Mobility affects copulation and oviposition dynamics in Pieris brassicae in seminatural cages
Abstract
When, how often and for how long organisms mate can have strong consequences for individual fitness and are crucial aspects of evolutionary ecology. Such determinants are likely to be of even greater importance in monandrous species and species with short adult life stages. Previous work suggests that mobility, a key dispersal-related trait, may affect the dynamics of copulations, but few studies have investigated the impact of individual mobility on mating latency, copulation duration and oviposition latency simultaneously. In this paper, we monitored the copulation dynamics of 40 males and 40 females, as well as the oviposition dynamics of the females of the Large White butterfly Pieris brassicae, a facultative long-distance disperser butterfly. Individuals from a breeding were selected to create a uniform distribution of mobility and we recorded the timing, number and duration of all copulations in a semiexperimental system. We showed that mobility, measured as the time spent in flight under stressful conditions (a proxy of dispersal tendency), correlates with all aspects of copulation dynamics: mobile males and females mated earlier and for shorter periods than less mobile individuals. In turn, late mating females increased the time between copulation and oviposition. These results feed the previously described mobility syndrome of P. brassicae, involving morphological and physiological characters, with life-history traits. We suggest that the reduction of mating latency and copulation duration has an adaptive value in dispersing individuals, as their life expectancy might be shorter than that of sedentary individuals.
Introduction
In sexual organisms, mating is a necessary condition to increase fitness through the production of offspring. Despite the obvious benefits of increased sexual activity, increasing the number and duration of copulations is energetically costly, decreases the time available for feeding, and increases predation and infection risk (Magnhagen, 1991; Kemp, 2012). As a result, selection may influence the timing, number, and duration of copulations. These pressures may be of even greater importance for species experiencing small numbers of copulations, for example, species with a short adult phase, or monandrous species.
In lepidopterans, most females mate soon after their emergence, in part because protandry is a common phenomenon in this taxon (Wiklund & Fagerström, 1977). However, some females remain virgin up to several days (Scott, 1972), that is, a significant part of their adult life. Copulating late bears several costs, for example, lower fecundity, increased preoviposition period (Jones & Aihara-Sasaki, 2001; Mori & Evenden, 2013), and reduced attractiveness to males by older females (Xu & Wang, 2009). However, delayed copulation may hold an adaptive value if females select specific characteristics in males, as ejaculates contain not only sperm, but also nutrient-rich spermatophores (Svärd, 1985; Bissoondath & Wiklund, 1996). Spermatophore quality decreases with the number of copulations and is age-dependent (Vande Velde et al., 2012). Females can assess male condition based on several cues, for example, whether males can defend a territory (Wong & Candolin, 2005), or the production of vibratory signals (Andrade & Mason, 2000). Copulation duration can also represent a significant proportion of insects' adult phase (Scott, 1972). It is influenced mostly by males’ spermatophore size (Edvardsson & Canal, 2006), because it generally reflects the physical constrains of transferring spermatozoa and nutrients to females. Male fertility itself varies with mating history (Hughes et al., 2000) and age (Wedell & Cook, 1999; Vande Velde et al., 2012). Males can also adjust their investment into courtship and copulation based on the condition and mating status of females (Bonduriansky, 2001). Mating status can be assessed, for example, via sperm storage cues or evidence of recent copulation such as mating plugs (Wedell et al., 2002).
Mobility is crucial for diverse aspects of an individual's life, for example, to search for food or sexual partners, to escape predators, and is generally correlated with many other phenotypic characteristics including dispersal (O'Riain et al., 1996; Hoset et al., 2011; Ducatez et al., 2012). In insects, reproduction is often thought to be traded-off against mobility, according to the so-called oogenesis-flight syndrome (Johnson, 1969), which describes a negative correlation between the relative investment in reproductive and flying abilities. The generality of this syndrome has been debated recently, especially in Lepidopterans where it is either documented (Karlsson & Johansson, 2008; Gibbs & van Dyck, 2010), or refuted (Hanski et al., 2006; Jiang et al., 2010). Several arguments have been put forward to explain this discrepancy, as it may depend, for example, on habitat characteristics (Gibbs & van Dyck, 2010), or species-specific life histories (Stevens et al., 2013, 2014). At the intraspecific level, associations between mobility and reproductive output may also vary between males and females, or depend on the type of trait (Legrand et al., 2016).
Correlations between dispersal and life-history traits are receiving increasing attention at the individual level (Clobert et al., 2009). Mechanisms like life history trade-offs between growth and mortality or between current and future reproduction are often invoked to explain behavioral polymorphism within populations (Wolf et al., 2007; Réale et al., 2010). The underlying theory is that phenotypic traits such as exploration should be positively correlated with mortality risk, and therefore should favor early sexual maturity and higher investment in reproduction early in life (Wolf et al., 2007; Biro & Stamps, 2008). As dispersers within a population are likely to face higher mortality risk, especially during the transient stage (Bonte et al., 2012), they should start investing in reproduction earlier than their sedentary conspecifics, for example, to secure enough time for egg-laying (females), or for potential subsequent copulations (males and females). Cues of life expectancy have been shown to affect reproductive decisions in a moth (Javoiš & Tammaru, 2004), so it is possible that a similar mechanism drives dispersing individuals to shorten their reproduction time, although little information is available.
To study the influence of mobility on reproductive decisions, our study model was the Large White, a facultative long distance disperser (Baguette et al., 2014) that exhibits interindividual variation in mobility across Europe (Ducatez et al., 2013), with a bimodal distribution corresponding to disperser and resident phenotypes under experimental conditions (Legrand et al., 2015). Such distribution suggests that natural populations are composed of a mixture of dispersing and sedentary individuals (Ducatez et al., 2013; Larranaga et al., 2013). In this species, mobility measured as the time spent in flight under stressful conditions in laboratory conditions covaries with a suite of morphological, behavioral and physiological traits including wing morphology, exploratory behavior, flight direction at emergence and, importantly, dispersal (Ducatez et al., 2012, 2013; Larranaga et al., 2013; Legrand et al., 2015, 2016). Mobility, exploration, and orientation are also consistent over time (Spieth et al., 1998; Ducatez et al., 2012; Larranaga et al., 2013). P. brassicae females typically mate rapidly after emergence and do not re-mate for a period of 5 d, while males can copulate more than one time per day (David & Gardiner, 1961). After copulation, females lay their eggs mostly on many plant species belonging to the Brassicaceae family (Feltwell, 1982) in large clutches of up to hundreds of eggs. Both males and females seem to copulate before dispersing (Trochet et al., 2013).
In this study, we monitored groups of P. brassicae and followed several aspects of their copulation dynamics under seminatural conditions. Our aim was to provide some insight to the oogenesis-flight syndrome, by identifying possible relationships between mobility, the timing of copulations, and the timing of ovipositions. We used a mixture of mobility assessment in the laboratory and direct observations of matings in seminatural environments. Based on the costs associated with mobility (developmental, mortality, etc., Bonte et al., 2012), and on the oogenesis-flight syndrome, we expected mobile individuals to decrease their total reproductive time. As P. brassicae copulates before dispersing, mobile individuals should copulate earlier, and for shorter periods. Mobile females should also lay eggs earlier. Late copulation may (Mori & Evenden, 2013) or may not be associated with longer adult lifespan (e.g., Jones et al., 2008). If late-mating females do live longer, they should be more likely to lay eggs late, compared with early-mating females. If not, they should have limited time for oviposition, and should lay eggs early.
Materials and methods
Breeding conditions
Clutches of P. brassicae used for this study were selected from our laboratory breeding established in August 2011 in Moulis (Ariège, southwestern France). The founding 48 clutches originated from three locations in Ariège (mean distance between locations: 10 km) and one in Vaucluse (Southeastern France). Based on high densities of P. brassicae in Ariege (pers. obs.), and large time intervals (one to several weeks) between collections of clutches in those regions, it is likely that all clutches collected originated from different females. Hence, our breeding was likely established by a total of 48 founding females. A set of 210 individuals from 10 clutches of the second generation of this breeding were reared in the laboratory after artificially induced diapause (from December 2011 to May 2012, photoperiod 14 L:10 D, 20 ± 1 °C during light periods and 12 ± 1 °C). Of those 210 individuals, 80 were selected for this study, based on their mobility. Eggs and larvae were reared in similar conditions, that is, 14 L:10 D cycle, 23 ± 1 °C during light and 18 ± 1 °C during dark) in Moulis.
All clutches were kept separately. Adults emerged gradually during the course of the experiment and were maintained under common garden conditions. During the 24 h following emergence, newly emerged butterflies were marked with a unique number (blue and red pen for males and females, respectively), after their wings had dried and they were capable of flight. Then, butterflies were submitted to the flight performance test described in Ducatez et al. (2012, see below). Emergence was checked twice each day (from 06:00 to 08:00 and from 18:00 to 19:00) and no emergence occurred at night. Most butterflies emerged from 06:00 to 08:00. This means that all individuals were marked and tested within 11 h following their emergence, and most of them within 2 h. Individuals were then transferred to a 1 × 1 × 1 m breeding cage with a water point and nectariferous flowers and remained in the cage (25 °C) until being transferred to the experimental cages. To prevent any sexual interaction, males and females were kept separately. The 80 individuals used in the mating experiment were selected based on their score to the mobility test. We prioritized the maximization of variability in mobility, so some butterflies remained in the laboratory longer than others. Hence, individuals spent on average 2 d (i.e., 37–50 h) in the laboratory (range = 1–6, but only four individuals were kept for more than 4 d) before being released in the experimental cages.
Mobility test
Mobility was assessed by a test monitoring flight endurance under stressful conditions (Ducatez et al., 2012). Each butterfly was placed individually in a 250 × 100 × 100 mm plastic chamber fixed to a rapid agitator (Vortex Genie 2, Scientific Industries Bohemia, New York, NY, USA). All tests were performed under similar conditions (between 07:00 and 08:00 AM, temperature = 25 ± 1 °C). Each individual spent 30 s in the chamber before the beginning of the test. The agitator was then turned on for 1 min, which shook the chamber. An individual could only fly or lay uncomfortably at the bottom of the chamber. All individuals were able to fly before being tested and had wings of good quality. The time spent in flight was measured with a timer and used as a proxy of mobility (Ducatez et al., 2012, 2013; Larranaga et al., 2013). This mobility proxy indeed correlates with individual dispersal decisions in experimental metapopulations with low and high performers at this mobility test being most often residents and dispersers, respectively (Legrand et al., 2012, 2015). The time spent in flight in the chamber correlated with other surrogates of mobility, including the willingness to fly in a dark tunnel and the total distance travelled in a greenhouse (Ducatez et al., 2012). Hence, our measurements should give a relatively accurate representation of mobility in seminatural conditions. Such measurement may be harmful to butterflies and reduce lifespan. However, we ensured that the wings of butterflies with low mobility scores were not damaged.
Mating experiments
The experiments started on April 19, 2012 and finished on May 12, 2012. Experiments were performed only during periods of sunny days. We used two of the large 10 × 10 m outdoor unconnected cages of the Metatron (Legrand et al., 2012), which provides excellent seminatural conditions for behavioral observations of butterflies. Four groups of butterflies were released during uninterrupted periods of bright sunny days (i.e., without rain). For each group, we selected ten males and ten females to create a continuous uniform distribution of mobility in each sex. Within a group, individuals belonged to at least four different families. To stimulate copulation and provide opportunity for egg-laying, a fresh cabbage was placed inside each cage. Egg-laying events were spread over 5 d and clutches were removed less than 15 min after the last egg was laid. We stopped observations when no sexual activity was recorded for 1 d, and after all mated females had laid eggs or had shown no interest in oviposition for several days. This period corresponded to the death of more than half of the individuals. Experiments were terminated when all individuals had died (5 d in two groups, 6 d in the other two). Every day during the experiment, from 09:00 to 18:00, the same observer inspected the cage every 30 min and recorded copulations for about 15 min. Both partners were identified and the length of the copulations was estimated to the closest 15 min. The observer left the cage after 15 min to limit the disturbance caused by its presence on copulations. When eggs were laid, the observer identified their mother by slowly approaching the oviposition site. Unmated females never laid eggs and mating never occurred during the two first hours or during the last hour of observation each day. Therefore, we are fairly confident that all matings and egg-laying events were recorded during the course of this experiment.
Statistical analyses
Mobility was expressed as the time spent in flight during the mobility test in seconds. Mating latency was defined as the time between introduction in the cage and first copulation in hours. Copulation duration was defined as the time between the first observation of a copulation and the first observation after which it stopped in minutes. Second copulations of males were not considered in the analyses of male mating latency and copulation duration. Oviposition latency was defined as the time between the end of a copulation and oviposition in females in hours.
Low numbers of emergences in our breeding also implied that siblings had to be selected within the same group. Siblings also shared a common larval environment, which can affect their development as well as reproductive decisions (e.g., inbreeding avoidance). Although inbreeding avoidance can be low in butterfly species (Haikola et al., 2004), this could affect the results. We tackled this issue by (1) including family as a random factor in our analyses and (2) evaluating the difference in mating latency and copulation duration between sibling and unrelated pairs.
- (1) Mating latency ∼ Mobility + Age at release + family (random) + group (random)
- (2) Copulation duration ∼ Mobility + Mating latency + Age at release + family (random) + group (random)
- (3) Oviposition latency ∼ Female mobility + Mating latency + Copulation duration + Age at release + family (random) + group (random)
Significant effects were determined using a descendant selection procedure (Hocking, 1976), that is, the least significant factor was dropped until all remaining factors had a significant P. None of the variables was different among groups, nor among families (ANOVA, P > 0.1 in all cases). The effect of mating latency and copulation duration on the likelihood to lay eggs, as well as the difference in mating latency and copulation duration between sibling and unrelated pairs were investigated using Wilcoxon signed-rank tests.
Our experimental design implies that a copulation could potentially start 15 min before it was first detected, and could finish 15 min before we considered it stopped. Therefore, we may over- or underestimate its duration by up to 15 min (maximal copulation duration = 3 h). We replicated analyses using bootstrapped distributions of durations, by adding random periods within a uniform distribution where minimum = –15 and maximum = 15 min (1000 iterations), to prevent the possibility of statistical artifacts. The proportion of tests with significant P in the 1000 tests was used as a discriminant value to reject the null hypothesis. Mating and oviposition latency were also systematically underestimated by up to 15 min, although this bias is thought to be less important than for copulation duration because they occurred over the course of several days. Nevertheless, we also addressed this potential detection error by adding random values from a uniform distribution (minimum = 0 and maximum = 15 min) in the bootstraps. As bootstrap procedures never changed the outcome of the model selection, we only present the results using the original values of all variables.
Another statistical issue is the presence of few extremely short copulations in our dataset (20–30 min), which are not compatible with the transmission of spermatophores in P. brassicae. Those interrupted matings could result from random perturbations or be related with post-mating incompatibilities or choices. We thus replicated the analyses using only copulations that lasted more than 30 min. All but one result remained significant after removing short copulations from our dataset, we thus present the model including all copulations, but also report the result that was not significant after removing short copulations. Females mating with nonvirgin males were not excluded from the dataset, but a second set of analyses was performed to evaluate how it may affect our results. Significant effects remained significant after removing those females.
As we expect mobility and mating latency to be correlated, as well as mobility and copulation duration, several of the models contain potentially correlated predictors. However, in all cases, only one of these predictors had an effect on the response variable, and the other(s) never had an effect, even separately from other covariates. Hence, multi-collinearity is unlikely to be a significant issue in our dataset.
All statistical analyses were performed using the software R 3.0 (R Core Team, 2013) and we used the “lme4” package to perform linear mixed models (Bates et al., 2008).
Results
Overall, adult lifespan was 6.25 ± 1.78 d in the experimental cages, and was not different between males (6.35 ± 1.85 d) and females (6.15 ± 1.73 d, Wilcoxon signed-rank test, P = 0.703). We did not detect a negative correlation between mobility and lifespan in the 80 selected individuals, neither overall (P = 0.481), nor during the experimental study (P = 0.367). The relationship remained nonsignificant after separating males from females (P > 0.05 in both cases).
Timing of copulation
Thirty-three and 26 of the 40 released females and males mated, respectively, and 7 males mated twice. Copulations occurred on average 32.91 ± 24.88 h after the beginning of each experiment. Copulations lasted on average 75.45 ± 36.82 min. On the 33 copulations, 11 occurred between siblings, which suggests that inbreeding avoidance was low. Mating latency and copulation duration were similar for sibling and unrelated pairs (Wilcoxon signed-rank test, P = 0.702 and 0.782, respectively). Five copulations lasted 30 min or less, which may suggest they were deliberately interrupted.
There was a negative correlation between both female and male mobility and first mating latency (P = 0.005 and 0.013 for males and females, respectively, Fig. 1A and B, Table 1). In other words, males and females with low mobility waited longer to mate after their first contact with potential partners. The association between mobility and mating latency remained significant in females after removing the seven females that mated with nonvirgin males (P = 0.005). Similarly, mobility and mating latency were significantly correlated based only on copulations that lasted longer than 30 min. Age at release did not affect mating latency, neither in males, nor in females.
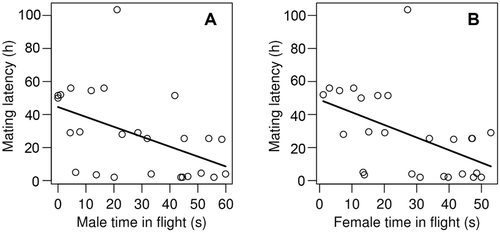
| Response variable | Explanatory variable | Estimate | Std error | df | P value |
|---|---|---|---|---|---|
| Mating latency (males) | Mobility | –0.723 | 0.229 | 19.139 | 0.005 |
| Mating latency (females) | Mobility | –0.843 | 0.267 | 21.090 | 0.013 |
| Copulation duration (males) | Mobility | –0.830 | 0.380 | 19.908 | 0.017 |
| Copulation duration (females) | Mobility | –1.226 | 0.397 | 22.145 | 0.005 |
| Oviposition latency (females) | Mating latency | 1.515 | 0.314 | 12.000 | <0.001 |
Copulation duration (for first matings only) was negatively correlated with both male and female mobility (P = 0.017 and 0.005 for males and females respectively, Fig. 2A and B). The relationship remained significant after removing females mating with nonvirgin males from the analysis (P = 0.007). However, excluding copulations that lasted 30 min or less made the relationship nonsignificant in males (P = 0.120 and 0.036 for males and females, respectively). Neither mating latency nor the age at release affected copulation duration in either males or females.

Oviposition latency
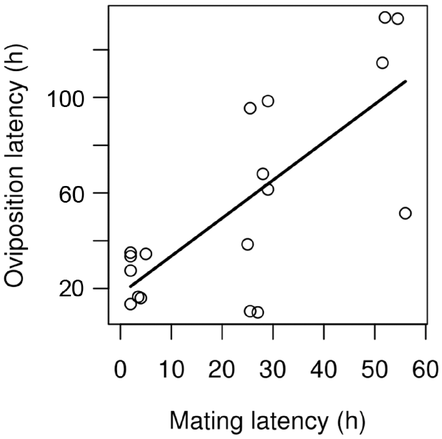
Among the 33 mated females, 18 laid eggs. Oviposition occurred on average 41.19 ± 52.58 h after copulation. All five females that copulated for 30 min or less laid eggs. Among the seven females mating with nonvirgin males, three did not lay eggs, hence a similar proportion to females mating with virgin males. Oviposition latency was similar between sibling and unrelated pairs (Wilcoxon signed-rank test, P = 1). Females mating late were less likely to lay eggs (Wilcoxon signed-rank test, P = 0.018), indicating a cost of late copulation. However, copulation duration did not affect whether or not mated females laid eggs (Wilcoxon test, P = 0.450). Oviposition latency increased with increasing mating latency (P < 0.001, Table 1, Fig. 3). There was however no evidence that female mobility influenced oviposition latency. Similarly, copulation duration had no significant effect on oviposition latency (Table 1). Female age at release had no significant effect on oviposition latency.
Discussion
During this study, females had relatively short longevity, similar to previous experiments in the same cages (Legrand et al., 2012; Trochet et al., 2013). Females of P. brassicae typically do not re-mate for a period of at least 5 d after first mating under laboratory conditions (David & Gardiner, 1961), so it is not surprising that females did not re-mate in our system. Low numbers of copulations (average = 1.28) have also been reported for females P. brassicae by Wiklund et al. (2001) in the laboratory. Although some males copulated a second time, re-mating rate remains low compared to previous observations of males copulating several times in 1 d (David & Gardiner, 1961). This means that the timing of copulations was critical in our experimental system for such short living species, and thus most likely subject to high selective pressures.
Mating latency
According to our predictions, high mobility shortened the age at reproduction in both males and females, which could have several nonmutually exclusive causes. A possible explanation is that more mobile individuals were less choosy and accepted copulations more easily. This would be an adaptive response to shorter lifespans for individuals investing heavily on mobility, and would provide arguments in favor of the oogenesis-flight syndrome. Cues of life expectancy can affect reproductive decisions in Lepidopterans (Javoiš & Tammaru, 2004), so the costs associated with mobility (predation risk, energetic costs of flying, potentially not finding new habitats, etc.) may drive mobile P. brassicae to copulate earlier than their less mobile counterparts.
Our results may also reflect the fact that mobility, which is an accurate predictor of movements in seminatural settings (Ducatez et al., 2012), may help find mates more rapidly, be detected more easily or can be used as a cue for sexual selection (Husak & Fox, 2008; Kalarus et al., 2013). In a previous study, virgin females of Pararge aegeria were flying more often and therefore were detected by males more often (Bergman et al., 2011). However, this relates to individual activity, and thus may not relate to mobility per se. In addition, butterflies were confined in a cage of moderate size and could potentially detect all other individuals in a short time. Locomotor performances can serve as reproductive cues both during male–female interactions (courtship) and male–male interactions (sexual competition, Husak & Fox, 2008). Mobility is correlated with several morphological traits in butterflies such as wing morphology (Ducatez et al., 2012) and melanization (Ellers & Boggs, 2004), so mobile females might be courted preferentially based on a different cue than mobility. We cannot conclude firmly on the origin of the association between mobility and mating latency.
Copulation duration
Copulations were shorter for both mobile females and mobile males. Such results had been reported for species with dimorphism in wing length, for example, the pygmy grasshopper Tetrix subulata in which long-winged individuals copulate for 1 min against 19 min for short-winged individuals (Steenman et al., 2015). Our results suggest that the same pattern can arise in insects with less extreme polymorphism in flying ability. It is unclear if only males, females, or both control copulation duration (Ward et al., 1992). Dispersing females may attempt to halt copulation if they need to disperse, or may be more efficient at doing so if higher locomotor abilities make this behavior more efficient. On the other hand, copulation duration may be influenced mostly by spermatophore size (Edvardsson & Canal, 2006). Actively flying males have smaller spermatophores (Vande Velde et al., 2012) and should copulate faster. Spermatophore size also increases with age and mobile males copulated at a younger age (i.e., potentially with smaller spermatophores). However, we did not measure spermatophore size and this information will be necessary to formally validate the links between copulation duration, mobility and ejaculate characteristics.
In promiscuous species, males usually increase their reproductive success by mating multiple times or by increasing copulation duration to prevent their current partner from being courted by other males (Parker, 1970). As the two tactics are in part mutually exclusive, time budgets may play an important role in the partitioning of time and the evolution of the ratio between said tactics. In this context, mating latency and copulation duration should be traded-off and we could expect a negative correlation between these two components of reproduction (e.g., Singh & Singh, 2014). We did not find such correlation (neither positive, nor negative), but our results suggest that both mobile males and mobile females decreased mating latency and copulation duration in concert. This could indicate that time budgets are important determinants of behavioral decisions for species with high variability in mobility.
Oviposition latency
Our results suggest that oviposition latency (time between copulation and oviposition) was not influenced by female mobility, contrary to our prediction, but depended on mating latency. This could indicate that the timings of both mating and egg-laying depend on the same plastic or genetic determinants. For example, mobile females might be less selective for both mates and oviposition sites, or could be more efficient at detecting both of these resources. This result, however, should be interpreted with caution, because our design did not allow mobile individuals to disperse to new habitats. Such experimental constraints should have less impact on the timing of copulations because Pieris females typically mate before dispersing (Jones et al., 1980; Ohsaki, 1980; Hirota, 2004). However, mobile females were forced to lay eggs where they mated. Hence, the time usually devoted to disperse cannot be measured. Future experiments are needed to investigate how mobility affects the timing of oviposition in a more complex design allowing for the occurrence of dispersal events. Importantly, roughly half of the mated females laid eggs in this study, which is relatively low. This could indicate strong competition for the only spawning site (one cabbage per cage), but this is inconsistent with the frequency of oviposition events (i.e., only two per day on average). Alternatively, the negative correlation between mating latency and oviposition latency may suggest that mating late significantly decreases oviposition opportunities, for example, if females have limited time to find or secure spawning sites.
Conclusion
Mobility is a critical determinant of the timing of copulations in P. brassicae. We suspect that this should be the case for species with similar characteristics, that is, species experiencing scramble competition, with high variability in dispersal strategies and a short adult phase. Overall, our results are in agreement with the oogenesis-flight syndrome, that is, mobile individuals seem to invest less in reproduction by being less selective for mates and copulating for shorter periods. However, we cannot conclude whether the patterns we observed reflect this syndrome as a cause (costs of flight as a cue of life expectancy) or a consequence (e.g., mobile individuals finding mates more rapidly). Future research should focus on the adaptive nature of such effect, for example, by measuring the life expectancy and reproductive output of individuals with contrasting mobility using different pair types (mobile–mobile, sedentary–mobile and sedentary–sedentary), and allowing for dispersal movements. It would also be interesting to test how forcing a delay to copulation for both males and females affects other aspects of reproduction (copulation duration and oviposition latency) as well as individual fitness.
Acknowledgments
The authors want to thank A. Fingerle, S.Ó. Steingrímsson, C.A.L. Leblanc, and several anonymous reviewers for helpful comments on previous versions of this manuscript. This study was funded by the Agence Nationale de la Recherche through DIAME (open call, 2007), MOBIGEN (6th extinction call 2009), and INDHET (open call, 2012) grants. This work is part of the Laboratoire d'Excellence TULIP (ANR-10-LABX-41).
Contribution of Authors
All authors participated in designing the experiment. D.L. and O.C. carried out the laboratory work for diapausing butterflies. N.L. set up the experiment, collected, and analyzed the data. N.L. wrote the first draft of the manuscript and all authors made improvements on the manuscript.
Disclosure
The authors declare no conflict of interest.




