Knockin of the G275E mutation of the nicotinic acetylcholine receptor (nAChR) α6 confers high levels of resistance to spinosyns in Spodoptera exigua
These authors contributed equally to this work: Ya-Yun Zuo and Yu-Xin Xue.
Abstract
Spinosyns, including spinosad and spinetoram, act on the insect central nervous system, gradually paralyzing or destroying the target insect. Spinosad resistance is associated with loss-of-function mutations in the nicotinic acetylcholine receptor (nAChR) α6 subunit in a number of agricultural pests. Using gene editing, nAChR α6 has been verified as a target for spinosyns in five insect species. Recently, a point mutation (G275E) in exon 9 of nAChR α6 was identified in spinosad-resistant strains of Thrips palmi and Tuta absoluta. To date, no in vivo functional evidence has been obtained to support that this mutation is involved in spinosyn resistance in lepidopteran pests. In this study, the G275E mutation was introduced into the nAChR of Spodoptera exigua using clustered regularly interspaced short palindromic repeats (CRISPR) / CRISPR-associated protein 9 (Cas9) gene-editing technology. Reverse transcriptase-polymerase chain reaction and sequencing confirmed that this mutation was present in exon 9 of the nAChR transcripts in the edited 275E strain. The results of bioassays showed that the 275E strain was highly resistant to spinosad (230-fold) and spinetoram (792-fold) compared to the unedited background strain, directly confirming that the G275E mutation of the nAChR α6 subunit confers high levels of spinosyn resistance in S. exigua. Inheritance analysis showed that the resistance trait is autosomal and incompletely recessive. This study employs a reverse genetics approach to validate the functional role played by the G275E mutation in nAChR α6 of S. exigua in spinosyns resistance and provides another example of the use of CRISPR/Cas9 gene-editing technology to confirm the role played by candidate target site mutations in insecticide resistance.
Introduction
Insecticides are among the most commonly utilized methods for managing many of the most dangerous arthropod pests in the world. However, arthropod pests may grow and develop resistance to these insecticides with exceptional capacity, either through a de novo mutation or through resistance allele selection, which is genetically present in populations of pests (Hawkins et al., 2019). Although it is well-known that target-site resistance is a common mechanism underlying insecticide resistance, we are not aware of specific gene alleles' accurate contribution to the resistance phenotype, particularly in agricultural pests (Douris et al., 2020). To prevent the failure of insecticide-based pest control, it is necessary to detect and functionally characterize putative resistance genes or mutations in specific organisms.
The nicotinic acetylcholine receptor (nAChR) α6 subunit of insects is targeted by spinosyns, a special class of natural insecticides derived from Saccharopolyspora spinosa secondary microorganism metabolites (Douris et al., 2020). At present, two of the most heavily marketed and studied spinosyns are spinosad and spinetoram (Sparks et al., 2012). Spinosad, a kind of microbial pesticide with good insecticidal activity against a variety of pests, is the first commercial product of spinosyns and comprises spinosyn A and spinosyn D (Sparks et al., 1999; Salgado & Sparks, 2010). The second commercial product of spinosyns is spinetoram (a mixture of two semi-synthetic spinosyn derivatives), which has considerably more insecticidal activity against pests (Kirst, 2010; Dripps et al., 2011; Geng et al., 2013).
Functional loss mutation in the nAChR α6 subunit gene of Drosophila melanogaster (Dα6) has been proven to be associated with resistance to spinosad for the first time, which indicates that mutations in Dα6 orthologous genes may be responsible for the resistance of other insects to spinosad (Perry et al., 2007). Many studies have reported that spinosad resistance is associated with truncated α6 transcripts causing mis-spelling, insertions, deletions and point mutations in Plutella xylostella (Baxter et al., 2010; Rinkevich et al., 2010), Bactrocera dorsalis (Hsu et al., 2012) and Ceratitis capitate (Ureña et al., 2019); with three amino acid residues missing in the TM4 domain in P. xylostella (Wang et al., 2016); and with exon 3 skipping in α6 transcripts in Tuta absoluta (Berger et al., 2016). Moreover, the resistance of spinosad in Frankliniella occidentalis (Berger et al., 2016), Thrips palmi (Bao et al., 2014), and T. absoluta (Bao et al., 2014) was reported to be associated with a point mutation (G275E) at the top of TM3 in the nAChR α6 subunit, which does not lead to functional loss. Insect nAChRs expressed in heterologous systems, such as Xenopus oocytes, are notoriously difficult to achieve (Zimmer et al., 2016; Douris et al., 2020). However, the analogous mutation (A275E) of human α7, when successfully expressed in Xenopus oocytes, had an effect on the modulation of agonist responses by spinosad, but the in vivo contribution of the G275E mutation of nAChR α6 to spinosyn resistance remains unknown (Puinean et al., 2013). A population of T. absoluta with the G275E mutation was identified and exhibited high levels (284-fold) of spinosad resistance (Silva et al., 2016). However, this analogous mutation (G275E) was introduced into Dα6, which resulted in 66-fold resistance to spinosad (Zimmer et al., 2016). Although this model system (D. melanogaster) has many advantages for insect toxicology studies, there is no 1:1 orthology between D. melanogaster genes and the genes of other species, and the bioassay methods of analyzing insecticides in D. melanogaster studies may not accurately represent the appropriate biological assays used against certain species of pests (Adolfi et al., 2019; Douris et al., 2020). Thus, it is necessary to validate the functional role played by the G275E mutation in specific pests in spinosyn resistance to prevent the failure of insecticide-based pest control.
Clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) is a significant method for the study of the function of genes and the contribution of the resistance phenotypes of mutations (Zuo et al., 2017; Zuo et al., 2018). For example, knockout of nAChR α6 mediated by CRISPR/Cas9 has been functionally validated in vivo as being associated with high levels of resistance to spinosyns in D. melanogaster (Zimmer et al., 2016), P. xylostella (Wang et al., 2020a), S. exigua (Zuo et al., 2020), Helicoverpa armigera (Wang et al., 2020b) and Aedes aegypti (Lan et al., 2020). To date, no functional validation of G275E in a non-model species has been performed. The CRISPR/Cas9 technique has enabled researchers to study the function of point mutations in specific species (Guest et al., 2019). Thus, in this paper, the contribution of the G275E mutation of S. exigua nAChR α6 (Seα6) to spinosyn resistance was confirmed using the CRISPR/Cas9 system.
Materials and methods
Insect strains and rearing
The WHS strain was obtained from the Laboratory of Insect Molecular Toxicology, Nanjing Agricultural University and kept in a laboratory without insecticide exposure after it was originally collected from Wuhan Province of Hubei in 1998 (China). The 275E strain, which was created by knockin of the G275E mutation, was homozygous for the G275E mutation of Seα6.
A diet dependent on wheat germ and soybean powder was used to raise the larvae of S. exigua. Adults were fed a sugar solution (10%). Insects were maintained in an artificial climate chamber at 26 ± 1 °C, 60% ± 10% relative humidity (RH) and 16 h (light) : 8 h (dark) photoperiod.
Preparation of a single guide RNA (sgRNA) and design of a single-stranded oligodeoxynucleotide (ssODN)
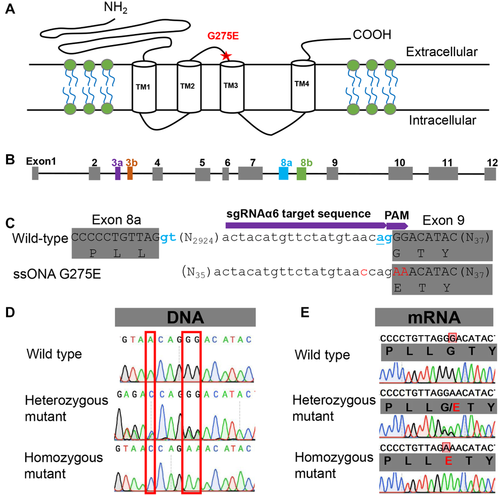
To improve homology-directed repair (HDR) efficiency, the sgRNA target sequence of Seα6 was chosen near the amino acid (G275) to be mutated. (Fig. 1C). The predesigned target primers for the Seα6 sgRNA template assembly are presented in Table 1. The sgRNA template was purified with a polymerase chain reaction (PCR) purification kit (Omega Bio-Tek, USA). The GeneArt Precision gRNA Synthesis (Thermo Fisher, Shanghai, China) kit was used for in vitro transcription of the sgRNA and purified sgRNA following the instructions of the manufacturer. A 102-nt ssODN for the introduction of the Seα6 G275E mutation was designed to provide a HDR template for Cas9-induced double-strand break repairs. The ssODN template presented two substitutions at the protospacer adjacent motif sequence site and a single insertion base at the target sequence of the intron region compared with the wild-type sequences of Seα6 (Table 1, Fig. 1C), which will result in the G275E mutation and prevent Cas9 recleavage after repair of the HDR. The ssODN was synthesized by TSINGKE Biological Technology (Nanjing, China).
| Name | Sequence (5′ > 3′) |
|---|---|
| sgRNA-α6R† | TAATACGACTCACTATAGACTACATGTTCTATGTAAC A |
| sgRNA-α6F | TTCTAGCTCTAAAACGTTACATAGAACATGTAGT |
| ssODN‡ | cataattaacttcatcaatatatgcaatgtgtaaaactacatgttctatgtaaccagAAACATACTTCAACTGCATCATGTTCATGGTAGCGTCGTCAGTGG |
| α6F | GGTGTTACAATCCTCCTGTCG |
| α6R | GCGGCATCTCATGTATATCTG |
| Full-α6F | GCTATGGCCCCTATGTTGG |
| Full-α6R | CACCCGAGGCACCTAGTTG |
- † The sequences targeted by sgRNA are underlined.
- ‡ Lowercase letters represent intron sequences. Capital letters represent exon 9 sequences. The bold capital letters (AA) are double substitution to introduce a codon substitution from glycine (G) to glutamatic acid (E). The single insertion base (bold lowercase letters c) prevents re-cleavage of Cas9 through a mismatch in the gRNA seed sequence (last 12 nt of gRNA).
Embryo microinjection
The newly laid eggs (within 2 h) on A4 paper from WHS strain were collected and washed with 1% (v/v) sodium hypochlorite solution, and the remaining sodium hypochlorite solution was rinsed with distilled water. Next, the eggs were placed on double-sided adhesive tape attached to a microscope slide. A mixture containing Cas9 protein (100 ng/μL, Thermo Fisher Scientific, Shanghai, China), sgRNA (300 ng/μL) and ssODN (400 ng/μL) was injected into eggs using PV820 Pneumatic PicoPump (World Precision Instruments Inc., New Haven, CT, USA). After injection, the injected eggs were placed at 26 ± 1 °C and 60% ± 10% RH until hatching.
G275E mutation identification
To identify the G275E substitution of Seα6, the AxyPrep DNA Extraction Kit (Axygen, Hangzhou, China) was used to extract genomic DNA of single pairs (after oviposition) for genotyping. Direct sequencing of PCR products by amplification with α6F/α6R (Table 1) was conducted by TsingKe (Xi'an, China) to identify the correct G275E mutation at the Seα6 target site.
Total RNA was extracted from the 3rd instar larvae of S. exigua with the reagent TRIzol® (Invitrogen, Carlsbad, CA, USA) in compliance with the instructions of the manufacturer to verify the mutation at the transcript level. The open reading frame of Seα6 in the 275E strain was amplified using Full-α6F/Full-α6R (Table 1). The PCR products were cloned into pGEM-TEasy (Promega, Madison, WI, USA), and positive clones were selected for sequencing.
Insecticides and bioassays
Spinosad (0.5% suspension concentrate [SC]) and spinetoram (6% emulsifiable concentrate [SC]) were obtained from Dow AgroSciences LLC (USA). Emamectin benzoate (5% EC) and chlorantraniliprole (5% EC) were obtained from the Plant Protection Institute, Guangdong Academy of Agricultural Sciences, China.
A series of gradient concentrations of insecticides diluted from stock solution with 0.1% (w/v) Triton X-100 water were prepared. In every well of the 24-well plate, a liquid artificial diet (∼120 μL) was supplied. One hundred microliters of the insecticide solution was added to the dietary surface of every single well after the diet cooled and solidified. A 3rd-instar larva was placed in each well after the wells dried. For each concentration, 24 larvae were treated. Triton X-100 (0.1%, w/v) was employed to process the control group. Two days later, larvae were considered dead if they did not move after gentle prodding. The data were analyzed by using DPS 7.05 software (Zhejiang University, China). When the 95% fiduciary limits of the median lethal concentration (LC50) values did not overlap, the LC50 values were considered to vary significantly.
Inheritance of spinosyns resistance in the 275E strain
More than 30 pairs of virgin female moths of the 275E strain were mass-crossed with male moths of the WHS strain and vice versa. The F1 progeny were mass-crossed to produce F2 progeny. The bioassay described earlier was used to assess the toxicological responses of F1 progeny from the reciprocal crosses to two spinosyn insecticides. According to Stone's formula (Stone, 1968), the degree of dominance (D) was calculated as (2X2 − X1 − X3)/(X1 − X3), where X1, X2 and X3 are log (LC50 values) of the resistant strain 275E, the F1 hybrids and the susceptible strain WHS, respectively. The D values range from −1 (completely recessive) to +1 (completely dominant).
Results
CRISPR/Cas9-mediated G275E mutation in Seα6 of S. exigua
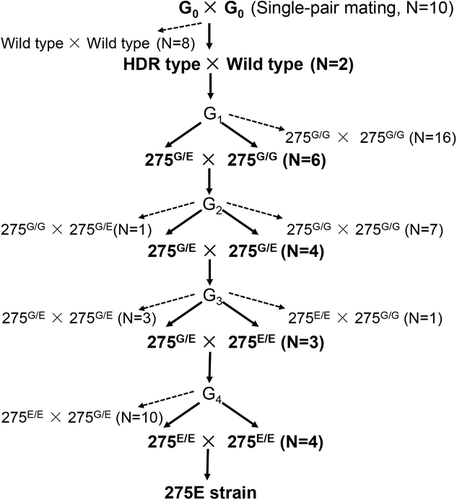
For the induction of the G275E mutation, a mixture of Cas9 protein, ssODN template and the specific sgRNA was injected into 376 eggs. Of those eggs, 67 hatched (17.8%), and 55 of the 67 larvae (82%) grew into adults (26 males and 29 females). The G0 moths were single-pair mated to lay eggs. After the G0 larvae laid eggs, sequencing of 20 individual DNA samples from 10 effective single-pairs indicated that one male adult and one female adult from different single-pairs were HDR mutant mosaics (Fig. 2). The rate of HDR mutant mosaics in a mutant mosaic of G0 was only 14.3% (Fig. 2). The progeny of both single-pairs at G1 were reared until adulthood (Fig. 2). Single-pair matings were made between the G1 moths to produce G2 progeny. After G1 oviposition, sequencing of 44 individual DNA samples from the 22 effective single-pairs indicated that four female adults and two male adults from six different single-pairs inherited the HDR-based G275E substitution (13.6% efficiency) (Fig. 2). The G2 moths produced G3 progeny via single-pair mating (Fig. 2). After G2 oviposition, both male and female moths in four of the 12 single pairs of G2 were determined to carry the heterozygous G275E mutation by direct sequencing of PCR products. The offspring of the four single-pairs were pooled and raised to emerge. A single-pair mating cross was made between the G3 moths to create the G4 progeny (Fig. 2). Progeny from three pairs of G3 (with one of the parents from each pair being heterozygous for the G275E mutation, and the other parent being homozygous for the G275E mutation) were pooled, and a single-pair mating cross was made between the G4 moths to create the G5 progeny. After G4 oviposition, the progeny from three G4 pairs (with the parents from each pair carrying the homozygous G275E mutation) were pooled, and their progeny were called the 275E strain (a G275E homozygous knockin strain) (Fig. 2). Sequencing of 275E strain complementary DNA (cDNA) has confirmed that the introduction of GG/AA codon substitution from glycine (G) to glutamic acid (E) and a single insertion base (c) does not interfere with the normal splicing of the Seα6 mRNA transcript (Fig. 1E).
Impact of G275E on S. exigua susceptibility to insecticides
The results indicate that the larvae of S. exigua with the G275E mutation are more resistant to spinosad and spinetoram than those carrying wild-type WHS larvae (Table 2). In the dose-response bioassays, the genome-edited 275E strain had LC50 values of 11.29 μg spinosad/cm2 and 1.9 μg spinetoram/cm2. The spinosad and spinetoram resistance levels of the 275E strain were estimated to be increased 230- and 792-fold, respectively (Table 2). The bioassays showed nonsignificant changes in LC50 (overlapping 95% fiducial limits) against emamectin benzoate and chlorantraniliprole between the two strains (Table 2). This result demonstrated that the Seα6 G275E substitution provided high levels of resistance to the two tested spinosyn insecticides in S. exigua but did not confer resistance to other forms of insecticides, such as benzoate emamectin and chlorantraniliprole.
| Insecticides | Strain | N† | Slope ± SE | LC50 (μg/cm2) | 95% FL‡ | RR§ | D¶ |
|---|---|---|---|---|---|---|---|
| Spinosad | WHS (Seα6275G) | 288 | 2.42 ± 0.24 | 0.049 | 0.041–0.060 | – | – |
| 275E (Seα6275E) | 288 | 3.40 ± 0.55 | 11.29 | 8.47–18.40 | 230 | – | |
| F1 (275E♂×WHS♀) | 288 | 1.95 ± 0.28 | 0.097 | 0.068–0.13 | 2.0 | −0.75 | |
| F1’ (WHS♂×275E♀) | 288 | 2.97 ± 0.44 | 0.12 | 0.097–0.16 | 2.4 | −0.67 | |
| Spinetoram | WHS (Seα6275G) | 288 | 2.35 ± 0.23 | 0.0024 | 0.0019–0.0029 | – | – |
| 275E (Seα6275E) | 288 | 2.67 ± 0.36 | 1.90 | 1.50–2.42 | 792 | – | |
| F1 (275E♂×WHS♀) | 288 | 1.99 ± 0.29 | 0.0084 | 0.0057–0.011 | 3.5 | −0.62 | |
| F1’ (WHS♂×275E♀) | 288 | 2.86 ± 0.40 | 0.0097 | 0.0072–0.12 | 4.0 | −0.58 | |
| Emamectin benzoate | WHS (Seα6275G) | 288 | 2.35 ± 0.23 | 0.0015 | 0.0013–0.0018 | – | – |
| 275E (Seα6275E) | 288 | 1.99 ± 0.28 | 0.0016 | 0.0012–0.0022 | 1.1 | – | |
| Chlorantraniliprole | WHS (Seα6275G) | 288 | 2.41 ± 0.23 | 0.020 | 0.017–0.024 | – | – |
| 275E (Seα6275E) | 288 | 2.27 ± 0.33 | 0.026 | 0.019–0.038 | 1.3 | – |
- † Numbers of larvae used in bioassay (larvae of the control group not included).
- ‡ Fiducial limits.
- § RR (resistance ratio) = LC50 (275E or F1) / LC50 (WHS).
- ¶ The degree of dominance (D) ranges from −1 (completely recessive) to +1 (completely dominant).
Inheritance of acquired resistance to spinosyns in strain 275E
The toxicological responses of the two spinosyn insecticides to the WHS and the genome-edited 275E strains and the F1 progeny from the reciprocal crosses are shown in Table 2. In the reciprocal crosses, the resistance levels of the F1 progeny were typically close to the resistance levels of the wild-type WHS strain, indicating that spinosad and spinetoram are autosomal and recessively resistant in the 275E strain without any major maternal or sexual effects (Table 2). The value of D is between 0 and −1 based on the data obtained from the two reciprocal crosses. The resistance to spinosyns exhibited by the 275E strain is incompletely recessive.
Discussion
Since G275E was initially identified as a target mutation of spinosad in F. occidentalis (Puinean et al., 2013), it has been found in two other species, T. palmi (Bao et al., 2014) and T. absoluta (Bao et al., 2014). The relative ease of expression of human nAChR α7 as a functional homomeric receptor is commonly used as a model to investigate the effect of nAChR mutations on insecticides (Puinean et al., 2013; Zimmer et al., 2016). Although the role played by the G275E mutation in spinosad resistance has been strongly demonstrated by surrogate receptor expression and introduction of homologous mutations in Drosophila experiments, direct in vivo functional validation has not been obtained to date due to a lack of genetic manipulation tools for agricultural pests. In the current study, CRISPR/Cas9 genome editing technology was employed to successfully knock in the G275E mutation linked to spinosad resistance in the Seα6 gene of S. exigua, and the phenotypic resistance of the mutant versus the wild-type form was compared.
CRISPR/Cas9, a powerful and efficient genome editing method, enables researchers to study the function of genes in non-model organisms with the same genetic background (Zuo et al., 2017). Although gene knockouts using CRISPR/Cas9 have been successfully achieved in many species of insects, gene knockin or HDR-mediated mutations by CRISPR/Cas9 are rather difficult to achieve, especially in non-model insect species (Taning et al., 2017). The frequency of HDR-induced mutations is inherently low compared with the frequency of gene knockout (Mali et al., 2013; Zhu et al., 2015). To facilitate the identification of the genotype of G275E individuals from G1, a single-pair mating strategy was employed in this study. The rate of HDR mutant mosaics in mutant mosaics of G0 was 14.3%, and the rate of inherited HDR mutant progeny was 13.6%. In S. exigua, the rate of inherited substitution for HDR-based G4946E was relatively low (3.3% efficiency) when using a mass-crossing strategy in G0 (Zuo et al., 2017). Thus, a single-pair mating strategy in G0 may be a better choice at the inherently low frequency of HDR-mediated mutations. Additionally, knockout of key proteins (e.g., Ku70, Ku80, and DNA Ligase 4) or inhibition of the activity of the main non-homologous end joining proteins is also a good way to increase the efficacy of HDR-induced gene knockin (Beumer et al., 2008, 2013; Yu et al., 2014; Zhu et al., 2015).
Our results confirmed that the G275E mutation of nAChR in S. exigua is related to high levels of resistance to spinosyns (Table 2), and this mutation would be sufficient to lead to control failure. A population of T. absoluta with the G275E mutation, divided from the Iraquara-BA population and subsequently reared without exposure to any insecticides, was 284-fold more resistant to spinosad than wild-type insects (Silva et al., 2016). In a laboratory-selected F. occidentalis strain, the G275E mutation was also identified and displayed high levels of spinosad resistance (Bielza et al., 2007a, 2007b). However, an editing G275E mutation D. melanogaster strain exhibited a 66-fold increased resistance level to spinosad (Zimmer et al., 2016). The analogous mutation (A275E) of human α7 successfully expressed in Xenopus oocytes had an effect on the modulation of agonist responses by spinosad but did not have any substantial impact on natural ligand acetylcholine activation (Puinean et al., 2013). The G275E mutation in insect nAChR α6 is homologous to the 338D of the glutamate-gated chloride channel (GluCl) of Caenorhabditis elegans, which is critical to form a van der Waals interaction with ivermectin (Hibbs & Gouaux, 2011). Based on the above results, the location of the G275E mutation in nAChR α6 may appear closely associated with the spinosad binding location (Puinean et al., 2013).
Notably, the resistance to spinosad and spinetoram imparted by G275E (230-fold) was slightly lower than that of the Seα6-KO strain (373-fold) with a Seα6 loss-of-function mutation. However, the resistance ratio of the Dα6 G275E knockin strain (62.2-fold) against spinosad was significantly lower than that of the Dα6 knockout strain (138.8-fold) in D. melanogaster (Zimmer et al., 2016). These results suggested that the resistance conferred by the G275E mutation against spinosad might be different in different species, which might be attributable to differences in their binding profiles on nAChR α6.
The G275E mutation is inherited as an incomplete recessive trait in S. exigua that completely matches the spinosad resistance inheritance mode mentioned in D. melanogaster and F. occidentalis (Bielza et al., 2007a; Zimmer et al., 2016), where only homozygous G275E mutants could survive under high doses of spinosad. In addition, P146S near the conserved Dα6 Cys-loop leads to moderate and incomplete resistance to spinosad in D. melanogaster. Hence, it is also suggested that mutations at different loci of nAChR α6 may result in different levels and/or different inheritance patterns for resistance to spinosyns.
In conclusion, we demonstrated a high degree of spinosad and spinetoram resistance in S. exigua is due to a Seα6 mutation. The findings helped to elucidate spinosyn resistance mechanisms in S. exigua and may supply basic data for the design of effective management programs for S. exigua control.
Acknowledgments
This work was funded by the National Natural Science Foundation of China (32001941 and 31972303), and the China Postdoctoral Science Foundation (2020M683586), the Research Fund for the Doctoral Program of Northwest A&F University (Z1090219195).
Disclosure
All authors have seen and agree with the contents of the manuscript and there is no conflict of interest, including specific financial interest and relationships and affiliations relevant to the subject of the manuscript.




