Genome-wide identification of target genes for transcription factor BR-C in the silkworm, Bombyx mori
Abstract
Transcription factor Broad Complex (BR-C) is an ecdysone primary response gene in insects and participates in the regulation of insect growth and development. In this study, we performed a genome-wide identification of BR-C target genes in silkworm (Bombyx mori) using chromatin immunoprecipitation followed by high-throughput sequencing (ChIP-seq). As a result, a total of 1006 BR-C ChIP peaks were identified, and 15% of peaks were located in the promoter regions of 133 protein-coding genes. Functional annotation revealed that these ChIP peak-associated genes, as potential BR-C targets, were enriched in pathways related to biosynthetic process, metabolic process, and development. Transcriptome analysis and quantitative real-time polymerase chain reaction (PCR) examination revealed that developmental changes in expression patterns of a portion of potential BR-C targets, including HR96 and GC-α1, were similar to those of BR-C. ChIP-PCR examination confirmed that BR-C could directly bind to the promoters of potential targets. Further, dual luciferase assays demonstrated that HR96 promoter activity was significantly upregulated following BR-C overexpression, and this upregulation was abolished when the binding motif in the promoter was truncated. This study will be helpful for deciphering the regulatory roles of BR-C during insect growth and development.
Introduction
Insect growth and development are cooperatively orchestrated by two endocrine hormones, ecdysone and juvenile hormone (Dubrovsky, 2005). The ecdysone as a steroid hormone controls insect embryogenesis, larval molting, metamorphosis with larva–pupa–adult transition, and reproduction (Schwedes & Carney, 2012; Yamanaka et al., 2013). In addition to receptor complexes including ecdysone receptor and ultraspiracle, a series of ecdysone primary response genes are involved in the ecdysone signaling cascade, including Broad Complex (BR-C), E75, HR3, HR4, and beta Ftz-f1 (Riddiford, 1993; King-Jones & Thummel, 2005; Ou & King-Jones, 2013; Yamanaka et al., 2013).
Insect BR-C is a transcription factor and contains two types of functional domains, two zinc finger domains that regulate target gene transcription by directly binding to their promoters and a Bric-a-brac-Tramtrack-Broad Complex (BTB) domain that mediates the protein-protein interaction (DiBello et al., 1991; Zollman et al., 1994; Cheng et al., 2014a). Increasing evidence demonstrates that BR-C is involved in numerous biological processes during insect development, including metamorphosis (Karim et al., 1993; Zhou & Riddiford, 2002; Erezyilmaz et al., 2006), organ remodeling (Spokony & Restifo, 2009), and programmed cell death (Lee et al., 2002; Uhlirova et al., 2003). BR-C knockdown in silkworm blocks the larval-pupal transition and silk gland degradation (Uhlirova et al., 2003).
Insect BR-C is transcriptionally induced by ecdysone and repressed by juvenile hormone (Zhou et al., 1998; Kayukawa et al., 2016). BR-C also directly regulates the transcription of several downstream targets, including wing cuticle protein 4 (WCP4) (Deng et al., 2015), WCP5 (Wang et al., 2009b), WCP10 (Wang et al., 2009a), Vitellogenin (Yang et al., 2014), Chitinase 5 (Zhang & Zheng, 2017), and Lebocin gene encoding an antimicrobial peptide (Mai et al., 2017). We previously reported that protein-protein interaction between silkworm BR-C and Receptor of Activated C Kinase 1 is required for protein kinase C-mediated phosphorylation of BR-C at Ser373 and Thr406, which mediates BR-C nuclear import and its transcriptional activity (Cheng et al., 2014b). However, protein kinase A-mediated BR-C phosphorylation at S186 inhibits its transcriptional activity (Qian et al., 2017).
Silkworm (Bombyx mori) is a model organism for the lepidopterans (Xia et al., 2014). To date, four different silkworm BR-C isoforms, Z1, Z2, Z3, and Z4, have been identified (Nishita & Takiya, 2004). Although the expression patterns and essential roles of silkworm BR-C have been well characterized (Uhlirova et al., 2003; Nishita & Takiya, 2004), its direct targets are largely unknown. In the present study, we performed a chromatin immunoprecipitation followed by high-throughput sequencing (ChIP-seq) analysis using an antibody against silkworm BR-C Z2 to identify novel direct targets of silkworm BR-C on a genome-wide scale. A total of 1006 ChIP peaks have been identified for silkworm BR-C. Further expression profiling, ChIP-PCR (polymerase chain reaction) examination, and luciferase reporter assays revealed that two genes of our interest, HR96, a nuclear hormone receptor, and GC-α1, a receptor for nitric oxide, are regulated by BR-C directly binding to their promoter regions.
Materials and methods
Silkworm rearing and cell culture
Silkworm strain Dazao were reared on fresh mulberry leaves at 25 °C under a photoperiod of 12 h light and 12 h dark with 70%–80% relative humidity. Silkworm embryo-derived BmE cells were cultured in Grace's insect medium (Gibco) supplemented with 10% fetal bovine serum at 27 °C.
ChIP-seq and ChIP-PCR
The ChIP assay was performed as previously described (Kayukawa et al., 2017; Cheng et al., 2018). In brief, BmE cells transfected with pSL1180-BR-C overexpression plasmid for 48 h were fixed with 37% formaldehyde to cross-link protein and chromatin, then sonicated to shear into DNA fragments of 200–1000 bp in length. The protein-chromatin complexes were immunoprecipitated using the antibody against silkworm BR-C Z2 (Zoonbio Biotechnology), which we have used previously (Cheng et al., 2014b; Qian et al., 2017). Anti-immunoglobulin G (IgG) antibody was used as a control. Following elution and cross-link reversal, DNA was purified by phenol-chloroform extraction and ethanol precipitation. Finally, purified DNA fragments from the immunoprecipitated products were used for DNA sequencing and PCR validation.
Input and ChIP DNA samples were PCR-enriched for paired-end sequencing on the Illumina HiSeq 2500 platform. For each sample, more than 16 million clean reads were obtained. All sequences have been deposited in the Short Read Archive of the National Center for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov/sra/) under the Sequence Read Archive (accession number PRJNA518741). Based on the sequencing data, clean paired-end reads were mapped to the silkworm reference genome downloaded from KAIKObase (http://sgp.dna.affrc.go.jp/ComprehensiveGeneSet/) using the BWA program (version 0.7.12-r1039). Only uniquely aligned reads were used. Highly enriched peaks were obtained by MACS2 using standard settings (q value < 0.05) (Zhang et al., 2008). For all ChIP-seq data sets, Wiggle files were generated with Bedtools (version 2.26.0) and were subsequently used for visualization by the Integrative Genomics Viewer (Robinson et al., 2011) and for obtaining average signal profiles (Cheng et al., 2018). MEME Suite 5.0.3 (http://meme-suite.org) was used for detecting sequence motifs.
Purified DNA samples were analyzed following the sequencing assay. To determine BR-C ChIP peak locations, we divided the genome into genic and intergenic regions. The genic region contained exons and introns, while the intergenic region contained upstream promoter (−3.0 kb to transcription start site, TSS), downstream (0–3 kb downstream of a gene), and distal intergenic (> 3 kb upstream of TSS and > 3 kb downstream of a gene) regions. Each peak could be assigned to these regions. All primers used in ChIP-PCR experiments were designed based on the ChIP peaks and are listed in Supplementary Table S1.
Data analysis
Using the Web Gene Ontology Annotation Plot (WEGO) online program, we performed Gene Ontology (GO) annotation enrichment analysis of all BR-C target genes. Silkworm fat body transcriptome data from our previous report (Peng et al., 2018) were used to profile target gene expression. Target gene expression patterns were calculated using the reads per kilobase million method (Qian et al., 2020).
RNA extraction and quantitative real-time (qRT)-PCR
As previously described (Peng et al., 2018), total RNA samples were extracted from fat bodies of silkworm larvae at four stages, the initiation of the wandering stage (W0), day 1 of the wandering stage (W1), prepupal stage (PP), and day 1 of the pupal stage (P1), using the Trizol regent. Complementary DNA was synthesized following the manufacturer's instructions for the Prime Script™ RT Reagent Kit (TaKaRa, Japan) using 2 μg total RNA. The qRT-PCR examination was performed using a 7500 FastReal Time PCR System (Applied Biosystems) and a SYBR Premix Ex Taq kit (Takara). The silkworm eukaryotic translation initiation factor 4A (eIF-4a) gene was used as the internal control. All primers used in qRT-PCR are listed in Table S1.
Dual luciferase assay
Full-length and peak truncated promoters of candidate genes were subcloned into the pGL3-basic vector (Promega) containing the firefly luciferase gene. A pRL-TK vector containing the Renilla luciferase gene was used as the internal control. The open reading frame of silkworm BR-C was subcloned into pSL1180 (Life Technologies) vector for gene overexpression. The newly constructed vectors containing different promoters were co-transfected with the BR-C overexpression vector. After transient transfection for 48 h, cells were collected for dual luciferase assay as described previously (Cheng et al., 2018). The primers used in dual luciferase assay are listed in Table S1.
Electrophoretic mobility shift assay (EMSA)
To confirm the direct binding of BR-C within the HR96 promoter, EMSA experiments were performed as described previously (Cheng et al., 2018). Following the EMSA/Gel-Shift Kit (Beyotime) manufacturer's instructions, DNA probes targeting the BR-C binding site in the HR96 promoter were biotin-labeled at the 5ʹ end. Then, 100 nmol/L biotin-labeled probes were co-incubated with different amounts of nucleoproteins extracted from silkworm BmE cells overexpressing BR-C in 10 μL of binding reaction system. For competition assay, 7 μg of extracted nucleoproteins was incubated with 1–50-fold molar excess of unlabeled probes before the addition of labeled probes. Subsequently, the binding systems were electrophoresed in 5% (w/v) polyacrylamide gels in TBE buffer (45 mmol/L Trisborate and 1 mmol/L ethylenediaminetetraacetic acid, pH 8.3) and transferred onto nylon membranes for chromogenic reactions. The primers used for EMSA are listed in Table S1.
Statistics
Statistical analyses were performed using the Student's t-test. Values are presented as the mean ± SE (error bars) of at least three independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001. For analysis of variance, bars labeled with different lowercase letters significantly differ (P < 0.05).
Results
ChIP-seq analysis of genome-wide binding sites of silkworm BR-C
To identify downstream targets of silkworm BR-C on a genome-wide scale, we performed BR-C overexpression in silkworm embryo-derived BmE cells and used anti-BR-C antibody to conduct ChIP-seq analysis. In total, 16 477 790 raw reads and 16 455 296 clean reads were obtained from BR-C immunoprecipitated products, and 16 297 532 raw reads and 16 281 588 clean reads were obtained from the input sample (NCBI accession number PRJNA518741). Compared with the input sample as a control (without BR-C immunoprecipitation), a total of 1006 unique BR-C ChIP peaks were identified from BR-C immunoprecipitated products (Table S2). After mapping with the silkworm genome, we found that approximately 63% of BR-C ChIP peaks were located in the distal intergenic region, 15% in the 3.0 kb promoter region upstream of TSS, 18% in the introns, 3% in the exons, and only approximately 2% in the downstream region (Fig. 1A). We also found a ChIP peak within the promoter region of the WCP10 gene, a known target of silkworm BR-C Z2 (Fig. S1)
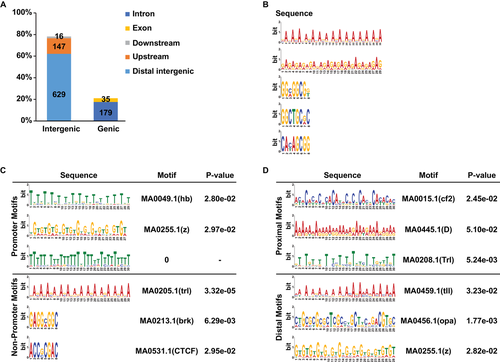
We further characterized the motifs within the BR-C ChIP peaks using MEME Suite 5.0.3. This revealed the most enriched motifs within BR-C ChIP peaks (Fig. 1B). Further prediction using Tomtom analysis revealed that enriched motifs within either promoter or non-promoter regions could be matched with motifs that are recognized by other zinc finger transcription factors, and that the short motifs mainly existed in non-promoter regions (Fig. 1C). We also observed that the motifs enriched in promoters differed between proximal (from −1.0 kb to the TSS site) of 39 peaks and distal regions (from −3.0 kb to −1.0 kb) of 108 peaks (Fig. 1D).
Functional annotation of novel silkworm BR-C target candidates
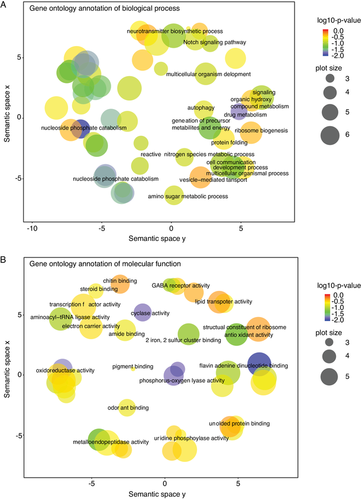
We further annotated the genes associated with BR-C ChIP peaks. A total of 829 protein-coding genes were confirmed to be associated with BR-C ChIP peaks, 133 of which have ChIP peaks in their promoters. GO enrichment analysis showed that these genes could be functionally classified into different categories (Fig. 2). Within the class of biological process, a high portion of genes were involved in biosynthetic process, catabolic process, metabolic process, ribosome biogenesis, developmental process, autophagy, cell communication, and protein folding (Fig. 2A). In addition, the categories of catalytic activity, binding, and structural constituent were mostly enriched in molecular function (Fig. 2B). This distribution is consistent with the main BR-C protein functions during insect growth and development.
Co-expression of BR-C and novel target candidates
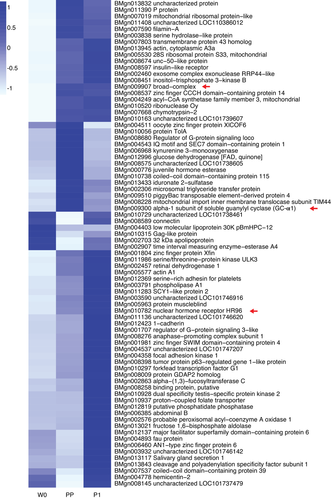
To identify downstream targets with transcription directly regulated by BR-C, we investigated co-expression patterns between BR-C and potential targets. Using the transcriptome data of silkworm fat body during metamorphosis we previously reported (Peng et al., 2018), we identified 76 genes with BR-C ChIP peaks in their promoters that were expressed in the fat body during silkworm metamorphosis (Fig. 3). Notably, BR-C exhibits low expression at the initiation of the wandering stage (W0) and is highly expressed from the prepupal (PP) stage to the first day of the pupal stage (P1). A total of 25 potential target genes, including two genes of our interest, nuclear hormone receptor HR96 (BMgn010782) and the nitric oxide receptor alpha-1 subunit of soluble guanylyl cyclase (GC-α1, BMgn009300), showed dynamic expression changes similar to that of BR-C.
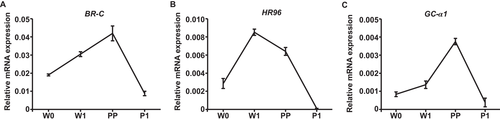
We further performed qRT-PCR analysis to confirm the expression of both HR96 and GC-α1 in the fat body during silkworm metamorphosis. HR96 and GC-α1 were highly expressed in the fat body at prepupal stage, which is consistent with BR-C expression (Fig. 4). These results, together with their relationship with BR-C ChIP peaks, suggested that HR96 and GC-α1 are likely direct targets of BR-C and may mediate BR-C signaling during silkworm metamorphosis.
Confirmation of BR-C binding to the promoters of novel target candidates
Previous reports demonstrate that silkworm BR-C regulates target gene transcription by directly binding to their promoters via specific motifs (Wang et al., 2009a; Wang et al., 2009b; Deng et al., 2015; Qian et al., 2017). Our ChIP analysis revealed that HR96 and GC-α1 promoters both contain BR-C ChIP peaks (Fig. 5A–B). We performed ChIP-PCR examination in silkworm BmE cells with BR-C overexpression to confirm whether BR-C could directly bind the HR96 and GC-α1 promoters. Compared with ChIP products with nonspecific IgG as a negative control, the DNA region covering the BR-C ChIP peaks within the promoters of novel targets could be detected from the ChIP products using an anti-BR-C antibody, showing a size same to that of input samples (Fig. 5C–D).
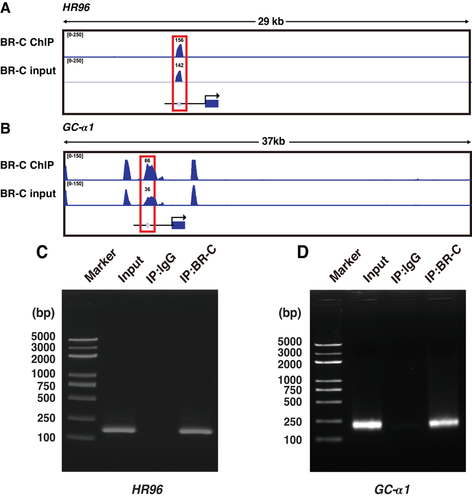
We next performed EMSA experiments to examine BR-C binding ability to the HR96 promoter. The oligonucleotide probes covering BR-C ChIP peak in the HR96 promoter were designed. EMSA results revealed that the nucleoprotein proteins from silkworm BmE cells with BR-C overexpression can bind the biotin-labeled probes in a dose-dependent manner (Fig. 6A). This binding was competitively suppressed by unlabeled cold probes and this was abolished when the BR-C ChIP peak region was deleted in the cold probe (Fig. 6A–B). Together, these results confirm that BR-C binds to a specific motif within the HR96 promoter.
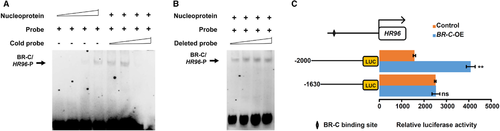
BR-C promotes the activity of the promoters of novel target HR96
We next performed dual luciferase reporter assays in BmE cells to determine how BR-C regulates HR96 gene transcription. As described previously (Meng et al., 2015), we first cloned the proximal promoter containing approximately 2.0 kb of sequence upstream of the HR96 gene TSS and generated constructs with a luciferase reporter gene driven by either complete or truncated promoters with or without potential BR-C binding sites, respectively. Subsequently, these constructs were separately co-transfected into BmE cells with the vector overexpressing silkworm BR-C. Further analysis revealed that, compared with the control overexpressing red fluorescent protein gene, the activity of the complete HR96 gene promoter significantly increased following BR-C overexpression, and that this increase was abolished when the binding site within the promoter was truncated (Fig. 6C). Taken together, these results show that BR-C positively regulates HR96 gene transcription through directly binding to a specific site within the promoter region.
Discussion
Insect BR-C functions as a transcription factor to mediate ecdysone signaling during growth and development, and is especially involved in specifying the pupal fate (Karim et al., 1993; Zhou & Riddiford, 2002; Dubrovsky, 2005; Wilson et al., 2006; Ou & King-Jones, 2013; Kayukawa et al., 2016). To date, few direct targets of insect BR-C have been reported, including several WCP genes, Vitellogenin, Chitinase 5, and Lebocin (Wang et al., 2009a; Wang et al., 2009b; Yang et al., 2014; Deng et al., 2015; Zhang & Zheng, 2017; Mai et al., 2017). Herein, we performed a genome-wide ChIP-seq analysis in silkworm and identified 1006 BR-C ChIP peaks, five of which were confirmed using ChIP-PCR (Fig. 5C–D and Fig. S2). These BR-C ChIP peaks were associated with 829 protein-coding genes. These genes are involved in multiple biological processes, including developmental process, autophagy, catabolic process, and ribosome biogenesis, and provide a useful resource for better understanding the roles and signaling cascade of BR-C during insect growth and development.
Consistent with the functions of insect BR-C as a pupal specifier during the larval-pupal transition (Zhou & Riddiford, 2002; Kayukawa et al., 2016), the expression of BR-C and 25 potential BR-C target genes showed similar dynamic changes in the fat body during silkworm larval-pupal transition. Two targets of interest, the HR96 nuclear hormone receptor and GC-α1 nitric oxide receptor, were directly and positively regulated by BR-C, and may be involved in the regulation of metabolic processes in the fat body. Previous reports in Drosophila revealed that HR96 is transcriptionally induced by ecdysone (Fisk & Thummel, 1995), and plays key roles in the control of triacylglycerol metabolism, maintenance of cholesterol homeostasis, and the regulation of xenobiotic stress responses (King-Jones et al., 2006; Sieber & Thummel, 2009; Horner et al., 2009; Sieber & Thummel, 2012; Afschar et al., 2016). Given that the insect fat body undergoes morphologic and metabolic changes during the larval-pupal transition (Arrese & Soulages, 2010; Peng et al., 2018; Li et al., 2019), we reasoned that BR-C and HR96 may cooperatively modulate energy storage and utilization in the fat body during the larval-pupal transition.
GC-α1 encodes soluble guanylyl cyclase, which consists of alpha and beta subunits and functions as a receptor for nitric oxide to catalyze the production of the second messenger cyclic guanosine monophosphate (cGMP) (Koesling et al., 1991; Denninger & Marletta, 1999; Murad, 2006). Interestingly, nitric oxide is produced in the Drosophila prothoracic gland, an organ for ecdysone synthesis, and regulates ecdysone biosynthesis and developmental transition (Reinking et al., 2005; Caceres et al., 2011). Given that BR-C is also an ecdysone primary response gene, the potential GC-α1 transcription regulation by BR-C in the fat body indicates a feedback regulation of ecdysone on the nitric oxide-cGMP signaling pathway in the fat body.
We further noted that changes in the anaphase-promoting complex subunit 1 (APC1) gene expression was opposite to that of BR-C during silkworm metamorphosis. APC1 is a subunit of the anaphase-promoting complex/cyclosome (APC/C) E3 ligase complex, which controls cell cycle progression by directly ubiquitylating and degrading its substrates (Zielke et al., 2013; Edgar et al., 2014). Our data suggest that BR-C might negatively regulate APC1 expression to modulate cell cycle progression. Taken together, our data show that further analysis to determine how BR-C cooperates with its targets to regulate various biological processes during insect growth and development is needed.
Acknowledgments
This research was funded by grants from the National Natural Science Foundation of China (31572464, 31772679, and 32070496), the Natural Science Foundation of Chongqing (cstc2019jcyj-msxmX0446), and the Fundamental Research Funds for the Central Universities (XDJK2020C008).
Disclosure
The authors declare no conflict of interest.




