological balance and endangers various life forms. Efforts to combat plastic pollution are underway, with a promising avenue being biological degradation facilitated by certain insects and their symbiotic gut microorganisms, particularly bacteria. This review consolidates existing knowledge on plastic degradation by insects and their influence on gut microbiota. Additionally, it delves into the potential mechanisms employed by insects in symbiosis with gut bacteria, exploring the bioconversion of waste plastics into value-added biodegradable polymers through mineralization. These insights hold significant promise for the bio-upcycling of plastic waste, opening new horizons for future biomanufacturing of high-value chemicals from plastic-derived compounds. Finally, we weigh the pros and cons of future research endeavors related to the bioprospection of plastic-degrading bacteria from underexplored insect species. We also underscore the importance of bioengineering depolymerases with novel characteristics, aiming for their application in the remediation and valorization of waste plastics.
Introduction
The Earth is burdened by substantial loads of a noxious polymeric substance referred to as plastics. Plastic polymers, derived from fossil oils, are ubiquitously employed in various industries such as construction, food packaging, automotive, electronics, health, and chemicals (Tilsted et al., 2023). The demand for plastic polymers has tripled during the last 3 decades, propelled by the advantages offered by various types of plastics over alternative materials like glass, wood, and metals (Table 1) (Dong et al., 2024). Over the past 5 decades, plastic production has surged exponentially (Bombelli et al., 2017; Bertocchini & Arias, 2023), resulting in an output exceeding 830 million tons (Bao et al., 2023). In 2021, alone, global plastic manufacturing reached over 390.7 million metric tons (Yang et al., 2023). The production of plastics is expected to increase significantly in coming years due to rising populations and continuous demands. In 2022, the global plastic market size reached USD 609 billion, and the annual compound growth rate is expected to increase by 4% between 2024 and 2030 (De Filippis et al., 2023).
| Sl no. | Type of the plastic | Composition | Applications |
|---|---|---|---|
| 1. | Acrylonitrile butadiene styrene (ABS) | Acetonitrile, butadiene, styrene | Pipes, electronics, vehicle spares, protective head gears |
| 2. | Polyamide | Amides, α,ω-amino acids, diamine, diacid | Carpets, garments, ropes, gears, seatbelts |
| 3. | Polyethylene | Ethylene | Wire insulations, bottles, toys, bags |
| 4. | Polyimide | Dianhydride, diamine, diisocyanate | Medical tubing, high-temperature adhesive |
| 5. | Poly-methyl methacrylate | Propylene, benzene | Construction, automotive industry, medical |
| 6. | Polypropylene | Propylene | Automotive industry, packaging for consumer products, textiles |
| 7. | Polystyrene | Styrene | Food packaging, laboratory ware, electronics, automotive parts |
| 8. | Polytetrafluoroethylene | Tetrafluoroethylene | Cookware, buildings, dental clinics, pipes, machinery, gaskets |
| 9. | Polyvinyl chloride | Vinyl chloride | Construction, health care, electronics, automobile |
| 10. | Acrylic resins | Acrylic acid, methacrylic acid, other related compounds | Decorative panels, adhesives, coatings, tiles |
| 11. | Epoxy resins | Epoxy monomers, acidic hydroxy groups, epichlorohydrin | Adhesives, plastics, paints, coatings, sealers |
| 12. | Polyester resin | Dibasic organic acids, polyhydric alcohols | Boats, flat roofing, pond building, arts, crafts, surfboards, custom mold |
| 13. | Polyurethane resin | Polyol, isocyanate | Furniture, automotive interiors, packaging |
| 14. | Silicone resin | Oligo-siloxanes | Automotive, electronics, industrial molds, packaging, artistic replications, toys |
| 15. | Vinyl resin | Styrene, methacrylic acid, epoxy | Water pipe, window frames, gutters and downspouts, tiles, electronics |
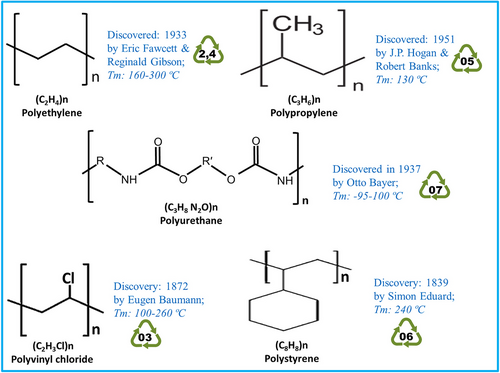
The overall production of plastics, dominated by single-use convenience products, has escalated at an alarming rate. Among plastics, 4 predominant polymers, polyethylene (PE), polypropylene (PP), polystyrene (PS), and polyurethane (PUR), contribute to more than 92% of total plastic production (Fig. 1). PE, comprising approximately 40% of the total demand for plastic products, is extensively used for packaging (Ritchie et al., 2023), resulting in the use of over a trillion plastic bags annually. PS, a synthetic polymer (poly[1-phenylethene]) of styrene monomer, commercially manufactured from petroleum, is employed in the packaging industry (Kim et al., 2020), apart from the production of various items such as CD cases, plastic cutlery, and Petri dishes (Maul et al., 2007). Constituting 6.6% of the total utilization of plastics, PS is used in the packaging industry, disposable cup production, and manufacturing of insulation products (Plastics Europe, 2018). The widespread usage of PUR gained momentum during World War II, replacing the then-expensive and hard-to-obtain rubber (Brzeska & Piotrowska-Kirschling, 2021). It can be tailored into rigid or flexible forms to suit end-user applications. It is commonly used as insulation in freezers, refrigerators, and cars, apart from adhesives, coatings, and sportswear (Das & Mahanwar, 2020). PP is a soft, flexible material, and more environment friendly than other polymers due to its recyclable nature, quick degradation, and release of fewer toxins than polyvinyl chloride (PVC). The most common applications for PP include yogurt cups, medicine, and water bottles with a hazy appearance (Traxler et al., 2024). Globally, an estimated 1 trillion plastic bags manufactured from PE are utilized, necessitating an annual production of over 45 million tons (Wagner, 2017). Throughout the years, over half of used plastics have been carelessly disposed of into the environment with the majority of these recalcitrant polymers finding their way into landfills or oceans, contributing to the accumulation of pollutants (Geyer et al., 2017). Due to the prevailing technological constraints, a substantial majority, approximately 49%, of the plastic materials are dumped or landfilled. Concurrently, 19% of plastic wastes are subjected to incineration, while a modest fraction of merely 9% undergoes recycling processes, showcasing a notable disparity in sustainable waste management for the plastics industry (Kumar et al., 2021; OECD, 2023). The limited recycling of plastics is attributed to various factors including cost, consumer acceptance, safety regulations related to recycled materials, and so forth (Wierckx et al., 2015). Additionally, the majority of plastic types are difficult to decompose due to their distinctive and complex molecular structures.
The continuous accumulation of plastics and their leaching into the soil and marine habitats have adversely affected the environment as well as life on Earth. Plastics are known to show negative impacts on the activity and diversity of soil organisms, impacting their reproductive processes (Lahive et al., 2019). Plastic waste also impacts aquatic ecosystems, especially it affects the sensation and endocrine system of marine animals (Jung et al., 2023). Consequently, many approaches have been applied for the mitigation of plastic pollution, including incineration, recycling, and landfill. However, each of these approaches owns a set of demerits. For example, the combustion of certain plastic polymers produces more hazardous and volatile chemicals such as furans, dioxins, heavy metals, and sulfides, all of which are carcinogenic (Verma et al., 2016). Similarly, the process of landfilling causes contamination of surrounding soil and water, affecting wildlife, besides occupying large space and being relatively slow for the complete decomposition of plastics (Kehinde et al., 2020). Recycling plastics has been noted as inefficient due to their elevated costs, as recycled products prove more expensive than virgin plastics (Gradus et al., 2017). Moreover, the down-cycling of plastics creates products of lower value and reduced functionality as compared to their virgin counterparts. Although some heat and photocatalytic methods have proved effective for the degradation of PS (Patnaik et al., 2020), these approaches themselves inflict environmental pollution. Additionally, the remediation of plastic pollution is far too big to rely on photocatalysis or thermal strategies alone.
In contrast, the use of enzymes for biodegradation is emerging as a promising strategy, being foreseen as more efficient, plausible, and sustainable. Correspondingly, there has been a significant focus in recent years on scientific research exploring biological systems as efficient alternatives for the biodegradation of these pollutants. The biological transformations are mediated through the extracellular enzymes that cleave the polymer into short-chain carbon-ring compounds, acids, and alcohols (Lee et al., 2023a). The discovery of these biocatalysts has demonstrated the feasibility of bio-upcycling plastic waste as an alternative substrate for chemicals like surfactants, and biopolymers for establishing a polymer-based circular economy (Lee et al., 2023b). However, the natural metabolic pathways are not the sole alternatives to address plastic waste pollution. The recent breakthrough in metabolic engineering and synthetic biology with genome editing techniques have opened up new avenues for incorporating exogenous pathways or creating entirely novel metabolic routes for plastic degradation (Tiso et al., 2022). Current scientific investigations are actively exploring the bio-upcycling of plastic waste into value-added commodity products like lactic acid, adipic acid, bioplastics, ethylene glycol, and so forth, aimed at a circular plastic economy (Ruthi et al., 2023).
The biodegradation process is mediated by some organisms that have developed a capacity to digest plastic polymers during their evolution. Biodegradation is influenced by several factors, including substrate availability, molecular weight, and surface morphology of the polymers (Cai et al., 2023; Yang et al., 2023). Additionally, the assessment of biodegradation has been conducted through diverse metrics, encompassing substrate weight reduction, alterations in structure or mechanical attributes of the polymer, and the quantification of carbon dioxide emissions (Mamtimin et al., 2023). Biodegradation involves the depolymerization of high molecular weight polymer chains by enzymes into intermediate compounds with differing properties which allow and enhance their assimilation by the organisms (Zhang et al., 2022). To date, several organisms, including bacteria (Lear et al., 2021), fungi (Koitabashi et al., 2012), and invertebrate animals (Peng et al., 2019; De Filippis et al., 2023), have been identified as capable of transforming plastic polymers into elemental molecules of CO2 and H2O.
Among invertebrates, insects represent the predominant group that has gained scientific attention. In recent years, the identification of insects capable of plastic degradation, such as mealworms (Liu et al., 2023), and wax moths (Sanluis-Verdes et al., 2022), has opened up promising avenues for research. The ability of wax moths, superworms, and mealworms, to ingest, break down, and mineralize various plastics has been proven in their larval phase, albeit with the augmentation from their gut microflora (Zhang et al., 2022). Hitherto, several microorganisms including bacteria are known to supplement the plastic degradation capacity of the host insect (Janakiev et al., 2023). Exploring the intestinal functional microorganisms associated with these insects has offered novel perspectives for enhancing the efficiency of plastic biodegradation, waste recycling, and the development of innovative approaches for the recycling and disposal of plastic waste. In line with the importance of microbes as plastic degraders, the present review offers in-depth insights into the functions of various insects and their enzyme-secreting gut bacterial symbionts in the biodegradation processes of synthetic plastics. Here, we provide an overview of recent advancements in the biodegradation of synthetic plastics including PE, PS, PP, PVC, polyethylene terephthalate (PET), and PUR specifically within the gut system of insects. Additionally, it delineates the enzymes pivotal to the biodegradation process. Moreover, comprehensive details of the potential mechanisms utilized by these natural biocatalysts, along with the factors that impact plastic degradation, are also highlighted. Although the mechanism of plastic biodegradation by microbes in the environment is well established (Bilal et al., 2021; Ali et al., 2021a; Ali et al., 2021b; An et al., 2023), the detailed understanding of the mechanism of plastic degradation within the gut systems remains largely elusive. Therefore, this article emphasizes the potential mechanism of plastic degradation within the gut system of insects. Undoubtedly, the frequency of plastic degradation within the gut of insects is relatively faster when compared to photocatalytics and biodegradation in the environment (Yang et al., 2023). We believe that this article would serve as a valuable reference for academics and policy-makers to develop innovative strategies based on insect–bacterial symbiosis to combat plastic wastes and convert it into monomers, carbon dioxide, and added-value byproducts.
Review methodology
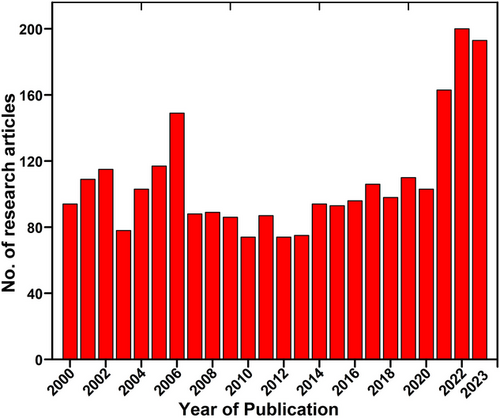
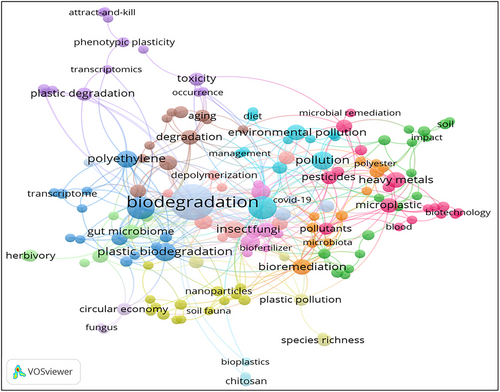
In this review, a total of 2 594 research articles were retrieved from the Scopus and Web of Science databases through a search using the keywords “plastic degradation” and “insects”. The articles included in this review were published during the years 2000 to December 16, 2023, except few examples that highlight the historical details of plastic degradation by insects (Fig. 2). The VOS viewer software was employed, along with an examination of pertinent keywords given by authors. The findings revealed that 10 represented the minimum frequency of keyword occurrences. Following this, a network map illustrating the terms frequently used in this research is presented in Fig. 3. The connections between the circles are represented by lines, with color and size denoting the cluster category and frequency of each specific keyword, respectively. The analysis indicates that these articles predominantly emphasize 3 aspects: (1) biodegradation, plastic biodegradation, and PE (blue cluster); (2) involvement of gut microbiome, impact, and herbivory (green cluster); and (3) microplastics. Biotechnology, heavy metals, biofertilizer, and fungi (pink cluster); (4) plastic pollution, soil fauna, and species richness (yellow cluster); and (5) phenotypic plasticity, plastic degradation, transcriptomics, and toxicity of plastics (purple cluster). These keywords underscore the remediation of plastics by symbiotic microbiota of insects, focusing on microbial representatives, and their mechanisms for the degradation and valorization of waste plastics. In addition, these key aspects highlight the hotspots of future research on the bioremediation and upcycling of plastic wastes.
Taxonomically diverse groups of insects can degrade plastics
In the realm of plastics, each polymer presents its unique molecular riddle for biodegradation which challenges scientists to discover novel techniques that are efficient, fast, and can degrade multiple types of plastic polymers. The degradation of plastics occurs very slowly in natural environments, taking several years for complete mineralization depending upon the nature and chemical composition of the polymer (Wilkes & Aristilde, 2017). In contrast, the degradation of plastics inside the gut environment of insects is comparatively faster, accomplished in just a few weeks (Bertocchini & Arias, 2023; Jung et al., 2023). It has been proven that plastics can be metabolized into biodegradable matter by some insects (Table 2), and microbes (Yang et al., 2014; Zhang et al., 2022). The discovery of plastic degradation by insects and their symbiotic gut microbiota, particularly bacteria, was a surprising one, as some researchers in China observed that the larvae of waxworms can chew and damage PE packaging bags (Yang et al., 2014). Subsequently, several researchers carried out multiple tests and observed that the insects in collaboration with gut symbionts were indeed accountable for the degradation of PE films (Yang et al., 2014; Zhang et al., 2022). Thus, the use of insects to transform plastics and other polymers is one promising strategy attracting attention worldwide (Urbanek et al., 2018; Danso et al., 2019).
| Taxonomic name | Common name | Order | Family | Natural diet | Metamorphosis | Plastic-based diet | References |
|---|---|---|---|---|---|---|---|
| Galleria mellonella | Greater wax moth | Lepidoptera | Pyralidae | Honeycombs | Complete | PE | Sanluis-Verdes et al. (2022); Spínola-Amilibia et al. (2023) |
| Alphitobius diaperinus | Lesser mealworm | Coleoptera | Tenebrionidae | Stored grains | Complete | PS | Cucini et al. (2020) |
| Tenebrio molitor | Yellow mealworm | Coleoptera | Tenebrionidae | Wheat flour or bran | Complete | PVC, PS, PP, face masks | Yang et al. (2015a, b); Yang et al. (2018a, b); Brandon et al. (2018); Aboelkheir et al. (2019); Peng et al. (2019); Wang et al. (2023); Xu et al. (2023) |
| Zophobas atratus | Superworm (giant mealworm) | Coleoptera | Tenebrionidae | Pupae, guano cannibalism | Complete | PBS, Styrofoam | Kim et al. (2020); Yang et al. (2020); Jung et al. (2023) |
| Zophobas morio | Superworm | Coleoptera | Tenebrionidae | Pupae, Guano Cannibalism | Complete | Styrofoam | Yang et al. (2020); Rumbos and Athanassiou (2021) |
| Tenebrio obscurus | Dark mealworm | Coleoptera | Tenebrionidae | Grains | Complete | PS | Peng et al. (2019) |
| Plesiophthophthalmus davidis | Darkling beetle | Coleoptera | Tenebrionidae | Omnivorous | Complete | PS | Woo et al. (2020) |
| Lasioderma serricorne | Cigarette beetle | Coleoptera | Ptinidae | Tobacco | Complete | PE, PS | Riudavets et al. (2007) |
| Rhizopertha dominica | Lesser grain borer | Coleoptera | Bostrichidae | Stored grains | Complete | ||
| Sitophilus oryzae | Rice weevil | Coleoptera | Curculionidae | Stored grains | Complete | ||
| Tribolium sp. | Flour beetle | Coleoptera | Tenebrionidae | Stored grains | Complete | Nylon | Elijah et al. (2015) |
| Sitophilus sp. | Rice weevil | Coleoptera | Curculionidae | Stored grains | Complete | ||
| Oryzaephilus sp. | Sawtoothed grain beetle | Coleoptera | Silvanidae | Stored grains | Complete | ||
| Tribolium confusum | Flour beetle | Coleoptera | Curculionidae | Stored grains | Complete | PS, PE, EVA | Abdulhay (2020) |
| T. castaneum | Red flour beetle | Coleoptera | Tenebrionidae | Stored grains | Complete | PS | Wang et al. (2023) |
| Uloma sp. | Darkling beetle | Coleoptera | Tenebrionidae | Omnivorous | Complete | PS | Kundungal et al. (2021) |
| Spodoptera frugiperda | Fall armyworm | Lepidoptera | Noctuidae | Grasses and tender crops | Complete | PVC | Zhang et al. (2022) |
| Tomares mauritanicus | Moroccan hairstreak | Lepidoptera | Lycaenidae | Vegetables/herbs | Complete | Plastics | Cline (1970) |
| Plodia interpunctella | Indian meal moth | Lepidoptera | Pyralidae | Grains and seeds | Complete | PE | Yang et al. (2015) |
| Corcyra cephalonica | Rice moth | Lepidoptera | Pyralidae | Cereals, dry seeds | Complete | LDPE | Kesti and Thimmappa (2019) |
| Achroia grisella | Lesser wax moth | Lepidoptera | Pyralidae | Honeycombs | Complete | HDPE | Kundungal et al. (2019b) |
| G. mellonella | Greater wax moth | Lepidoptera | Pyralidae | Honeycombs | Complete | PE | Kundungal et al. (2019a); Bombelli et al. (2017); Cassone et al. (2020) |
| Gryllodes sigillatus | Crickets | Orthoptera | Gryllidae | Vegetables/herbs | Incomplete | PE | Ritchie et al. (2023) |
| Gryllus bimaculatus | Crickets | Orthoptera | Gryllidae | Vegetables/herbs | Incomplete | PS | Khan et al. (2021) |
- EVA, ethylene-vinyl acetate; HDPE, high-density polyethylene; LDPE, low-density polyethylene; PBS, polybutylene succinate; PE, polyethylene; PP, polypropylene; PS, polystyrene; PVC, polyvinyl chloride.
Plastic-degrading Lepidopteran insects
Insects are recognized as the most diverse and successful group of animals on Earth (Bankar et al., 2018; Sollai & Solari, 2022). Insects are pivotal for the proper functioning of natural and managed ecosystems and exhibit numerous applications (Forister et al., 2019). Although insects face tremendous challenges while living in different habitats, they have evolved sophisticated gut systems to contend with the challenges of new habitats (Douglas, 2013). Insect gut systems are highly systematic and specialized microenvironments with distinctive functions due to which they are frequently used in biotechnological research (Brune, 2015; Dar et al., 2021a; Dar et al., 2021b; Dar et al., 2022a; Dar et al., 2022b). Recently, plastic degradation by insects has emerged as a fascinating discipline to debate over and remediate plastic pollution (Ali et al., 2021a; Ali et al., 2021b; Bertocchini & Arias, 2023). The larval forms of several insect species, primarily Coleopterans and Lepidopterans, are known to possess the remarkable ability of plastic consumption and degradation (Bilal et al., 2021). Correspondingly, a thorough analysis of the plastic biodegradation in the Indian meal moth (Plodia interpunctella) signified the contributions of symbiotic bacteria like Bacillus sp. YP1 and Enterobacter asburiae YT1 (Yang et al., 2014). This seemingly implied that the insect gut is not only a vessel for microorganisms capable of degrading low-density PR (LDPE); rather there might be functional relationships between the gut physiology of the host and its microbiome (Cassone et al., 2020). The Indian mealworms, P. interpunctella, for example, can eat and digest PE packaging bags (Yang et al., 2014). P. interpunctella is a major agriculture pest commonly referred to as stored grain pest.
The larvae of the Indian meal moth pierce the plastic bags and cause damage to the stored food grains. When the larvae of Indian mealworms were enclosed in the PE bags, they were found to chew and eat it (Yang et al., 2014) and the degradation was further revealed by various analytical and isotopic methods. The larvae of the fall armyworm, Spodoptera frugiperda, can also devour and survive on PVC (Zhang et al., 2022). In another study, the larvae of the wax moths that usually ravage beeswax of honeycombs were found to eat and digest shopping bags made of PE (Bombelli et al., 2017). Further analysis confirmed that wax moths can degrade PE plastics probably due to their similarity in structure with beeswax. The worms were observed to excrete ethylene glycol which is a moderately toxic compound commonly used in refrigerators or antifreeze instruments. Beeswax comprises a diverse array of lipid molecules, containing alkanes, esters, alkenes, and fatty acids. Being a rich source of CH2–CH2 bonds of hydrocarbons, bees wax shows similarities with the composition of PE plastics (Maia & Nunes, 2013). Although the mechanism of wax biodegradation requires a comprehensive investigation, it appears that the target of digestion is mostly the C–C single bond of the aliphatic molecules.
Plastic-degrading Coleopterans
Earlier, Riudavets et al. (2007) observed that Sitophilus oryzae, Rhyzopertha dominica, and the cigarette beetle, Lasioderma serricorne could degrade multilayer food packaging films made of PP, LDPE, and polyester. Likewise, Elijah et al. (2015) investigated the biodegradation of nylon for 6 weeks by 3 species of insects, namely, Tribolium confusum, Sitophilus zeamais, and Oryzaephilus surinamensis, using the number of borings or holes as a measure of plastic degradation. The authors stated that the number of boring by insects increased significantly with time, being highest after 6 weeks of treatment by Oryzaephilus, indicating its higher efficiency among the tested insects. However, it is important to note that the increased number of holes may primarily indicate mechanical fragmentation rather than true degradation of the polymer. Recently, Abdulhay (2020) observed a mass loss of 51.92% for PS by T. confusum. Based on the findings, these authors stated that weevils initiate the biodegradation of the nylon polymer by making borings that could subsequently serve as attachment sites for bacteria to attack and degrade the polymer (Elijah et al., 2015). Digestion of plastics by the darkling beetle (Plesiophthalmus davidis) which feeds on PS suggests that plastics may be broken down by other insects that devour old wood (Woo et al., 2020). The superworms are reported to digest and metabolize polybutylene succinate as the energy source, resulting in a weight gain of 5.13% and a 23.23% increase in body protein content, when compared to the initial values of the insect (Jung et al., 2023). In addition, Yang and colleagues concluded that super worms (Zophobas atratus) could also chew and eat Styrofoam as a sole diet like the mealworm, Tenebrio molitor (Miao & Zhang, 2010). The yellow mealworms, T. molitor, are also known to dwell on face masks composed predominantly of PP polymer (Wang et al., 2023). The Styrofoam degradation by superworms was observed to be 0.58 mg/d per worm, which was 4-fold higher than the activity of mealworms (0.12 mg/d per mealworm; Yang et al., 2015a). Moreover, the survival rate of Styrofoam-eating super worms did not differ significantly from the insects fed on a normal diet. In addition, it was found that the continuous consumption of a Styrofoam diet for 28 d by superworms was not hindered, rather it abetted their metabolic activities and overall survival. The PS molecules were depolymerized into low molecular weight degradable products, which could be subsequently assimilated and mineralized into CO2 during their passage through the insect gut systems. The larvae of T. molitor can also eat and digest PE and PS (Brandon et al., 2018; Yang et al., 2018a). The ubiquitous degradation of plastics by T. molitor larvae was also confirmed by Yang et al. (2018b) who feasted the insect on PS, which forms the basis of Styrofoam. These mealworms transformed the PE and PS plastics into CO2, cell biomass, and other biodegradable compounds (Brandon et al., 2018; Yang et al., 2021). The rate at which these worms consume PS is overwhelming. Every day, 100 mealworms digested about 34–39 mg of Styrofoam which is equivalent to the weight of a pill (Mamtimin et al., 2023). Further, the excreta of the worms were safe, causing no toxicity to plant soils. Using different feeding assays, the authors observed that none of the mealworms that digested Styrofoam instead of bran showed any harm or physiological damage (Yang et al., 2021). These findings elucidated that plastic-degrading insects are widespread in natural ecosystems (Yang et al., 2020). However, it remains to be investigated whether superworms can degrade and mineralize ingested Styrofoam in the same way as mealworms since the 2 species share similar mandibulate features despite having 20.9% genetic differences (Park et al., 2013).
The literature survey revealed that the plastic degradation capacity of insects is species-specific (Spínola-Amilibia et al., 2023; Wang et al., 2023). When the rate of plastic degradation was compared between superworms and mealworms, the former showed better potential than their counterparts. In just 21 d, superworms devoured 1.42 g of the PS while mealworms consumed only 0.22 g of the polymer. Moreover, the mean weight and length measurements for superworms were recorded at 0.4648 g and 3.64 cm, respectively. In contrast, the mealworms exhibited average weight and length values of 0.0454 g and 1.70 cm (Kim et al., 2020). Additionally, 12 strains of mealworms were evaluated for plastic degradation (Yang et al., 2018a). Insects like yellow mealworm T. molitor are also found to chew and wad Styrofoam types of plastics, which are subsequently depolymerized and mineralized across the passage through gut systems (Engel & Moran, 2013; Yang et al., 2015a; Yang et al., 2015b; Yang et al., 2018a) probably in collaboration with the proteins contributed by gut symbionts. Moreover, the rate of plastic consumption by wax moths was even faster than in mealworms, where 100 worms digested about 92 mg of PE in just 12 h which is equivalent to the weight of 3 pills. Scientists studying these drab worms are now trying to identify and extract the secreted biocatalysts that are responsible for the decomposition of complex polymeric plastics within the worm's gut.
Several other insect species have been documented to transform plastic wastes into cellular energy (Fig. 4). Some authors stated that when wax worms, Galleria mellonella were fed on PE films, the worms started to develop almost 2.2 holes per insect per hour within 40 min of exposure only (Bombelli et al., 2017). Further, the gravimetric analysis of the PE films consumed by worms revealed a considerable mass loss of 13% after 14 h of treatment, indicating an average degradation rate of 0.23 mg/cm2/h (Bombelli et al., 2017). This phenomenon could be attributed to the endogenous secretions found in the saliva of G. mellonella, that belong to the hemocyanin/prophenoloxidase superfamily. The authors renamed these proteins as Demetra/Ceres (heterohexamer), Cibeles (hexamerin aJHSP1, XP_026756459), and Cora which is a methionine-rich protein bJHSP1 (Spínola-Amilibia et al., 2023). These hexamerin family proteins present in the saliva of wax worms oxidize PE (Sanluis-Verdes et al., 2022). The degradation rate by G. mellonella was significantly higher than the degradation efficiency reported for a microbial consortium that has been tested in vitro (Yoshida et al., 2016). It is fascinating to observe that PE degradation is rapid under in vivo conditions existent inside the insect guts as compared to the bacteria-mediated in vitro degradation of plastics (Yang et al., 2015a). This, in other words, signposts that the accelerated breakdown of plastics in the insect gut system is a complicated process involving synergism between the microbiome and the host. However, more research is needed to investigate the in vivo degradation of plastics, specifically how PE breakdown affects the caterpillar's physiology, genetics, and metabolic functions (Cassone et al., 2020). Even though wax worms have been discovered to cause far more damage to plastics than mealworms, the mechanism of in vivo biodegradation is still unclear. This has puzzled scientists to unravel whether the chemical reaction is catalyzed by a single protein or a complex of enzymes that demonstrate synergism for complementary actions. Considering the involvement of proteins, the biotechnology-based engineering of the enzymes should be feasible and replicable on a large scale. Further, it would be easier, accessible, and cost-effective than breeding wax worms. New evidence is emerging regarding the origin of these proteins and pathways responsible for hydrocarbon-digestion activity in G. mellonella (Réjasse et al., 2022; Sanluis-Verdes et al., 2022). Future research could focus on elucidating the genetic and biochemical mechanisms underlying the production and regulation of these enzymes in G. mellonella. By investigating the synthesis pathways and regulatory factors involved, we can gain a deeper understanding of their role in plastic degradation. Moreover, exploring the potential for genetic manipulation or enhancement of these pathways may provide new avenues for improving plastic degradation efficiency in waxworms. These efforts hold promise for advancing our knowledge of insect-mediated plastic degradation and developing innovative strategies for sustainable waste management. Furthermore, these findings will not only pave the way for using waxworms to degrade other types of discarded plastics similar to PE or rubber but they will also serve as a springboard for further research into identifying other insects viable for plastic waste biodegradation (Bombelli et al., 2017; Peng et al., 2019; Kundungal et al., 2019b).

Plastic-degrading insects harbor a tremendous diversity of bacterial symbionts
Undoubtedly, the endogenous systems inherent to insects exert a notable influence on the biodegradation and metabolic processing of plastic materials (Boschi et al., 2023). However, the contribution of gut microbial communities, including bacteria, to the metabolic processes of their insect hosts cannot be disregarded. The successful survival of insects is attributed to their remarkable adaptations, exploiting diverse nutritional resources, together with hosting a militia of microbiota that contributes to their digestion and other metabolic processes (Dar et al., 2018; Spínola-Amilibia et al., 2023; Xian et al., 2023). The gut symbiotic bacteria supply essential nutrients like amino acids, vitamins, and sterols to the host when the diet of the insect is specialized and deficient in some components or when the insects colonize novel habitats lacking their metabolic repertoire (Dar et al., 2022a). Apart from the digestion and absorption of nutrients, symbiotic bacteria residing in insect guts perform diverse functions that are continuously gaining scientific attention. Some gut microorganisms including bacteria can actively participate in the breakdown of hazardous chemicals like plastics and pesticides, thereby augmenting their hosts to adapt to new and extreme environments (Itoh et al., 2018).
Culture-independent techniques like metagenomics have established that Proteobacteria, Firmicutes, Actinobacteria, and Bacteroidetes are the most prevalent bacterial phyla found in insects (Jones et al., 2013; Yun et al., 2014; Xian et al., 2023). It is anticipated that some of these microorganisms, although not all, may be actively and directly involved in plastic degradation. Correspondingly in recent years, the bioprospection of plastic-degrading microbial candidates from insect gut systems has gained considerable interest. So far, more than 29 species of gut bacteria (Table 3) have been recognized to degrade plastic polymers (Pathak & Navneet, 2017; Kundungal et al., 2019b). Among the gut bacteria, the Gram-negative bacterium, Klebsiella sp. EMBL-1, has been recently characterized and evaluated on PVC as a sole source of carbon for 10 d (Zhang et al., 2022). The bacterium was found to consume and degrade the plastic by encoding 77 genes for PVC metabolism. In superworms and mealworms, many strains of the rod-shaped bacterium, Pseudomonas aeruginosa, 0.5 μm in diameter and 2 μm long, were found most dominant, accounting for 35% of the total bacteria. Particularly, many members of Pseudomonas sp. DSM 50071 attach to the PS surfaces and catalyze degradation (Kim et al., 2020). Pseudomonads are well-known for their adeptness in degrading synthetic plastics due to their highly efficient arsenal of catabolic enzymes (Tiso et al., 2015; Wierckx et al., 2015; Xian et al., 2023). Many strains of P. putida are recognized for their ability to metabolize a diverse array of substrates, encompassing aromatics like terephthalic acid (Kenny et al., 2008) and other related chemicals. A survey of the 12 strains of mealworms from different geographical locations like the USA, China, and Northern Ireland demonstrated a high diversity of PS-degrading bacteria in their gut systems. The treatment of the mealworms with the antibiotic compound, gentamycin, caused the collapse of the gut microbiota and a likely reduction in PS-degradation capacity, indicating their possible role in polymer degradation (Yang et al., 2018a). Further, Tang et al. (2017) also isolated PS-degrading Aeromonas sp. TM1 and Klebsiella pneumoniae ZM1 from yellow mealworms and superworms, respectively. The 2 most common bacteria found in the gut of P. interpunctella are E. asburiae YT1 and Bacillus spp. YP1 (Yang et al., 2015) which has been acclaimed for PE degradation. The whole-genome sequencing of the Bacillus sp. YP1 revealed several genes responsible for PE biodegradation (Yang et al., 2015). Yang et al. (2015a) elucidated that over a period of 16 d only, mealworms possibly in symbiosis with gut bacteria transformed 47.7% of the PS into CO2. The Exiguobacterium sp. YT2 obtained from the gut system of yellow mealworms developed a biofilm on the surface of PS during an incubation period of 28 d. The biofilm formation reduced the surface hydrophobicity of the plastic material, altered its surface morphology, and resulted in the formation of pits and cavities measuring 0.3–0.4 μm deep as shown by scanning electron microscopy and atomic force microscopy analyses (Yang et al., 2015b).
| Polymer | Bacteria | Host insect | Reference |
|---|---|---|---|
| Polypropylene | Lactococcus sp., Morganella spp., Pseudomonas spp., Bacillus spp., Gordonia spp., Caldicoprobacter spp., Shigella spp. | Tenebrio molitor | Wang et al. (2023); Xian et al. (2023) |
| Polyvinyl chloride | Klebsiella sp. EMBL-1; bacterial consortium | Spodoptera frugiperda; Tenebrio molitor | Zhang et al. (2022); Xu et al. (2023) |
| Polystyrene, polyphenylene sulfide | Pseudomonas sp., P. aeruginosa | Zophobas atratus | Kim et al. (2020); Lee et al. (2023a) |
| Polystyrene and polyethylene | Citrobacter sp., Kosakonia sp. | T. molitor | Brandon et al. (2018) |
| Polystyrene | Aeromonas sp., Klebsiella pneumoniae | Zophobas morio | Tang et al. (2017) |
| Polystyrene |
Aeromonas sp., K. pneumoniae, Exiguobacterium sp. YT2, Spiroplasma sp. Enterococcus sp., Staphylococcus sp. Pantoea sp., Klebsiella sp. |
T. molitor | Yang et al. (2015b); Tang et al. (2017); Peng et al. (2019); Urbanek et al. (2020) |
| Polyethylene |
E. asburiae YT1, Bacillus sp. YP1 Pseudomonas sp., Klebsiella sp., Serratia sp. P. aeruginosa YHPJ1, K. pneumoniae THPJ3 K. granulomatis THPJ2, B. cereus NMJ4 B. stratesphericus NMJ3, E. cancerogenes NMJ2 E. tabaci, B. subtilis subsp. Spizizenii |
P. interpunctella | Yang et al. (2014); Mahmoud et al. (2021) |
| Polystyrene |
Escherichia sp., Spiroplasma sp. Enterococcus sp. |
T. obscurus | Peng et al. (2019) |
| Polyethylene | Sebaldella termitidis | Termite | Harmon-Smith et al. (2010) |
| Polystyrene | Serratia sp. WSW | P. davidis | Woo et al. (2020) |
| Polyethylene |
Aeromonas sp., Ottowia sp. Acinetobacter sp. |
G. mellonella | Cassone et al. (2020) |
| Polystyrene | Acinetobacter sp. AnTc-1 | T. castaneum | Wang et al. (2023) |
| Vulcanized rubber | Acinetobacter sp. BIT-H3 | Mealworm | Cheng et al. (2023) |
The metagenomic analysis of the gut bacteria in T. molitor revealed that 2 operational taxonomic units (OTUs) representing Citrobacter sp. and Kosakonia sp. were strongly associated with PE and PS (plastic)-based diets (Brandon et al., 2018). Both of these groups are classified within the family Enterobacteriaceae, to which the PE-degrading E. asburiae YT1 is also affiliated (Yang et al., 2014). These OTUs use oxygen which provides more evidence for their involvement in plastic breakdown, as oxygen incorporation is critical for the fast degradation of PE and PS (Shah et al., 2008a; Tokiwa et al., 2009). In addition, Sebaldella termitidis and Brevibacterium sp. were shown to be verily associated with PE-fed microbiomes. The S. termitidis is an anaerobic bacterium commonly observed in the posterior gut of termites (Harmon-Smith et al., 2010) while the members of Brevibacterium spp. are aerobic and have been linked to the breakdown of hydrocarbons, particularly n-alkanes. In addition, 7 other microbial groups, like Listeria sp., Pedomicrobium sp., Candidatus Nitrospira defluvii, Aquihabitans sp., unclassified Saprospiraceae, unclassified Xanthomonadaceae, and unclassified Burkholeriales, have been linked with PS-fed gut microbiomes (Brandon et al., 2018). The biodegradation of these resistant polymers is a fascinating discovery that provides promise for the natural bioremediation of places contaminated with PET, PE, and PS, plastic wastes in the environment (Jacquin et al., 2019).
In another study, the darkling beetle larvae that are indigenous to the Korean peninsula can eat PS and break it into short-chain oligomers, thus reducing its molecular weight (Woo et al., 2020). The researchers isolated Serratia from the digestive tract of Plesiophthophthalmus davidis larvae and stated that the isolated gut bacteria could oxidize PS films and affect their surface properties. The abundance of Serratia was increased by 6-fold when the larvae were fed on PS continuously for 2 weeks, accounting for 33% of the total bacterial load in the insect. Furthermore, unlike the gut flora of other PS-digesting insects, the larvae of P. davidis contained fewer bacterial species (<6) in the gut. Recently, Cassone et al. (2020) employed different strategies to demonstrate the impact of gut bacteria on plastic degradation. They observed that antibiotic-treated caterpillars excreted a reduced proportion (40%) of liquid excreta while demonstrating a high number of gut bacterial communities when fed on PE. The authors observed 89 bacterial phylotypes in the gut of G. mellonela when the caterpillars were fed on a plastic-based diet (Cassone et al., 2020). Among 89 bacterial phylotypes, the metagenomic analysis revealed the dominance of Escherichia–Shigella (28.5%), Asaia (20.3%), and Acinetobacter (13.1%). They further concluded that the gut microorganisms including the members of Acinetobacter sp. might be effective for the breakdown of PE and glycol synthesis in insects. Members of the genus Acinetobacter have consistently garnered acclaim for their ability to degrade PE and vulcanized rubber (Montazer et al., 2018a; Montazer et al., 2018b; Cheng et al., 2023). This genus, being strictly aerobic, can be correlated with the oxidation of the stable carbon–carbon double bond of PE (Restrepo-Flórez et al., 2014) that requires the presence of oxygen. Considering the sustenance of Acinetobacter for 1 year on a PE-based diet, the authors speculated that it is indeed involved in catalyzing plastic biodegradation (Cassone et al., 2020). However, a thorough characterization of the metabolic efficiencies and expression of the bacterial genes to degrade PE remains to be studied.
Gut symbionts contribute to the plastic degradation by insects
In insects, it is assumed that gut symbionts, rather than the host's endogenous enzymes, perform the majority of plastic degradation (Jin et al., 2023). In the natural state, the larvae of G. mellonella ingest beeswax, which is similar in composition to PE plastics for being a polymer of diverse long-chain hydrocarbons (Spínola-Amilibia et al., 2023). This in other words indicates that similarities in structure or chemical composition of plastics with food of insects may be related to their plastic-eating tendency. Since ethylene glycol is a byproduct of PE degradation, the insects fed on an artificial diet containing antibiotics excreted less ethylene glycol than the insects fed on the artificial diet without antibiotics which in other words indicated the important role of the gut microbiota in PE metabolism (Cassone et al., 2020). Further, the inhibition of the gut microbiome due to antibiotic treatment caused reduced depolymerization of plastics, thereby indicating the poor metabolic capacity of a host organism to assimilate plastic on its own (Peng et al., 2019; Cassone et al., 2020; Yang et al., 2020). On the other hand, in vitro testing of Acinetobacter sp. isolated from the gut of G. mellonella, and T. molitor, showed slow mineralization of the plastics (Cheng et al., 2023). These experiments suggest that plastic degradation entails a synergistic interplay between the insect and its gut microbiome (Lear et al., 2021) a relationship that warrants comprehensive exploration. Nonetheless, the degree to which the larvae influence the structure and composition of the ingested plastics or their additives also deserves scientific attention. Although the utilization of gut bacteria seems plausible for plastic remediation, replicating the conditions prevailing in the gut systems of insects is not an easy task. Therefore, continuous efforts are required to meet the challenges of plastic remediation by using the gut flora of the PE-degrading insects.
Bacterial enzymes involved in plastic degradation
Bacteria are often referred to as the “engines” of the Earth's biogeochemical cycles because they play a crucial role in nutrient transformation and recycling. They have been extensively studied for their vital functions in bioremediation. Bacteria have demonstrated a tremendous ability to break down complex materials, including petroleum, plastics, metal compounds, antibiotics, and other substances that have become prevalent in the Anthropocene age. Through different methodologies such as culturing, metagenomics, and systems biology, several potential bacteria like Pseudomonas, Exiguobacterium, Comamonas, Bacillus, and so forth, have been demonstrated to show plastic degradation (Gan & Zhang, 2019). Bacteria perform remediation of wastes by expressing a diverse range of enzymes and cofactors.
The enzymatic biodegradation of plastics is a tricky and challenging task due to several constraints. A critical factor is the absence of hydrolysable functional groups within the C–C backbone of polyolefins including PE (Wei & Zimmermann, 2017). However, PET is an exception due to its polyester structure, which renders it less resilient to microbial action (Bertocchini & Arias, 2023). Plastic degradation involves various secreted enzymes that facilitate the formation of biofilms. A good example of plastic-degrading bacteria isolated from insect guts is Pseudomonas species. In Pseudomonas spp., several distinct enzymes that are responsible for plastic breakdown have been identified. The most typical ones are esterases, lipases, and cutinases which exhibit hydrolytic properties (Wilkes & Aristilde, 2017). Many esterases, laccases, peroxidases, hydroxylases, and reductases have been identified to degrade PE (Fig. 5). Alkane hydroxylase is secreted by Pseudomonas sp. E4 and was found crucial in the degradation of PE which was further validated through gene cloning and tested in Escherichia coli (Yoon et al., 2012). Subsequently, alkane hydroxylase, rubredoxin, and rubredoxin reductase were identified as pivotal components in the degradation of low molecular weight PE by P. aeruginosa (Jeon & Kim, 2015). After secretion by the bacteria, these enzymes adhere to the plastic surface and catalyze fragmentation (Nakamiya et al., 1997; Roohi et al., 2017). When Pseudomonas sp. DSM 50071 was grown on PS, the authors observed an upregulation of enzymes responsible for the consumption and maximum assimilation of the compound. Similarly, reverse transcription – polymerase chain reaction (RT-PCR) analysis by Kim et al. (2020) revealed higher expression of S-formylglutathione hydrolase (EC 3.1.2.12) and serine hydrolase (SH) in Pseudomonas sp. DSM 50071 when cultured on a PS-added nutrient medium. Particularly, 7- and 2-fold increased upregulations were observed for SH and S-formylglutathione hydrolase respectively when compared to their control sets (Kim et al., 2020). In addition, several depolymerases derived from Bacillus and Pseudomonas species were involved in PS breakdown (Mohan et al., 2016).

Other proteins involved in the process were phenylacetaldehyde dehydrogenase, styrene monooxygenase, phenyl acetyl coenzyme A ligase, and styrene oxide isomerase which can mineralize styrene, a monomer of PS. These enzymes transform styrene in a cascade of reactions (Ho et al., 2018) including depolymerization of the large polymer to styrene and its subsequent oxidation to phenylacetate. Peroxidases are heme-containing proteins that oxidize many organic and inorganic substrates utilizing hydrogen peroxide as an electron acceptor (Pathak & Navneet, 2017). In addition, 3 distinct hydrolases such as 6-aminohexanoate hydrolase, 6-aminohexanoate-cyclic-dimer hydrolase, and endo-type 6-aminohexanoate-oligomer hydrolase found in Flavobacterium and Pseudomonas strains are capable of catalyzing the metabolism of 6-aminohexanoate, an intermediate product formed in nylon metabolism (Negoro, 2000). Under optimum conditions, these enzymes work in tandem to convert the nylon intermediate to its monomeric form, 6-aminohexanoate (Amobonye et al., 2020). Subsequent research has discovered enzymes in Arthrobacter sp. KI72 also exhibit comparable characteristics to the aforementioned enzymes (Takehara et al., 2017). After the study of Yasuhira et al. (2007) who succeeded in cloning the nylon-oligomer degrading genes from Agromyces sp. KY5R, many authors have reported several enzymes with similar characteristics as found in Arthrobacter sp. KI72.
Recently, a global search based on the hidden Markov model (HMM) motif for PET hydrolases revealed 800 potential PET hydrolases in the existing genomic and metagenomic databases of bacteria and archaea (Danso et al., 2018). From these databases, many enzymes like PET2, PET4, PET6, and PET12 have been functionally characterized. Some recent studies suggested that genes encoding PET hydrolase are present in both marine and terrestrial metagenomes, including the guts of insects (Danso et al., 2018). Among Gram-negative bacteria, members of the genus Pseudomonas were most commonly associated with PUR activities. One of the first enzymes discovered to act on PUR was PueB lipase of Pseudomonas chlororaphis (Howard et al., 2001). Another enzyme, PueA, that breaks down PUR was also reported from the same bacterium (Stern & Howard, 2000). Being lipases, both these enzymes act on and degrade PUR as revealed by nuclear magnetic resonance and infrared (IR) analyses (Biffinger et al., 2015). Furthermore, several B. subtilis and Alicycliphilus spp. have been shown to degrade PUR (Oceguera-Cervantes et al., 2007; Shah et al., 2013). The enzyme-mediated hydrolysis of the PUR polymer is a surface erosion process that depends on the firm adhesion of the biocatalyst to the plastic (Mahajan & Gupta, 2015). When a solid polyester PUR was incubated with a polyamidase derived from Nocardia farcinica, and fused to the hydrophobic polymer binding module of the polyhydroxyalkanoate depolymerase of Alcaligenes faecalis, an enhanced yield in breakdown products was reported (Gamerith et al., 2016).
Proposed mechanism of plastic degradation within the insect gut
Plastic biodegradation involves the intricate biochemical transformation of complex compounds into simpler derivatives, thereby facilitating their facile recycling through biogeochemical cycles. Alterations in the physical characteristics of plastics, notably reductions in molecular weight, diminished mechanical strength, and alterations to the polymer surfaces, serve as discernible indicators of the biodegradation process (Ho et al., 2018). Despite the breakdown of large plastic polymers into smaller units, the overall biological degradation of plastics is at its nascent stage. PE is exceptionally resistant, taking weeks to years for biodegradation under in vitro conditions by isolated microbes, and requires physical processing to speed up mineralization (Restrepo-Flórez et al., 2014; Bombelli et al., 2017). In contrast, the in situ degradation of PE within the gut systems of insects is comparatively faster which involves a synergism of multiple species (consortium) and factors rather than a single organism (Nauendorf et al., 2016). Plastic may be degraded fast and efficiently by symbiotic bacterial consortia. During the coculturing of gut microbes, maximum weight reductions have been achieved for polyester PU film pieces within 20 d, concomitant with an elevated release of CO2, as revealed by the Strum test (Shah et al., 2016). In a microbial consortium, interactions among microorganisms can synergistically boost their degradation capacities. Despite their inability to grow individually on PVA, the cocultivation of P. putida VM15A, and Pseudomonas sp. VM15C demonstrated vigorous proliferation in PVA (Shimao, 2001). This symbiotic growth is attributed to the synthesis of growth factors like pyrroloquinoline quinone by P. putida VM15A which supports the metabolism of PVA by Pseudomonas sp. VM15C (Shimao, 2001). Similarly, when Flavobacterium sp. was cocultured with Pseudomonas sp., it exhibited the breakdown of polyethylene glycol, potentially preventing the feedback inhibition of the former strain through the removal of harmful byproducts generated during polyethylene glycol metabolism (Gu, 2003).
Although many studies have supported the notion that plastic degradation by insects is achieved through symbiotic associations with gut microbiota (Yang et al., 2015a; Yang et al., 2015b; Yang et al., 2018b; Peng et al., 2019), the relative roles of the host and its microbiome in plastic degradation are still not very clear. How well these systems could coordinate and which system prevails over another is not clear yet. Grinding the bigger plastic components into minute particles possibly increases the surface area, which allows better chances for microbes to attach to the particles. In insects, grinding and mincing of the plastics before ingestion acts as “peanut butter on the cracker” making the attachment of microbes to microplastics easier, as well as providing favorable microenvironments suitable for the proliferation of bacterial growth (Helmberger et al., 2020). Although the exact mechanism of plastic degradation by insects is still elusive, broadly it involves multiple steps such as biodeterioration, biofragmentation of the large molecules into smaller residues, assimilation, and lastly mineralization by the host and symbionts (Fig. 6) each of which comprises distinct biochemical routes for plastic biodegradation.
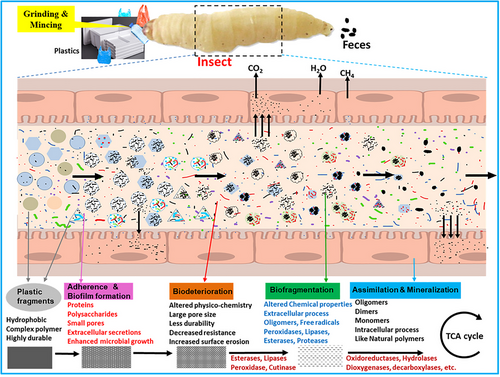
Biodeterioration of plastics inside the gut
Biodeterioration is the primary step that includes a mechanical, superficial breakdown of the polymer and changes in its chemical composition due to the physical forces like munching and chewing by insects and enzymatic actions of bacteria, or other biological agents (Anjana et al., 2020). During biodeterioration, bacteria adhere to the polymer surfaces and initiate colonization that reduces the resistance and durability of the plastics. In insects, this phenomenon is partly accomplished by the grinding action of mouth parts. Once the plastic is ingested and swallowed by the insect, symbiotic bacteria like Pseudomonas, Bacillus, and Enterobacter initiate the biofilm formation on the surface of particles (Puglisi et al., 2019). Biofilm formation accelerates the oxidation and overall biodegradation of the compound by enhancing firm contact between bacteria and the polymer particles (Tribedi & Sil, 2013). Since plastics are hydrophobic, the attachment of the bacteria mediates the insertion of hydrophilic functional groups which further promotes the surface interaction of bacterial cells with the polymer (Nauendorf et al., 2016). In the case of polymers with high surface hydrophobicity like PE, biofilm formation is immense to maximize the interaction of bacteria with polymer surfaces (Schwibbert et al., 2019). The nature of the biofilm formation depends on the structure and chemical nature of the plastic polymers, bacteria type, as well as the surrounding environment. Hence, some bacteria like Pseudomonas which form biofilms attach firmly to the polymer and rapidly depolymerize the low-density PE as compared to other species in the planktonic state (Tribedi et al., 2015).
Biofilms also protect bacterial communities from external fluctuations, thereby increasing their persistence in different environments like insect gut systems (Tribedi et al., 2015). The penetration of the microbes into the polymer surface is also enhanced by the extracellular compounds that influence hydrophobic interactions. These extracellular chemicals also promote the growth of microbial populations as well as the rate of biodeterioration (Mohan et al., 2016), which in turn enhances the rate of overall polymer degradation, as the process of biodeterioration is more successfully carried out by a consortia of microbes through synergistic mechanisms (Ali et al., 2021b). Once the bacteria attach to the plastic surfaces, they start to proliferate using the polymer as a sole source of carbon, partly supported by the secondary metabolites and pH maintained by the host and other symbionts. Sometimes the growth of the bacteria is enhanced by additives such as plasticizers that are easily and quickly metabolized by the microorganisms (Ru et al., 2020). Gupta and Devi (2020) stated that P. aeruginosa maintained active biofilms on PE surfaces for 60 d due to the presence and consumption of low molecular compounds attached to the polymer. Similarly, exopolysaccharides are also found to aid in stronger biofilm development by the bacteria and enhance the biodeterioration of plastics (Anjana et al., 2020). In some cases, the extracellular polymers act as surfactants, facilitating the interchange of hydrophilic and hydrophobic phases to aid penetration of the microbes (Lucas et al., 2008). The process of biodeterioration is followed by fragmentation, during which the polymer is further cleaved into its oligomers or monomeric units.
Biofragmentation of plastics within the gut environment
Biofragmentation is the depolymerization phase in which bacteria cleave the deteriorated polymer into simpler units via enzymatic reactions involving free radicals (Jenkins et al., 2019). Biofragmentation involves the biodevulcanization process which begins by breaking the S–S and S–C bonds of the polymer, thus reducing its degree of cross-linking (Aboelkheir et al., 2019). Biofragmentation is based on 2 main reactions, that is, reduction in molecular weight of the polymer and subsequent oxidation of the released compounds (Amobonye et al., 2020). Within the insect gut, the process of biofragmentation is augmented through endogenous secretions of the host systems. For instance, in the larvae of G. mellonella, the salivary secretion (GmSal) contains 2 hexamerin/prophenoloxidase family proteins, arylphorin and hexamerin (renamed as Demetra and Ceres by authors), which can rapidly oxidize PE films within hours (Sanluis-Verdes et al., 2022). The discovery of these proteins represents a pivotal breakthrough as they are the first animal enzymes capable of oxidizing PE at ambient temperature and neutral pH. Moreover, this strategy constitutes a viable alternative to the abiotic oxidation of plastic polymers (Boschi et al., 2023). These reactions are important to allow downstream degradation processes where microbial enzymes attack smaller compounds (Restrepo-Flórez et al., 2014). Many other enzymes cleave ester, and glycosidic bonds of plastics via a nucleophilic attack on the carbonyl carbon. Biofragmentation can be further categorized into 2 different modes involving exo- and endo-attacking enzymes. Exo-enzymes produce oligomers and monomers, for example, ethylene glycol and terephthalic acid, that can be instantly absorbed by the bacteria whereas endo-type enzymes such as oxidoreductases, lipases, hydrolases, and esterases generate intermediate products which must undergo further oxidation before being assimilated by the microorganisms (Pathak & Navneet, 2017). One of the best examples to depict the effects of biofragmentation is Rhodococcus rhodocrous, which could degrade nearly all previously oxidized oligomeric intermediates from PE (Gravouil et al., 2017). Moreover, the chemical bonds present in plastic monomers bear similarity to natural polymers, for example, lignocellulose. Consequently, it is plausible to hypothesize that the majority of the lignocellulases, particularly the lignin-attacking enzymes, can also break down some of the intermediate products of plastic degradation (Chen et al., 2020).
Assimilation by insects and symbionts
For assimilation, the products of biofragmentation are transported into the cytoplasm of the bacteria or host's cells via active or passive mechanisms as in the case of hydrocarbons (Amobonye et al., 2020). For example, at higher concentrations, octadecane (Hua & Wang, 2012; Shahnawaz et al., 2019) is easily taken up by Pseudomonas sp. DG17 via passive diffusion, while at lower concentrations, it is shuttled through energy-dependent active transport (Hua et al., 2013). Further, the movement of these compounds into the cytoplasm is facilitated by different membrane transport systems. In the Comamonas species, the inward transportation of terephthalic acid is carried out by specific transporters (Hosaka et al., 2013). Likewise, porins have been identified to transport the compounds like PE glycol (product of LDPE biodegradation), into the cytoplasm for downstream metabolic processing (Duret & Delcour, 2010). During the assimilation of PE-oligomeric intermediates in R. rhodocrous, Gravouil et al. (2017) observed the upregulation of different transporter proteins associated with the ATP-binding cassette family and major facilitator superfamily. The authors further suggested that certain transporters might play a dual function in intermediate trafficking as well as dehydrogenase activity in β-oxidation. The PS breakdown by enzymes of Pseudomonas sp. DSM 50071 is thought to begin with oxygen insertion into the methylene C–H bonds to form alcohols (C–OH), followed by subsequent oxidation to the carbonyl group (C = O), and ultimately fragmentation into tiny molecules by enzyme-mediated hydrolysis. However, more research is needed to discover plastic-degrading enzymes released by Pseudomonas sp. DSM 50071 that are involved in PS breakdown (Kim et al., 2020). In the cytoplasm, the plastic derivatives undergo a cascade of enzymatic reactions that culminate in their complete breakdown into oxidized metabolites such as CO2, CH4, N2, and H2O (Ho et al., 2018). The oxidation of the plastic polymer leads to the formation of many hydroxyls or carbonyl groups via β-oxidation (Bode et al., 2000; Mooney et al., 2006). These compounds then enter the tricarboxylic acid (TCA) cycle and contribute to the energy metabolism in bacteria, as illustrated in Fig. 7. The production of carbonyl groups is a well-known primary indicator for the breakdown of PS and other plastics (Yousif & Haddad, 2013). Moreover, alcoholic compounds have been recognized as byproducts of PE biodegradation in T. molitor and lesser wax moth, Achroia grisella (Brandon et al., 2018; Kundungal et al., 2019a). Many researchers are convinced recently by the fact that glycol is possibly a derivative of PE metabolism, although further research is desired to determine the precise chemistry including aliphatic diol and other metabolic intermediates.
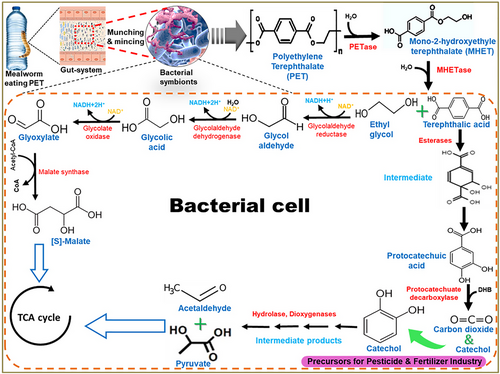
Mineralization and metabolism of the degraded products
Mineralization of the plastics is detected through isotopic tracing while the release of CO2 is measured by Strum's method (Yang et al., 2020). The labeling studies involving 13C-labeled polystyrol indicated that the carbon component is preferentially employed to form lipids (Yang et al., 2015). Sometimes, the intermediates are degraded through different metabolic pathways. For example, it has been observed that PE degradation forms acetic acid, which can undergo oxidation in the Krebs cycle or be redirected toward lipid production (Wilkes & Aristilde, 2017). Correspondingly, succinate is also produced in Pseudomonas sp. AKS2 (Tribedi & Sil, 2014) by the action of esterase on polyether sulfones. In another example, styrene (monomeric unit of PS) is oxidized to phenylacetate, which is subsequently incorporated into Kreb's cycle as phenyl acetyl coenzyme A for further metabolism (Ho et al., 2018). Similarly, catechol generated from the breakdown of terephthalic acid by esterases undergoes a series of reactions to form acetaldehyde and pyruvate (Weng et al., 2021). Once the aromatic ring is deformed by dioxygenases into pyruvate, acetyl coenzyme A, and succinate, they enter into the TCA cycle (Fig. 7) for further metabolism (Kasai et al., 2004). These secondary metabolites resulting from the assimilation of plastics are extracellularly transported, potentially serving as substrates for other bacteria engaged in mineralization processes. However, within the gut systems, some of the monomers are not assimilated due to the membrane permeability barrier. These molecules could be utilized by the microbial cells through enzyme-catalyzed biotransformation within the insect gut (Lucas et al., 2008). Further, some of the large particles or monomeric units that cannot cross the cell membrane and are not absorbed, are consequently removed through feces (Kale et al., 2015).
Although the carbon from plastics is absorbed into the body of invertebrates, the precise contribution of the higher organism or its associated microbiome to the breakdown of plastic polymers requires further investigation. Moreover, many knowledge gaps still exist like how well do these players collaborate, and which species of microorganisms are more pivotal for plastic biodegradation in the insect gut systems? Furthermore, the extent of synergism existing between the host and its gut microbiome, and how it affects the biodegradative processes also await exploration. Additionally, the direct implications of plastic degradation on the host's physiology, metabolism, and genetics remain to be comprehensively elucidated. Nonetheless, gut microorganisms evolve under highly complex but natural conditions; they constitute sophisticated bioresources that can be tailored for use in bioremediation and industrial settings. Overall, the continuous research on insect–microbial symbiosis may prove beneficial by providing biotechnologically useful molecules and enzymes, apart from uncovering the fascinating mechanism of host–symbiont interactions. In addition, the targeted search for chemicals having industrial applications such as detoxifying enzymes, lipases, antibiotics, peroxidases, and so forth, may leverage insights from host–symbiont ecology. This understanding could facilitate the design of biomimetic models to address the pertinent challenges and demands of a sustainable future.
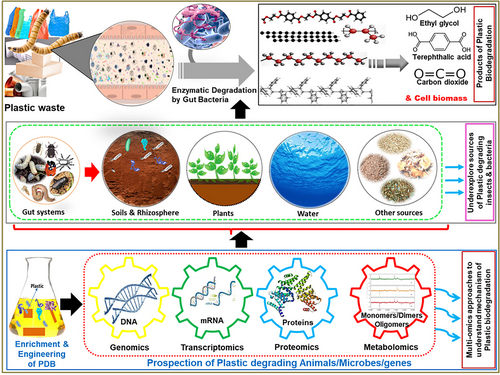
Challenges and future perspectives
Despite the hype surrounding the potential of some insects to ingest and decompose plastics, numerous scientists have emphasized that addressing plastic pollution is a more intricate and nuanced challenge than it appears (Dris et al., 2020; Bertocchini & Arias, 2023). Since natural selection has honed the insects and their gut symbionts for millions of years through evolutionary processes, the continuous bioprospection of more groups of animals and their symbiotic bacteria might provide some potential candidates for the mitigation and remediation of plastic pollution. Undoubtedly, there is a critical need for additional research to explore a diverse array of enzymes and microorganisms proficient in degrading these recalcitrant and persistent polymers (Vanapalli et al., 2020). To achieve this, a strategic approach involves harnessing global metagenomes and transcriptomes of uncultivated microorganisms, coupled with the exploration of dark matter proteins through the application of systems biology methodologies (De Filippis et al., 2023). Moreover, the implementation of -omics research like genomics, transcriptomics, metatranscriptomics, proteomics, and metabolomics holds considerable promise for providing thorough understandings of the complex biological networks covering genes, transcripts, proteins, metabolites, as well as external environmental factors integral to plastic degradation within the gut milieu of insects (Zhang et al., 2022). Thus, a holistic comprehension of the symbiotic interplay between insects and microorganisms may offer innovative avenues for addressing the imperative demands of the sustainable world through precise intervention/manipulation of symbionts or host–symbiont interactions.
Additionally, a comprehensive understanding of the mechanism of plastic degradation by bacteria within the insect gut systems is paramount to delineate the rate-limiting steps and engineering of the viable candidates for possible applications (Zhang et al., 2022). Molecular techniques like genetic engineering can help to integrate function-specific genes into the genomes of potential microorganisms suitable for the degradation of particular plastics. Plastics have macromolecular structures with higher crystallinity and cross-linking networks which pose impediments to enzymatic processes (Bertocchini & Arias, 2023). The physical pretreatments mimicking the mechanical grinding of insects and gamma irradiation might aid in the disintegration of macromolecular aggregates, thereby augmenting the enzymatic breakdown. The role of intermediate products that can inhibit metabolism is still largely unclear. For example, the mono-(2-hydroxyethyl) terephthalate produced from the hydrolysis of PET, inhibits Thermobifida fusca KW3 enzymes, while glyoxylic acid prevents PE glycol breakdown by Flavobacterium sp. (Gu, 2003; Barth et al., 2016). These bits of information may also be useful in the development of biodegradable plastics and the effective management of accumulated polymers in the environment.
-
Ongoing research is crucial to significantly enhance the diversity of enzymes and microbes proficient in the degradation of persistent plastic pollutants. Tapping into global metagenomes and transcriptomes using -omics studies is essential for uncovering potential candidates.
-
A thorough understanding of plastic degradation mechanisms by bacteria in insect gut systems is crucial for determining rate-limiting steps and engineering potential candidates. A comprehensive understanding of insect–microbial symbiosis is essential for targeted bioengineering and manipulation of symbionts or host–symbiont interactions that may open new avenues for sustainable solutions.
Conclusion
Although plastics offer numerous benefits, their extreme durability and environmental accumulation have transformed them into an environmental burden. Consequently, various species of insects, their gut microbiota, and enzymes have been implicated in mitigating plastic waste. The contemporary scientific focus is dedicated to elucidating the fundamental mechanisms underpinning the biodegradation of plastics by insects and their symbiotic bacteria. A plethora of information has consistently reported the involvement of several microorganisms and invertebrates, specifically insects, in plastic biodegradation, thereby accentuating their indispensable role in the bio-upcycling of waste polymers. In our review, we emphasize the importance of pretreatment (chewing/munching) as the initial and most essential stage in the microbial breakdown of plastic polymers in the gut system. Additionally, we shed light on plastic-eating insects and their gut symbiotic bacteria, positing them as vital and outstanding agents for plastic biodegradation in the future. The achievement of an efficient, affordable, environment friendly, and socially acceptable technology for plastic degradation remains an outstanding challenge. Therefore, it is imperative to pursue further research aimed at unraveling the complexities of the action mechanisms underlying plastic degradation by insects, their symbionts, and enzymes.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (32250410285), the National Key R&D Program of China (2023YFC3403600), and Foreign Expert Program, Ministry of Science and Technology (MoST) of China (WGXZ2023020L). The funders had no role in study design, data collection, analyses, decision to publish, or preparation of the manuscript.
Disclosure
The authors declare they have no conflict of interest.




